Automated Acoustic Dispensing for the Serial Dilution of Peptide Agonists in Potency Determination Assays
Summary
Peptide adsorption to plasticware during traditional tip-based serial dilutions can significantly impact potency determination and confound the understanding of structure-activity relationships used for lead identification and lead optimization phases of drug discovery. Here methods for automated acoustic non-contact serial dilution of peptide samples are described.
Abstract
As with small molecule drug discovery, screening for peptide agonists requires the serial dilution of peptides to produce concentration-response curves. Screening peptides affords an additional layer of complexity as conventional tip-based sample handling methods expose peptides to a large surface area of plasticware, providing an increased opportunity for peptide loss via adsorption. Preventing excessive exposure to plasticware reduces peptide loss via adherence to plastics and thus minimizes inaccuracies in potency prediction, and we have previously described the benefits of non-contact acoustic dispensing for in vitro high-throughput screening of peptide agonists1. Here we discuss a fully integrated automation solution for non-contact acoustic preparation of peptide serial dilutions in microtiter plates utilizing the example of screening for peptide agonists at the mouse glucagon-like peptide-1 receptor (GLP-1R). Our methods allow for high-throughput cell-based assays to screen for agonists and are easily scalable to support increased sample throughput, or to allow for increased numbers of assay plate copies (e.g., for a panel of more target cell lines).
Introduction
The GLP-1R is an established drug target in the treatment of type 2 diabetes2. The native peptide agonist for this receptor, GLP-1, has an in vivo half-life of 2-3 min3. The binding of GLP-1 to its G protein coupled target receptor results in the downstream production of the second messenger cAMP through native G protein coupling to the activation of adenylyl cyclase. Measurement of the accumulated cAMP provides a robust assay to monitor receptor activation and to screen for active GLP-1 analogues with preferred physicochemical properties. Such an assay requires the serial dilution of test samples to construct concentration-response curves, and this is particularly complicated when handing peptide samples. Potential errors from tip-based serial dilution preparation have been described previously1,4,5. Peptides will adsorb to plasticware, resulting in unreliable potency estimations. Peptide loss can be minimized through the inclusion of bovine serum albumin (BSA) in buffers and the use of siliconized plasticware, yet protein binding remains unpredictable. In particular, the variation in binding of GLP-1 to experimental containers has been described6. There is a further complication in that stabilization agents used in laboratory plasticware can leach from tips and microtiter plates into aqueous assay buffers and interfere with protein function7, 8. Therefore, methods to reduce exposure to plasticware are necessary to increase the accuracy of measurements.
Acoustic liquid dispensers focus a high-frequency acoustic signal onto the surface of a fluid sample, resulting in the ejection of precise nanoliter droplets into an adjacent assay plate9. The use of acoustic ejection is standard in the pharmaceutical industry for the preparation and screening of large synthetic compound libraries, and the technology has been well validated for small molecules10. To our knowledge, we are the first group to describe acoustic dispensing for the preparation of recombinant and synthetic peptides and we have previously reported the improved accuracy compared to conventional tip-based methods1.
This article describes the integration of the preparation of peptide serial and direct dilutions by non-contact acoustic transfer onto a fully automated plate handling robotics system. A number of methods encompassing acoustic transfer of samples have been described previously11. We utilize a two-step method to prepare intermediate stock concentrations and to serially dilute peptide analogues for the generation of the full dose-response curve. The prepared peptides are incubated with cells expressing the target mouse GLP-1R, and we use a commercially available homogenous time-resolved fluorescence (HTRF) assay to measure cAMP accumulation within these cells as a readout of peptide agonist activity. The assay is robust and amenable to a high-throughput 384-well format and routinely applied to both assay development and drug screening projects12.
Protocol
1. Peptide Serial Dilution
- Prepare assay buffer: Hanks buffered salt solution (HBSS) supplemented with 25 mM HEPES, 0.1% BSA and 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), pH 7.4.
- Use a bulk reagent dispenser to systematically add 5 µl of assay buffer to each well of five 384-well low volume assay plates.
- Use internal software to create a dispensing program for 5 µl volume addition to every well of a 384-well plate as per manufacturer's instructions.
- Immerse dispensing cassette tubing in assay buffer and prime fluid.
- Place 384-well low volume assay plate on plate carrier.
- Press start.
- Dilute all peptide samples, regardless of storage vehicle, into assay buffer to produce a 100x peptide stock.
NOTE: Peptides requiring screening may be provided in Phosphate-buffered saline (PBS) or Dimethyl sulfoxide (DMSO) as is deemed appropriate (e.g., DMSO will have a deleterious effect on peptides with secondary modifications such as PEGylation). - Dispense 25 µl 100x peptide stocks into columns 1-5 of an acoustically qualified 384-well polypropylene microplate. This plate is designated 'source plate A'. Ensure that source plates, but not destination plates, are flat bottomed and conform to specific acoustic tolerances as defined by the manufacturer of acoustic instruments.
- Dispense 25 µl 100x reference control into wells A23 and A24 of source plate A.
- Dispense 10 µl assay buffer into columns 11-15 and 30 µl assay buffer into columns 21-22 of source plate A.
- Dispense 10 µl assay buffer into columns 6-10 and columns 16-20 of a second acoustically qualified 384-well polypropylene microplate. This plate is designated 'source plate B'.
- Centrifuge source plates A and B at 300 x g for 1 min. Include an appropriate balance plate.
- Use acoustic fluid dispensers integrated into an automated robotic system to prepare three sequential 1:100 intermediate dilutions (in assay buffer) of the 100x peptide stocks from column 1 in source A.
NOTE: Programming requires plate reformatting and dose response software (as provided by the manufacturer of the acoustic fluid dispenser) to allow three acoustic transfers between source A and source B (see Figure 1 for plate layouts). Step 1.9 details specifically the use of fluid dispensers used in this laboratory under automated robotic control (see Materials Table):- Load source plates and assay plates into plate hotel section of robotics.
- Open automated robotic software and load plate reformatting and dose response program expansion protocols. Click 'Run'.
NOTE: Automation instructs an acoustic fluid dispenser to transfer 250 nl from columns 1-5 of source plate A into columns 6-10 of source plate B, and a second acoustic fluid dispenser capable of handling larger fluid volumes to backfill with 15 µl assay buffer to mix. Automation then transfers source plate B to integrated centrifuge and centrifuges at 300 x g for 1 min (an appropriate balance plate is included for all centrifugation steps). Source plate B is returned to the acoustic fluid dispenser for the transfer of 250 nl from columns 6-10 of source plate B into columns 11-15 of source plate A and backfill with 15 µl assay buffer to mix. Automation transfers source plate A to integrated centrifuge and centrifuges at 300 x g for 1 min. Source plate A is then returned to the acoustic fluid dispenser for the transfer of 250 nl from columns 11-15 of source plate A into columns 16-20 of source plate B and backfill with 15 µl assay buffer to mix. Automation then transfers source plate B to integrated centrifuge and centrifuges at 300 x g for 1 min.
NOTE: Finally the dose response protocol directs acoustic dispensing to transfer the required volume from each of the 4 serially diluted source plate wells (in both source plate A and source plate B) to construct a full 11-point curve in duplicate in assay plates (pre-filled with 5 µl assay buffer in step 1.2 above). Automation transfers assay plate to integrated centrifuge and centrifuges at 300 x g for 1 min.
2. Cell Preparation
- Thaw cryopreserved Chinese hamster ovary (CHO) cells expressing the target mouse GLP-1 receptor rapidly in a 37 ºC water bath and resuspend in 20 ml assay buffer.
- Centrifuge cell suspension for 5 min at 200 x g at room temperature (RT). Include an appropriate balance.
- Discard supernatant and resuspend cell pellet in 10 ml assay buffer.
- Dilute cell stock 1:1 in Trypan blue and determine viable cell density using an automated cell counter. Resuspend cells in assay buffer at 1.6 x 106 cells per ml (equivalent to 8,000 cells per well of assay plates).
- Use a bulk reagent dispenser to add 5 µl of cell suspension to each well of the assay plates (containing serially diluted peptides in 5 µl assay buffer) and incubate at RT for 30 min.
3. HTRF cAMP Detection Assay
- Bring HTRF cAMP assay kit to RT for 30 min prior to use.
- Prepare each HTRF reagent (cryptate and d2) separately at 1:20 dilution in lysis buffer (proprietary formulation, provided by manufacturer of cAMP detection assay).
NOTE: CAUTION: Lysis buffer contains potassium fluoride (KF) which is toxic and a teratogen13. For disposal, assay plates containing KF should be sealed and incinerated, and any liquid waste must be diluted to <1 mmol/L with water prior to disposal down the sink. - Use a bulk reagent dispenser to add 5 µl cryptate reagent to all wells of the assay plates.
- Use a bulk reagent dispenser to add 5 µl d2 reagent to columns 1-22 of the assay plates.
- Immediately manually pipette 5 µl lysis buffer into wells A23-D24 of the assay plates to give non-specific binding (NSB) control wells, and 5 µl of d2 reagent into wells E23-P24.
- Centrifuge the assay plates for 1 min at 200 x g at RT to mix the wells.
- Cover the assay plates to minimize evaporation and photo-bleaching and incubate at RT for 1 hr.
- Measure fluorescence resonance energy transfer (FRET) signal using excitation at 320 nm and emission at 620 nm and 665 nm using a plate reader.
4. Data Analysis
- Use mean NSB to subtract background from all wells.
- Calculate %Delta F from the 665 nm/620 nm ratio as follows:
Ratio = (A665nm/A620nm) x 104
Delta F = ((Sample Ratio – RatioNSB)/RatioNSB)) x 100
where RatioNSB = wells with no d2 reagent. - Calculate % activation between unstimulated cells and cells stimulated with maximum GLP-1 ligand as follows:
% activation = (%Delta Fsample – %DeltaFunstimulated cells)/(%DeltaFGLP-1 stimulated cells – %DeltaF unstimulated cells) x 100 - Analyze concentration-response curves via 4-parameter logistical analysis, and graph as % activation as defined by reference control1.
Representative Results
We routinely use a two-step method to dilute peptides via acoustic transfer. For the first step, an acoustic dispenser aligned with automation is used to create four stock peptide intermediate dilutions across two source plates (Figure 1a, b). For the second step, we use an acoustic dispenser to further dilute stock dilutions from source plates A and B to create an 11-point concentration range for each test peptide (Figure 1c). Each peptide concentration range is fired in duplicate, left to right across the assay plate, allowing 16 different peptides to be screened per 384-well assay plate (Figure 1c). Assay buffer backfill is performed to ensure a constant volume per well across the assay plate (Figure 1d). Reference peptide positive controls are also transferred into destination plates at maximal effective concentration.
Plates are read using a fluorescent plate reader, and a heat map for an example plate containing 16 peptides in duplicate is shown (Figure 2a). The cAMP accumulation assay used is an inverted competition assay, thus low values (shown as purple) represent a high concentration of cAMP. Reproducibility of duplicate points is evident when data is expressed as concentration-response curves (Figure 2b). Sample 1 (row A, Figure 2a) represents a positive control for the assay. Samples 2, 3 and 4 (rows B, C and D respectively, Figure 2a) were inactive and equate to the flat lines in Figure 2b. Samples 5-16 (rows E to P) represent active test peptides. The method allows for the generation of full concentration-response curves across a broad potency range.
A Flow Chart detailing the entire work flow is shown in Figure 3.
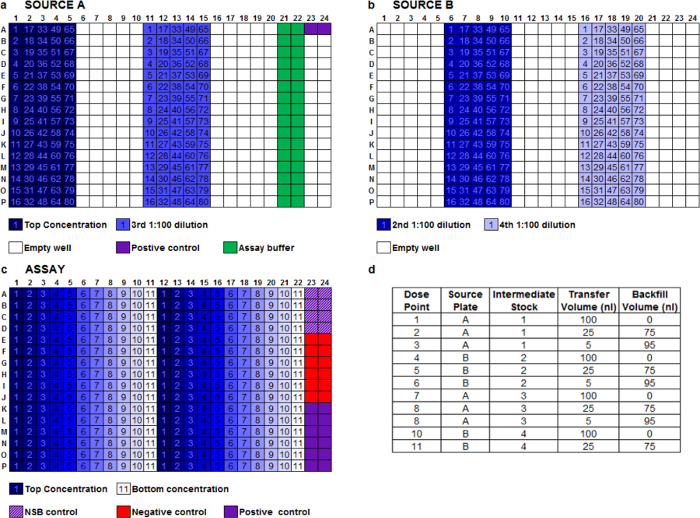
Figure 1: Plate Layouts. Description of the acoustic dispensing serial dilution method. (a, b) Layout of a 384-well source plates A and B containing 25 µl of 100x stock concentration of each peptide (example with 80 peptides possible per source plate), 100x positive control, assay buffer-only wells for backfill, and three 1:100 serial dilutions of each peptide prepared by automated acoustic transfer and backfill between both source plates. (c) A single assay plate layout showing 11-point concentration range for 16 peptides in duplicate across the plate. (d) Typical dispense protocol for an 11-point concentration range in a 10 µl final assay volume. All destination wells have an assay buffer backfill to provide a constant final transfer volume of 100 nl per well. Please click here to view a larger version of this figure.
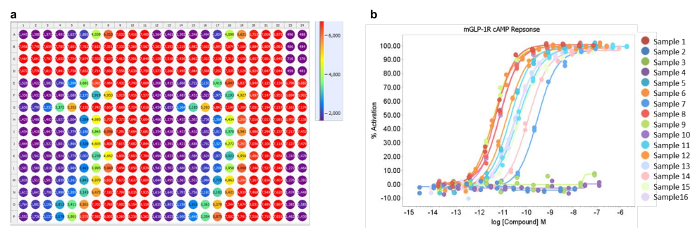
Figure 2: Heat map and dose-response curves. Heat map showing fluorescence at A665 nm. (a) 11-point concentration range for 16 peptides in duplicate across the plate. Purple indicates a high concentration of cAMP (max), red indicates a low concentration of cAMP (min). (b) Representative peptide concentration-response curves from a single plate assay showing a range of GLP-1 receptor agonist peptide activities against mouse GLP-1R expressing cells (16 compounds). Please click here to view a larger version of this figure.
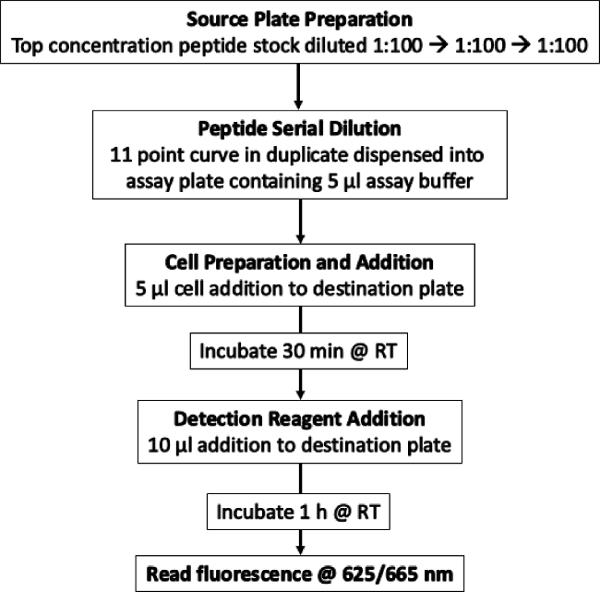
Figure 3: Flow Chart of the screening process. Detail of the entire workflow. Please click here to view a larger version of this figure.
Discussion
This protocol describes the successful application of automated acoustic dispensing to serially dilute peptide samples over a concentration range of 3 x 106 requiring less than 1 µl of sample. The major advantage of this method is to increase data quality through minimizing peptide adsorption to plasticware via reduced exposure of samples to experimental containers and plasticware (such as pipette tips) that are normally required for reagent transfer and mixing. While acoustic dispensing does not completely rule out the use of plasticware, it does significantly reduce it. Furthermore, the removal of tip-based pipetting also eliminates sample carryover, and errors are not propagated throughout the entire serial dilution, allowing for both reliable and reproducible preparation of concentration-response curves14,15.
Other groups have reported the direct acoustic transfer of test compounds solubilized in DMSO to cells for long-term assays (>48 hr) with no mixing steps required other than inversion of the assay plate and incubation over time16,17. DMSO is highly polar and is much more amenable to mixing with aqueous solutions than biological samples that must be prepared and stored in PBS. Therefore, when adopting an automated method for the acoustic transfer of peptide samples, we have employed additional backfill and centrifugation steps to ensure thorough mixing, allowing for accurate sample serial dilution. Our experience shows that centrifugation alone is not sufficient to ensure complete mixing of aqueous samples, and thus, a 'wet transfer' method was developed consisting of acoustic transfer into an existing 10 µl volume of aqueous buffer, followed by a 15 µl backfill of the same aqueous buffer. It is not possible to fire peptides into a dry source plate and then back fill as one might for small molecules, as the aqueous peptide solution will rapidly evaporate and the peptide will irreversibly adsorb to the dry plastic plate. Although it does not aid mixing of aqueous samples, centrifugation is still required to ensure a level sample meniscus for acoustic transfer. The inclusion of multiple intermediate transfer, backfill and centrifugation steps is somewhat time consuming, although less so than manual preparation of large numbers of samples, and the use of automation allows unsupervised throughput. Others have reported the need for manual compound mixing in 96-well plates17. Here we have fully automated the process and removed the need for manual intervention when the use of DMSO is not possible. The cost of instrumentation and consumables will limit the use of this process in some settings. If this is the case, we recommend tip-based serial dilutions are always performed in DMSO if the nature of the test sample is amenable to this (e.g., synthetic peptides). If samples cannot be treated with DMSO (e.g., PEGylated peptides) caution should be used when interpreting potency data, since we have previously shown even the inclusion of increasing concentrations of BSA will not completely prevent adsorption1.
Acoustic, tip-less transfer has additional benefits relating to assay miniaturization which is essential if samples are scarce/finite as is often the case for animal or human biological samples, and we have previously described the potential for reducing the volume of animal samples leading to a requirement for fewer animals per study1. It is important to remember that peptides are biological samples and should be handled as such; in order to maintain their integrity repeat freeze-thaw cycles should be avoided. Biologics is a rapidly growing area of novel therapeutics, and our automated high-throughput method for the preparation of peptides can be easily applied to other biological agents including recombinant proteins and antibodies.
Disclosures
The authors have nothing to disclose.
Acknowledgements
None.
Materials
| Hanks’ Balanced Salt solution | Sigma-Aldrich | H8264 | |
| HEPES | Sigma-Aldrich | H3375 | |
| Bovine Serum Albumin | Sigma-Aldrich | A9418 | |
| 3-Isobutyl-1-methylxanthine | Sigma-Aldrich | I7018 | Prepared as a 0.5 M stock in DMSO |
| GLP-1 (7-36) amide | Bachem | H-6795 | Prepared as a 1 mg/ml stock in PBS, referred to as '100X reference control' |
| Test peptides | Produced in-house at MedImmune | Supplied at various concentrations in DMSO or PBS as appropriate | |
| 100X peptide stock | Produced in-house at MedImmune | Test peptide diluted into assay buffer to 100X final required concentration | |
| Trypan Blue Solution, 0.4% | Thermo Fisher Scientific | 15250-061 | |
| Cedex XS Cell Analyzer | Innovatis | ||
| Corning 384 well plates, low volume | Sigma-Aldrich | 4514 | |
| Echo Qualified 384-Well Polypropylene Microplate | Labcyte Inc. | P-05525 | |
| Echo Qualified Reservoir | Labcyte Inc. | ER-0055 | |
| Echo 550 Liquid Handler | Labcyte Inc. | Droplet transfer volumes in increments of 2.5 nl | |
| Echo 525 Liquid Handler | Labcyte Inc. | Droplet transfer volumes in increments of 25 nl | |
| ACell Benchtop Automation | HighRes Biosolutions | MC522 | |
| Cellario Lab Automation Scheduling software for Life Science Robotics | HighRes Biosolutions | ||
| MultidropCombi Reagent Dispenser | ThermFisher Scientific | 5840300 | Referred to as 'bulk reagent dispenser' |
| HTRF cAMP Dynamic 2 kit | Cisbio Bioassays | 62AM4PEJ | |
| EnVision Multilabel Reader | PerkinElmer |
References
- Naylor, J., Rossi, A., Hornigold, D. C. Acoustic Dispensing Preserves the Potency of Therapeutic Peptides throughout the Entire Drug Discovery Workflow. J.Lab.Autom. 21 (1), 90-96 (2016).
- Campbell, J. E., Drucker, D. J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell.Metab. 17 (6), 819-837 (2013).
- Hui, H., Farilla, L., Merkel, P., Perfetti, R. The short half-life of glucagon-like peptide-1 in plasma does not reflect its long-lasting beneficial effects. Eur.J.Endocrinol. 146 (6), 863-869 (2002).
- Harris, D., Olechno, J., Datwani, S., Ellson, R. Gradient, contact-free volume transfers minimize compound loss in dose-response experiments. J.Biomol.Screen. 15 (1), 86-94 (2010).
- Ekins, S., Olechno, J., Williams, A. J. Dispensing Processes Impact Apparent Biological Activity as Determined by Computational and Statistical Analyses. PLoS ONE. 8 (5), 62325 (2013).
- Goebel-Stengel, M., Stengel, A., Tache, Y., Reeve, J. R. The importance of using the optimal plasticware and glassware in studies involving peptides. Anal.Biochem. 414 (1), 38-46 (2011).
- McDonald, G. R., et al. Bioactive Contaminants Leach from Disposable Laboratory Plasticware. Science. 322 (5903), 917 (2008).
- Belaiche, C., Holt, A., Saada, A. Nonylphenol ethoxylate plastic additives inhibit mitochondrial respiratory chain complex I. Clin Chem. 55 (10), 1883-1884 (2009).
- Sackmann, E. K., et al. Technologies That Enable Accurate and Precise Nano- to Milliliter-Scale Liquid Dispensing of Aqueous Reagents Using Acoustic Droplet Ejection. J.Lab.Autom. 21 (1), 166-177 (2016).
- Grant, R. J., et al. Achieving accurate compound concentration in cell-based screening: validation of acoustic droplet ejection technology. J.Biomol.Screen. 14 (5), 452-459 (2009).
- Turmel, M., Itkin, Z., Liu, D., Nie, D. An Innovative Way to Create Assay Ready Plates for Concentration Response Testing Using Acoustic Technology. J.Lab.Autom. 15 (4), 297-305 (2010).
- Butler, R., et al. Use of the site-specific retargeting jump-in platform cell line to support biologic drug discovery. J.Biomol.Screen. 20 (4), 528-535 (2015).
- Panchal, S., Verma, R. J. Effect of sodium fluoride in maternal and offspring rats and its amelioration. Asian Pac.J.Reprod. 3 (1), 71-76 (2014).
- Hanson, S. M., Ekins, S., Chodera, J. D. Modeling error in experimental assays using the bootstrap principle: understanding discrepancies between assays using different dispensing technologies. J.Comput. Aided Mol.Des. 29 (12), 1073-1086 (2015).
- Harris, D., Olechno, J., Datwani, S., Ellson, R. Gradient, Contact-Free Volume Transfers Minimize Compound Loss in Dose-Response Experiments. J.Biomol. Screen. 15 (1), 86-94 (2010).
- Chan, G. K. Y., Wilson, S., Schmidt, S., Moffat, J. G. Unlocking the potential of high-throughput drug combination assays using acoustic dispensing. J.Lab.Autom. 21 (1), 125-132 (2016).
- Roberts, K., et al. Implementation and challenges of direct acoustic dosing into cell-based assays. J.Lab.Autom. 21 (1), 76-89 (2016).

