High-Quality Seizure-Like Activity from Acute Brain Slices Using a Complementary Metal-Oxide-Semiconductor High-Density Microelectrode Array System
Summary
Here, we outline a protocol for using complementary metal-oxide-semiconductor high-density microelectrode array systems (CMOS-HD-MEAs) to record seizure-like activity from ex vivo brain slices.
Abstract
Complementary metal-oxide-semiconductor high-density microelectrode array (CMOS-HD-MEA) systems can record neurophysiological activity from cell cultures and ex vivo brain slices in unprecedented electrophysiological detail. CMOS-HD-MEAs were first optimized to record high-quality neuronal unit activity from cell cultures but have also been shown to produce quality data from acute retinal and cerebellar slices. Researchers have recently used CMOS-HD-MEAs to record local field potentials (LFPs) from acute, cortical rodent brain slices. One LFP of interest is seizure-like activity. While many users have produced brief, spontaneous epileptiform discharges using CMOS-HD-MEAs, few users reliably produce quality seizure-like activity. Many factors may contribute to this difficulty, including electrical noise, the inconsistent nature of producing seizure-like activity when using submerged recording chambers, and the limitation that 2D CMOS-MEA chips only record from the surface of the brain slice. The techniques detailed in this protocol should enable users to consistently induce and record high-quality seizure-like activity from acute brain slices with a CMOS-HD-MEA system. In addition, this protocol outlines the proper treatment of CMOS-HD-MEA chips, the management of solutions and brain slices during experimentation, and equipment maintenance.
Introduction
Commercially available high-density microelectrode array (HD-MEA) systems, which include an MEA chip with thousands of recording points1,2 and an MEA platform to amplify and digitize the data, are an emerging tool for electrophysiological research. These HD-MEA systems use complementary metal-oxide-semiconductor (CMOS) technology to record electrophysiological data with high sensitivity from cell cultures and ex vivo brain slice preparations. These MEA systems afford unprecedented spatial and temporal resolution to neurophysiological research via high electrode density and quality signal-to-noise ratios3. This technology has mostly been used to study extracellular action potentials, but it can also capture high-quality local field potentials (LFPs) from various neuronal brain slice preparations4,5,6,7,8,9,10,11,12,13,14,15. Due to the above-mentioned high-resolution recording capability of CMOS-HD-MEA systems, users can track electrophysiological activity with great spatial accuracy16,17,18. This capability is particularly relevant to tracking propagation patterns of network LFPs5,12,15,19,20,21. Therefore, CMOS-HD-MEA systems can provide an unprecedented understanding of the propagation patterns of physiological and pathological activity from various cell culture and brain slice preparations. Of particular note, these capabilities of CMOS-HD-MEA systems can allow researchers to contrast seizure patterns of different brain regions simultaneously and assay how various anti-epileptic compounds affect these patterns. By doing so, it provides an innovative method for studying ictogenesis and ictal propagation and for understanding how pharmacology disrupts pathological network activity7,10,14. Therefore, these novel capacities of CMOS-HD-MEA systems can contribute significantly to the research of neurological disorders, as well as aid in drug discovery research5,7,11,22. We aim to provide details on using CMOS-HD-MEA systems to study seizure-like activity.
When using CMOS-HD-MEA systems to study LFPs, such as epileptiform activity in acute brain slices, users can face many challenges, including debilitating electrical noise, keeping the slice healthy during experimentation, and detecting a quality signal from a two-dimensional (2D) CMOS-MEA chip that records only from the surface of the brain slice. This protocol describes basic steps for properly grounding the MEA platform and other equipment used in experimentation, a crucial step that may require individual customization for each lab setup. In addition, we discuss steps to aid in keeping the brain slice healthy during long recordings in the submerged chambers used with CMOS-HD-MEA systems23,24,25. Additionally, in contrast to more common electrophysiological recording methods, which record from deep in the brain slice, most CMOS-HD-MEA systems use 2D chips that do not penetrate into the slice. Therefore, these systems require a healthy neuronal outer layer to produce the majority of the recorded LFP signals. Other challenges include visualizing the massive amount of data generated by thousands of electrodes. To overcome these challenges, we recommend a simple but effective protocol that increases the likelihood of achieving high-quality network epileptiform activity that propagates across the brain slice. We also include a brief description of a publicly available graphic user interface (GUI) we developed with associated resources to aid in data visualization10.
Previous publications have provided related protocols for the use of MEA recording systems26,27,28,29. However, this work aims to assist experimenters using CMOS-HD-MEA systems with 2D chips, specifically those seeking to study high-quality epileptiform activity from brain slices. In addition, we compare two of the most common solution manipulations for induction of seizure-like activity, namely the 0 Mg2+ and 4-AP paradigms, to help users identify the most appropriate convulsant media for their specific application. Although the protocol is focused primarily on the generation of seizure-like activity, it can be modified to explore other electrophysiological phenomena using brain slices.
Protocol
Procedures involving mice were approved by the Institutional Animal Care and Use Committee (IACUC) at Brigham Young University. Male and female (n = 8) C57BL/6 mice aged to at least P21 were used in the following experiments.
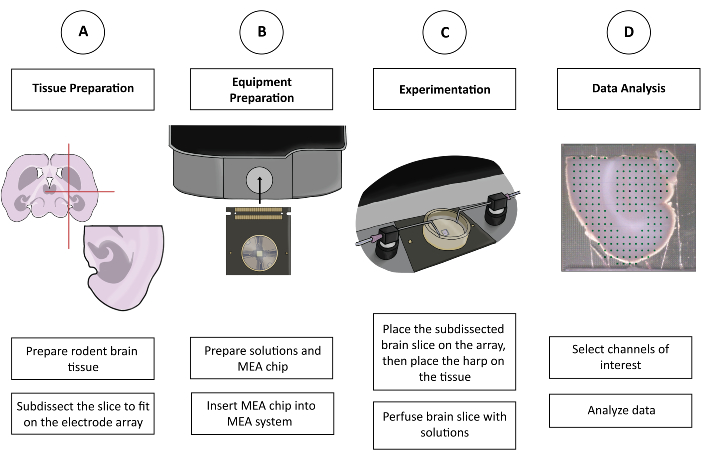
Figure 1: Schematic figure of CMOS-HD-MEA experimentation. (A) The brain slice is prepared by one's preferred cutting method and sub-dissected to fit on the MEA. (B) Prepare the solutions and the CMOS-HD-MEA chip. (C) The sub-dissected brain slice is placed on the electrode array and bathed in the appropriate solutions. (D) Relevant channels are selected from the collected data. Data is then prepared for analysis in the preferred program of the user. Please click here to view a larger version of this figure.
| Name | Concentration (mM) | g/L | ||
| Sodium Chloride (NaCl) | 126 | 7.36 | ||
| Potassium Chloride (KCl) | 3.5 | 0.261 | ||
| Dihydrogen Sodium Phosphate (NaH2PO4) | 1.26 | 0.151 | ||
| Sodium Bicarbonate (NaHCO3) | 26 | 2.18 | ||
| Glucose (C6H12O6) | 10 | 1.80 | ||
| Magnesium Chloride (MgCl2) | 1 (from 1 M stock) | 1 mL | ||
| Calcium Chloride (CaCl2) | 2 (from 1 M stock) | 2 mL | ||
Table 1: aCSF solution.
1. Preparing solutions
- Prepare the experimental solutions.
- Prepare 1 L of artificial cerebrospinal fluid (aCSF) (see Table 1 for details).
- Prepare 1 L of pro-convulsant solution.
NOTE: Solutions used to generate seizure-like activity in this protocol utilized either aCSF with 100 µM 4-Aminopyridine (4-AP) or aCSF free of magnesium ions. - Carbogenate all solutions for at least 10 min before use using porous stones.
- Obtain a beaker for discarding solutions.
- Place 1 L of aCSF, 1 L of pro-convulsant solution, and the discard beaker on a surface close to the perfusion system.
- Preparing the cutting and holding chamber solutions
- Prepare 0.5 L of aCSF, omitting CaCl2 and adding 3 mM MgCl2 (as opposed to 1 mM) to create a unique cutting solution. Retain this solution for use in rodent brain slice preparation by placing it near the acute brain slice preparation area.
- Prepare 0.5 L of aCSF and place it in a tissue-holding chamber that will be used to retain slices for use in experimentation. Insert porous stones into the tissue holding chamber and carbogenate the solution for at least 10 min before placing the sub-dissected slices in the chamber.
2. Preparing rodent brain slices
- Prepare all solutions as described above (see section 1). Ensure that all solutions are properly carbogenated during the preparation process.
NOTE: The cutting solution (see section 1.2) is recommended, but other cutting solutions can be used to obtain quality brain slices, such as a sucrose cutting solution30. - Use a vibrating microtome to make 350 µm rodent brain slices. Protocols for using these instruments are listed in the references30,31,32,33.
- Prepare the rodent brain slices to fit on the recording electrode area of the MEA chip (see Figure 2). Use a size 10 surgical blade to sub-dissect the slices, as shown in Figure 1A and Figure 2A , by gently rolling the blade back and forth on the brain slice. Perform the sub-dissection in the vibrating microtome cutting chamber. Use caution not to damage the sub-dissected brain slice.
- Place the sub-dissected slices in a tissue-holding chamber filled with aCSF. Ensure that the chamber has been carbogenated for at least 10 min before adding the sub-dissected slices.
3. Equipment preparation
- Preparing the MEA chips and system (Figure 2 and Figure 3)
NOTE: Hard materials like plastics and metals can easily damage the MEA chip if they forcefully contact the bottom of the chip well. When using pipettes to transfer the solution into or out of the chips, do not contact the bottom of the chip well with the pipette, especially the reference electrodes on the sides of the chip well and the recording electrodes in the center of the chip well (Figure 2C). Instead, add the solution by hovering over the bottom of the chip well or by contacting or approximating the plastic edges of the chip well. To pull out all solution easily, tilt the chip slightly to allow the solution to pool to one side of the chip well and remove it from the top of the pooled solution, or use an anti-static wipe to dab lightly at areas that still have the solution. Do not transport the chip by holding the chip well or the chip pins (Figure 2C). The chip well can hold approximately 4 mL of solution. For subsequent steps, fill the chip well with approximately 2 mL of solution if not otherwise specified.- Designate transfer pipettes for various tasks before beginning chip preparation. Label one transfer pipette for ethanol, one for waste, another for aCSF, and others for any remaining solutions to prevent unintentional mixing.
- Fill the well of the MEA chip with 190-proof ethanol so that the bottom of the chip well is completely covered (Figure 3). Let the ethanol sit for 30-60 s, then remove it with a discard pipette.
- Fill the well of the MEA chip with aCSF and remove it with a discard pipette to rinse residual ethanol out of the chip well. Add and remove aCSF from the chip well three times, using the previously designated waste and aCSF pipettes. After washing the chip well three times, add aCSF and let it stand for at least 30 s.
NOTE: The recording electrodes exhibit the least amount of noise from hydrophobic interactions when aCSF remains in the chamber for at least 45 min after rinsing with ethanol and aCSF. - Before docking the MEA chip, wet an anti-static wipe with 190-proof ethanol and use it to wipe the chip's pins (Figure 2C).
- Gently slide the MEA chip into the MEA platform and engage the docking mechanism to lock the chip into place.
- Check the recording and reference electrodes for bubbles (Figure 2C). If bubbles are present, take a clean paintbrush and lightly sweep over the electrodes to remove them.
- Check the chip for noise using the CMOS-HD-MEA software34 and visually scan the false color map for bubbles, non-biological oscillations, or spikes caused by electrical interference. Ground the MEA system appropriately to negate any encountered noise.
NOTE: The grounding setup will depend on the recording environment. For the experiments in this protocol, the MEA platform and the perfusion system were grounded.
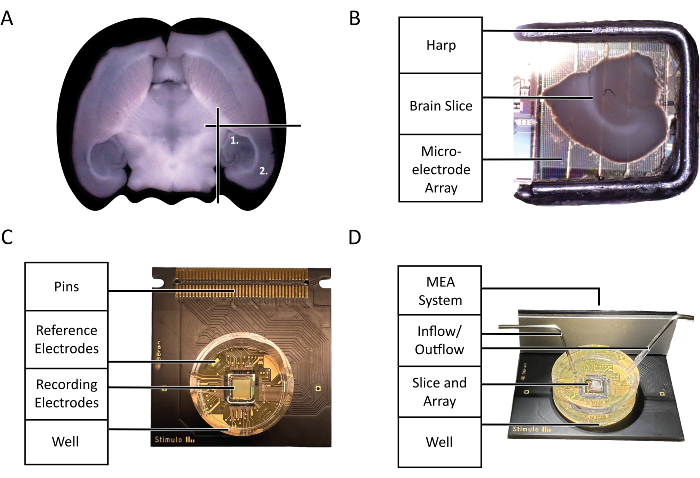
Figure 2: Configuration and technology diagrams. (A) Diagram of the selection of acute mouse brain slices used in the experiment highlighted by this protocol. (1) Hippocampal region (2) Neocortex region. (B) The proper placement of an acute mouse brain slice and harp on the microelectrode Array (MEA). (C) The anatomy of a 3Brain Accura CMOS-HD-MEA chip. (D) The proper configuration of perfusion inlets and outlets. Input should be deep in the chip well, whereas the output should be on the opposite side of the inlet at the top of the chip well to guarantee a constant flow of fresh, oxygenated aCSF. Please click here to view a larger version of this figure.
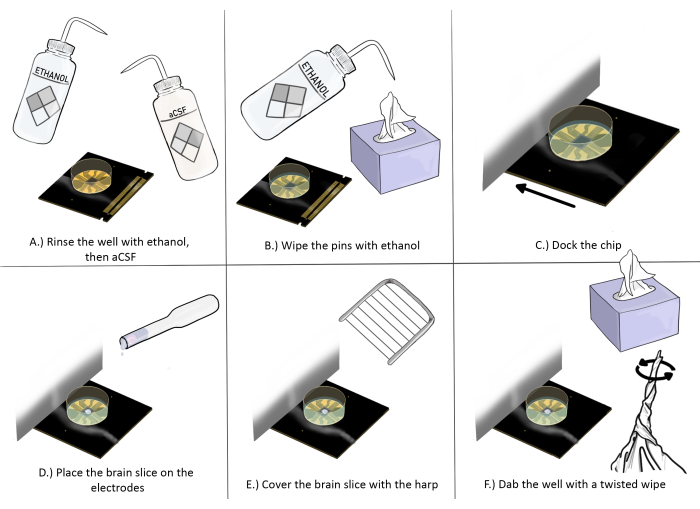
Figure 3: Schematic diagram of chip preparation and placement for brain slice experiments. (A) Rinse the chip well with ethanol once, then aCSF three times. (B) Wipe the pins with ethanol using an anti-static wipe. (C) Dock the chip. (D) Place the brain slice on the electrodes. (E) Place the harp on the brain slice (see Figure 2 for proper placement guidelines). (F) Dab the corner of the recording electrodes' well near the brain slice with a twisted anti-static wipe. Please click here to view a larger version of this figure.
4. Experimentation
- Placing the brain slice
- Place a platinum harp in a weigh boat (or another clean surface) near the MEA platform. Cover the harp with about 3mL of aCSF to reduce its hydrophobic tendencies.
- Use scissors to remove the thin tip portion of a transfer pipette. Cut off a third, about 1.5 in, of the pipette tip.
NOTE: This modified pipette will not constrict or damage the brain slice during collection and transportation. - Collect a brain slice from the slice-holding chamber with the modified pipette. Gently dispense the brain slice and any solution in the pipette into the chip well. To position the slice properly, gently release more aCSF from a transfer pipette to manipulate the position of the brain slice or use a soft paintbrush to create a current in the solution that pushes the brain slice onto recording electrodes. Limit contact with the recording electrodes or brain slice to minimize damage.
- Using forceps, gently place the harp over the brain slice with the threads downwards to press the slice onto the recording electrodes. Avoid contacting the electrode array with the harp. Orient the harp so that the side without a frame faces toward the inflow needle, and the frame of the harp does not contact any of the recording electrodes (Figure 2C, D).
- Take a discard pipette and remove excess aCSF. Take an anti-static wipe, twist a corner to create a tip, and use it to soak up the remaining aCSF surrounding the recording electrodes without touching the recording electrodes, brain slice, or harp (Figure 2).
- Using a designated aCSF pipette, quickly add enough carbogenated aCSF to cover the brain slice, about 2 mL.
- Repeat step 4.1.5. and 4.1.6. two more times.
- Fill the well with carbogenated aCSF until the well is roughly ¾ full, about 3 mL.
- Take a picture of the brain slice on the MEA chip with a microscope or camera. Ensure that the photo has a high enough resolution to see the borders of the recording electrode array and the anatomy of the brain slice.
- Running the experiment
- Operating the perfusion system
- Place the inflow and outflow tubes into the beaker filled with aCSF and the inflow and outflow needles into the chip well. Place the inflow needle close to the bottom of the chip well just outside the recording electrodes. Place the outflow needle close to the top of the chip well towards the edge so that the liquid rises almost to the brim of the chip well, about 4 mL, and the slice is submerged throughout the experiment (Figure 2D).
- Set the perfusion inflow to 5 mL/min and the perfusion outflow to 7 mL/min.
NOTE: It is recommended that the outflow rate exceeds the inflow rate to avoid solution overflow out of the chip well and to create a solution current over the brain slice. - Turn on the inflow and outflow. Remove the inflow needle from the chip well until the needle begins to output solution instead of air. Then, place the needle back to its position inside the chip well as described in step 4.2.1.1.
- Use a solution heater to keep the solution at or near physiological temperature, around 34-37 °C.
- Let the aCSF perfuse over the brain slice for 10 min. This will allow the slice to adjust to the recording environment.
- After 10 min has elapsed, move the outflow tube to the discard beaker. Then, move the inflow tube to the beaker containing the pro-convulsant solution. Allow the non-convulsant aCSF to be flushed out of the perfusion system into the discard beaker for 10 min.
- Transfer the outflow tube into the beaker containing the pro-convulsant solution.
- Let the pro-convulsant solution cycle until the experiment finishes.
- If the slice recording duration exceeds 2 h, consider preparing another pro-convulsant solution to offset the glucose consumption of the active brain slice.
- Exchanging brain slices
- Turn off the inflow. Turn off the outflow 10-15 s afterwards.
- Use forceps to remove the harp and place it onto a weigh boat or other surface.
- Use a modified pipette to extract and discard the brain slice. Do not touch the reference electrodes or the recording electrode array (Figure 2C).
- Place the inflow tube into the beaker containing the non-convulsant aCSF solution. Place the outflow tube in the discard beaker. Leave the inflow and outflow needles in the chip well. Run the perfusion system for 10 min to remove all residual pro-convulsant solution from the chip well and perfusion system. Begin the experimentation process again with a new brain slice (beginning at step 4.1).
- Operating the perfusion system
- Finishing the experiment
- Cleaning the rig
- Remove the harp, brain slice, and solution from the well, respectively.
- Undock the MEA chip, place it onto a clean surface, and fill the chip well with deionized water.
- Discard the deionized water to clear any salts left from the aCSF solutions.
- Using a transfer pipette, fill the chip well with a detergent solution. Hover the tip of the transfer pipette in the chip well and point it at the recording electrodes. Quickly and repeatedly squeeze and release detergent into the transfer pipette to vigorously wash detergent over the recording electrodes for 1 min. Let the detergent rest for 5-10 min.
- Remove the detergent, then rinse the chip well 4-6 times with deionized water to remove the detergent solution and any remaining solutes.
- Remove all the water from the chip well, then place an anti-static wipe over the MEA chip and leave overnight to allow the chip well to dry.
- Place a weigh boat or another watertight plate onto the MEA recording platform where the MEA chip previously rested. Place the harp in the center of the plate and place the inflow and outflow needles on either side of the harp.
- Clean the perfusion system and harp by sending 50 mL of water, 50 mL of 140-proof ethanol, and 200 mL of water, respectively, through the tubing into the discard beaker.
NOTE: At this time, perfusion speeds can be increased to decrease cleaning time, but the outflow speed must always exceed the inflow speed to avoid flooding. - Clean the carbogen stones by placing them in a beaker of distilled water and sending the carbogen through the tubing for 5 min. Set them out to rest on a dry, clean surface overnight and cover them to avoid dust accumulation.
- Cleaning the rig
5. Data analysis
NOTE: There are a variety of analysis packages used for analyzing electrophysiological data produced by CMOS-HD-MEAs, including BrainWave from 3Brain, Yet Another Spike Sorter (YASS), and custom Python tools34,35,36,37. We extracted data from the BrainWave data file format for use in the Xenon LFP Analysis platform to generate the data presented in Figure 4 and Figure 5. Custom Matlab code was used to analyze the data in Figure 6. Protocols for the Xenon LFP Analysis platform are publicly available10. The following protocol steps are specific to recordings made with Brainwave 438; for other systems, refer to supporting documentation related to those systems34,35,36,37. An overview of the analysis steps taken to produce the data with this protocol is provided below. For complete details of how to export, visualize, and analyze data, including tutorial videos and all relevant code files, see39.
- Export the recording file for analysis.
- Create a folder for the data recording file(s) that will be downsampled.
- Crop the image taken during step 4.1.9 to include only the recording array with the brain slices.
- Open the ExportToHDF5-ChannelSelection.py file (this code file can be accessed on GitHub under xenon-lfp-analysis/code-files/3Brain-processing40) and copy and paste the URL listed at the top of the window into an internet browser.
- Input the file path for the data recording file and upload the cropped image of the array associated with the recording.
- Under Select Channels for Export, use the lasso select tool to select the hippocampal and neocortical regions shown on the cropped image of the array.
- Set both the number of rows to skip and the number of columns to skip to 2.
NOTE: Recording files are often large; if appropriate for the desired analysis, the exported data can be downsampled. Options exist to downsample spatially (by skipping rows and columns of recording electrodes) and temporally (by downsampling to a lower sampling frequency). In the analysis, every 2 rows and 2 columns are skipped; this allows downsampling of the data spatially without being biased to certain channels. Each channel is far enough apart that the differences in activity could be significant between them; therefore, no channel averaging is performed. When skipping rows and columns of the electrodes, the data from unselected channels are not included when the output file is exported. - Set the downsampling frequency to 300 Hz.
- Click the Export Channels button to generate the channel selection file.
NOTE: The software will automatically save the channel selection file to the folder where the data recording file is saved. - Open the ExportToHDF5-ExtractDownsample.py file (also found on GitHub under xenon-lfp-analysis/code-files/3brain-processing40), paste the file path of the folder containing the channel selection file(s) and the data recording file(s), and push enter on the keyboard to generate the downsampled version of the data recording file.
- Using the analysis platform
- In the computer's command prompt, type run_lfp_analysis and hit enter to open the analysis platform.
- Input the file paths of the image and downsampled recording file and click submit.
- Under Select a Time Range for Analysis, select the entire recording.
- Under Select Channels for Plots, highlight the hippocampus as Group-1 and the neocortex as Group-2.
- Under Channel Raster, set the Threshold to 0.06 mV and the Time Duration to 0.02 s.
- Click Apply Settings and Generate Raster.
- Use the raster plot to explore different channels that show activity patterns of interest.
NOTE: See Figure 4 for examples of high-quality seizure-like activity (Figure 4A-D) versus suboptimal activity (Figure 4E-F).
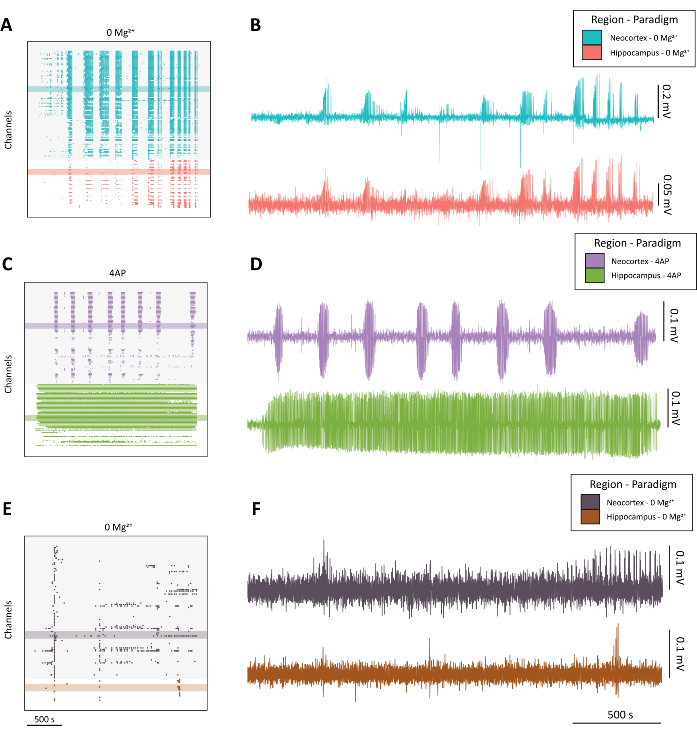
Figure 4: Example of evolving epileptiform activity from the 0 Mg2+ and 4-AP paradigms. (A) Example raster plot from the application of aCSF with 0 Mg2+ over approximately 40 min. (B) Example electrophysiology traces taken from the neocortex (blue) and hippocampus (red) demonstrating epileptiform activity from the 0 Mg2+ paradigm. (C) Example raster plot from the application 100 mM 4-AP over approximately 40 min. (D) Example electrophysiology traces taken from the neocortex (purple) and hippocampus (green) demonstrating epileptiform activity from the application of 4-AP. (E) Example raster plot from the application of aCSF with 0 Mg2+ over approximately 40 minutes showing bursting activity as opposed to seizure-like activity as found in the other representative traces. (F) Example electrophysiology traces taken from the neocortex (dark purple) and hippocampus (rust) demonstrating sub-optimal activity from the 0 Mg2+ paradigm intended for comparison to the quality seizure-like activity found in B and D. Please click here to view a larger version of this figure.
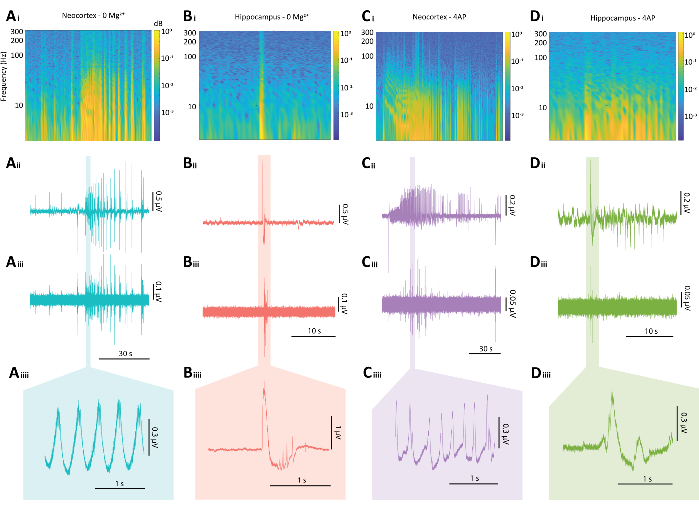
Figure 5: Representative results of epileptiform discharges from both the 0 Mg2+ and 4-AP paradigms. (A) Example plots of a typical neocortical seizure-like event induced by the 0 Mg2+ paradigm including (Ai) a spectrogram from a seizure-like event, (Aii) the associated electrophysiological trace, (Aiii) an 80 Hz high pass filter applied to the trace from Aii, (Aiiii) and a magnified section of the trace from Aii. (B) Example plots of a typical hippocampal epileptiform burst induced by the 0 Mg2+ paradigm including (Bi) a spectrogram of the epileptiform burst, (Bii) the associated electrophysiological trace, (Biii) an 80 Hz high pass filter applied to the trace from Bii, (Biiii) and a magnified section of the trace from Bii (C) Example plots of a typical neocortical seizure-like event induced bythe 4-AP paradigm including (Ci) a spectrogram of epileptiform activity, (Cii) the associated electrophysiological trace, (Ciii) an 80 Hz high pass filter applied to the trace from Cii, (Ciiii) and a magnified section of the trace from Cii (D) Example plots of a hippocampal epileptiform burst under the 4-AP paradigm including (Di) a spectrogram of epileptiform activity, (Dii) the associated electrophysiological trace, (Diii) an 80 Hz high pass filter applied to the trace from Dii, (Diiii) and a magnified section of the trace from Dii. Please click here to view a larger version of this figure.
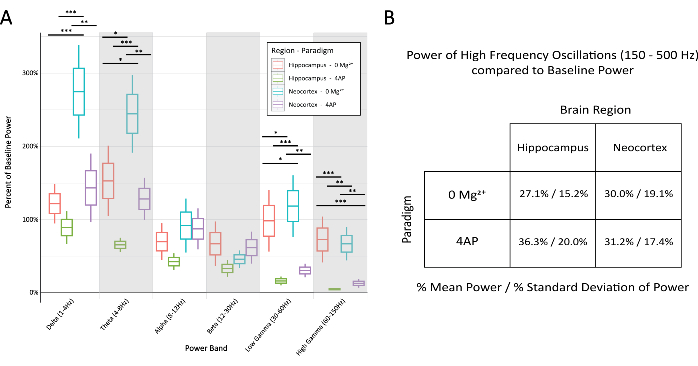
Figure 6: Comparison of percent of baseline power in the various bands across paradigm and brain region during stereotypical epileptiform discharges. (A) The power during epileptiform discharges was significantly different between the paradigms and brain regions for most frequency bands (2-way ANOVA with Tukey test, *P < 0.05, **P < 0.001, ***P < 0.0001). The middle line for each box represents the mean, the borders of the box ±1 standard error of the mean (SEM), and the outermost lines ±2 SEM. (B) Both paradigms and brain regions demonstrated limited power in bands related to high-frequency activity above 150 Hz. Please click here to view a larger version of this figure.
Representative Results
As is standard when visualizing activity from many channels1,4,5,10, we find it beneficial to first generate a raster plot of the data we acquire with the CMOS-HD-MEA (Figure 4A,C,E). This plot can create a bird's-eye view of the activity in all the recording channels in each brain slice by displaying each channel on the y-axis and time on the x-axis. In this raster plot, each signal point represents a channel that has exceeded the set LFP voltage threshold (see step 5.2.6 and a previously published work10). Such raster plots indicate when and where electrophysiological activity occurs across space and time (Figure 4A,C,E). Visualizing the data in this manner allows the user to take full advantage of the spatial and temporal resolution of the CMOS-HD-MEA system capabilities. Representative neocortical and hippocampal traces from channels corresponding to the raster plots demonstrate how the specific LFP data for each channel can also be visualized (Figure 4B,D,F). Zoomed-in events of epileptiform discharges are displayed for different brain regions and paradigms in Figure 5, and the comparison of the relative frequency powers we observed from the 0 Mg2+ and the 4-AP paradigms are summarized in Figure 6. Across all displayed frequency bands (1-150 Hz), seizure-like activity in both hippocampal and neocortical regions with both paradigms was significantly greater than baseline, following subtraction of all 60 Hz electrical noise (AC frequency in North America) with a digital notch-filter.
While using either the 0 Mg2+ or the 4-AP paradigm, powerful electrographic seizure-like activity was frequently observed in the neocortical regions. The hippocampal regions displayed more variability between brain slices, with some brain slices demonstrating seizure-like activity and others displaying shorter (~1 s) transient discharges (Figure 4 and Figure 5). The power for each recording was normalized to a 10 s "quiet" baseline section at the beginning of each recording in which no discharges were observed. All further discussion of "power" denotes spectral power compared to an in-sample control baseline. Neocortical activity was found to be more powerful in multiple frequency bands under the 0 Mg2+ paradigm when compared to the 4-AP paradigm. This was true for the delta (1-4 Hz), theta (4-8 Hz), low gamma (30-60 Hz) and high gamma (60-150 Hz) frequency bands (Figure 6). In addition, the hippocampal activity in the 0 Mg2+ paradigm demonstrated higher power in the high gamma frequency bands (60-150 Hz) when compared to the neocortex in the 0 Mg2+ paradigm and when also compared to both brain regions assayed in the 4-AP paradigm (Figure 6). Although the 4-AP paradigm exhibited less power in most frequency bands in our studies, compared to the 0 Mg2+ conditions, the hippocampal 4-AP samples tended to demonstrate more overall epileptiform discharges (Figure 4D). We did not observe any difference between brain regions or paradigms when analyzing high-frequency activity (Figure 6B). This is likely because the recordings are made on a 2D, non-penetrating recording chip, meaning that high-frequency activity might not be picked up due to the distance between the neurons producing the activity and the electrode recording sites. Taken together, the results presented here suggest that when using the CMOS-HD-MEA systems with 2D CMOS-MEA chips, one will generally achieve greater overall power during epileptiform discharges using the 0 Mg2+ paradigm, where quality data can be recorded up to the high gamma bands (from 1 up to 150 Hz). In addition, the data presented here suggests that the 4-AP model might not be a great choice with this system if one desires to study information from the gamma bands using this protocol. However, it provides rich data in the lower power bands while also displaying many epileptiform discharges. Importantly, both models will display high-quality seizure-like activity with the above protocol.
Differences in the seizure models likely result in characteristically unique patterns of induced seizure-like activity based on the cellular mechanisms of these paradigms and due to brain region microanatomy41. For example, 4-AP blocks certain classes of voltage-gated K+ channels (primarily the Kv3 family), extending the duration of neuronal excitability and resulting in an onset of epileptiform activity within a few minutes of exposure within the hippocampus42,43,44,45,46. However, the neocortex typically has a much longer delayed onset to epileptiform activity than the hippocampus when exposed to 4-AP41. On the other hand, the 0 Mg2+ paradigm induces epileptiform activity primarily by washing out the Mg2+ block of NMDA receptors in glutamatergic neurons, resulting in early neocortical epileptiform activity47,48,49,50. Although there were significant differences across brain regions and paradigms for many of the highlighted groups, some key similarities between the paradigms and brain regions also remained. The power in both alpha and beta frequency bands were comparable across brain regions and paradigms, and within hippocampal regions, the paradigms showed similar performance in the delta band (Figure 6). Additionally, an overall increase in power compared to baseline for all frequencies (1-150 Hz) was observed during epileptiform activity (Figure 5 and Figure 6), as expected. A final noteworthy observation of the neocortical areas during exposure to 4-AP was the striated pattern observed in the spectrograms depicting greater activity for higher frequencies during ictal start followed by a gradual decrease in frequency as the activity progressed (Figure 5C), possibly suggesting high interneuronal synchrony during the ictal start.
Discussion
This protocol includes specific guidelines related to acute brain slice management that address common problems faced by CMOS-HD-MEA users, namely noise development under the brain slice and maintaining a healthy environment for the brain slice. Noise development under the slice occurs when the slice is not properly adhered to the array; if the brain slice is not adequately adhered, air pockets can form underneath the slice, which results in noise. This will result in the inability to acquire data. To mitigate these challenges, the protocol emphasizes two critical steps: first, ensuring proper harp orientation during placement to prevent jostling by inflow perfusion (step 4.1.4), and second, using an anti-static wipe to remove excess aCSF around the electrodes, thereby promoting effective slice adhesion (steps 4.1.5-4.1.7). Another common issue addressed in the protocol involves chip hydrophobicity, which can lead to bubble formation and introduce noise during recordings. Steps such as ethanol treatment followed by rinsing with deionized water are recommended to alleviate these issues (steps 3.1.2, 3.1.3, and 3.1.6). However, if the issue persists, there are additional steps that can be taken. Fill the chip well with 140-proof ethanol and use a paintbrush to brush the array lightly for 30 s to 60 s. Remove the ethanol and rinse the chip well three times with deionized water. Afterward, fill the well with aCSF and continue the protocol at step 4.
Maintaining a healthy environment for the brain slice is vital and is managed through the proper placement of the inflow and outflow needles and the perfusion system flow rate (step 4.2.1). It is imperative to properly oxygenate the slice and find the most effective perfusion rate23,51. Due to these issues, interface chambers are commonly used for acute brain slice recordings and provide steadier oxygenation. However, recording with interface chambers has been reported to compress the extracellular space between neurons, which can change the physiology of the slice over time24,52,53. Surface tension dynamics from that altered physiology result in a higher baseline electrical sensitivity of the neurons54. This additional excitability increases the likelihood of seizure-like events and other LFPs of interest. In comparison, submerged chambers, which most CMOS-HD-MEA platforms operate with, can more efficiently deliver drugs and glucose to the slice23,51. To maintain a healthy environment for the slice during a recording session that would last over 2 h with CMOS-HD-MEA platforms, it is beneficial to have another pro-convulsant solution prepared (step 4.2.1.9). This will ensure the slice has enough glucose to continue to produce activity.
The lack of spatial resolution can be major limitations in electrophysiology23,55. For instance, patch-clamping is a technique often used in electrophysiology that can provide great detail about how a single cell behaves during network activity; however, one limitation of patch-clamping is that the recording is performed from a single cell and by default a single location. Patch-clamping can be utilized to study network activity, but it offers a rather limited spatial picture of the network activity41,56. Some imaging techniques do afford broad spatial detail of neuronal activity, such as genetically encoded fluorescent biosensors; however, they do so at the cost of temporal resolution57,58,59. Multimodal approaches, such as single or multichannel electrode recordings, can be combined with calcium imaging to achieve increased spatiotemporal resolution; however, the spatial resolution of the electrophysiological recording is still limited to a few locations60,61. When used properly, CMOS-HD-MEA technology allows users to overcome these limitations by recording with high spatial density (electrodes are typically spaced from 20-200 µm apart) from multiple brain areas simultaneously (Figure 4). CMOS-HD-MEA data can allow researchers to differentiate and characterize the propagating activity patterns across the brain slice with greater detail than most other recording techniques10,62,63. While CMOS-HD-MEA technology exhibits excellent spatial mapping ability with high temporal detail64, there are limitations, such as the inability to record reliably from single cells using cortical slices due to the use of non-penetrating 2D arrays and the inability to be used for in vivo experimentation. CMOS-HD-MEA systems typically record only local field potentials from cortical slices; however, they may capture extracellular action potentials on some channels with high-quality slice preparations, though this capability can be unreliable. Single-cell unit activity can be explored on the recorded channels from the CMOS-HD-MEA system using a high-pass filter (~50 Hz high-pass), combined with spike sorting software65,66,67. It is worth pointing out that it is possible to combine microelectrode recordings with CMOS-HD-MEA systems to possibly achieve single-cell information from a few locations, layered with the rich CMOS-HD-MEA information5. The current method with non-penetrating 2D chips is limited by difficulty in detecting higher frequency activity (150-500 Hz) in cortical brain regions. This is likely due to the distance between the neurons producing the activity and the recording electrodes (Figure 6B). This challenge could be overcome by using new penetrating 3D-CMOS-HD-MEA chips, which record from within the brain slice and not just at its surface68.
Using the above protocol, we generated example data to demonstrate the high-quality LFP signals in the form of seizure-like activity that can be achieved with these instruments (Figure 4 and Figure 5). We demonstrated that seizure-like activity can be observed with different recording paradigms (specifically 4-AP and 0 Mg2+) using CMOS-HD-MEA systems despite idiosyncratic differences between the paradigms.
In summary, the protocol and example data presented using the CMOS-HD-MEA provide a roadmap by which high-fidelity spatial and temporal LFP data can be collected. Future applications of this technique include applying this protocol to additional paradigms that induce epileptiform activity and integrating CMOS-HD-MEA with complementary techniques, like imaging. These studies will undoubtedly aid in our understanding of human seizure disorders using these simple brain slice models69. For example, augmentation of this protocol can provide insightful cross-examination of electrophysiological behavior and genetic mapping from various animal models of epilepsy with mutations in clinically relevant genes70. While we have focused on epileptiform activity, other important network activity, such as long-term potentiation, can also be studied with unprecedented spatial resolution using CMOS-HD-MEA systems. We hope this protocol and technology will be implemented to help others in the field overcome data collection challenges and develop more precise research methods to understand various neurological disorders, such as epilepsy.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank former and current Parrish lab members for their edits on this manuscript. We would also like to thank Alessandro Maccione of 3Brain for his feedback on this work. This work was funded by an AES/EF Junior Investigator Award and by Brigham Young University Colleges of Life Sciences and of Physical and Mathematical Sciences.
Materials
| 2D Workbench | Cloudray | LM04CLLD26B | |
| 4-Aminopyridine | Sigma-Aldrich | 275875 | |
| Accura Chip | 3Brain | Accura HD-MEA | CMOS-HD-MEA chip |
| Agarose | Thermo Fisher Scientific | BP160-100 | |
| Vibration isolation table | Kinetic Systems | 91010124 | |
| Beaker for the slice holding chamber, 270 mL | VWR | 10754-772 | |
| BioCam | 3Brain | BioCAM DupleX | CMOS-HD-MEA platform |
| Brainwave Software | 3Brain | Version 4 | CMOS-HD-MEA software |
| Calcium Chloride | Thermo Fisher Scientific | BP510-500 | |
| Carbogen | Airgas | X02OX95C2003102 | |
| Carbogen | Airgas | 12005 | |
| Carbogen Stones | Supelco | 59277 | |
| Compresstome | Precissionary | VF-300-0Z | |
| Computer | Dell | Precission3650 | |
| Crocodile Clip Grounding Cables | JWQIDI | B06WGZG17W | |
| Detergent | Metrex | 10-4100-0000 | |
| D-Glucose | Macron Fine Chemicals | 4912-12 | |
| Dihydrogen Sodium Phosphate | Thermo Fisher Scientific | BP329-500 | |
| DinoCam | Dino-Lite | AM73915MZTL | |
| Ethanol | Thermo Fisher Scientific | A407P-4 | |
| Forceps | Fine Science Tools | 11980-13 | |
| Hot plate | Thermo Fisher Scientific | SP88857200 | |
| Ice Machine | Hoshizaki | F801MWH | |
| Inflow and outflow needles | Jensen Global | JG 18-3.0X | |
| Inline Solution Heater | Warner Instruments | SH-27B | |
| Isofluorine | Dechra | 08PB-STE22002-0122 | |
| Kim Wipes | Thermo Fisher Scientific | 06-666 | |
| Magnesium Chloride | Thermo Fisher Scientific | FLM33500 | |
| Micropipets | Gilson | F144069 | |
| Mili-Q Water Filter | Mili-Q | ZR0Q008WW | |
| Paintbrush | Daler Rowney | AF85 Round: 0 | |
| Paper Filter | Whatman | EW-06648-24 | |
| Parafilm | American National Can | PM996 | |
| Perfusion System | Multi Channel System | PPS2 | |
| Pipetor | Thermo Fisher Scientific | FB14955202 | |
| Platinum Harp | 3Brain | 3Brain | |
| Potassium Chloride | Thermo Fisher Scientific | P330-3 | |
| Razor blade | Personna | BP9020 | |
| Scale | Metter Toledo | AB204 | |
| Scissors | Solingen | 92008 | |
| Slice Holding Chamber | Custom | Custom | Custom 3D Printer Design, available upon request |
| Sodium Bicarbonate | Macron Fine Chemicals | 7412-06 | |
| Sodium Chloride | Thermo Fisher Scientific | S271-3 | |
| Temperature Control Box | Warner Instruments | TC344B | |
| Transfer Pipettes | Genesee Scientific | 30-200 | |
| Tubing | Tygon | B-44-3 TPE | |
| Vibratome VZ-300 | Precissionary | VF-00-VM-NC | |
| Weigh Boat | Electron Microscopy Sciences | 70040 |
References
- Obien, M. E. J., Frey, U. Large-scale, high-resolution microelectrode arrays for interrogation of neurons and networks. Adv Neurobiol. 22, 83-123 (2019).
- Schroter, M., et al. Functional imaging of brain organoids using high-density microelectrode arrays. MRS Bull. 47 (6), 530-544 (2022).
- Miccoli, B., et al. High-density electrical recording and impedance imaging with a multi-modal CMOS multi-electrode array chip. Front Neurosci. 13, 641 (2019).
- Emery, B. A., Hu, X., Khanzada, S., Kempermann, G., Amin, H. High-resolution CMOS-based biosensor for assessing hippocampal circuit dynamics in experience-dependent plasticity. Biosens Bioelectron. 237, 115471 (2023).
- Ferrea, E., et al. high-resolution electrophysiological imaging of field potentials in brain slices with microelectronic multielectrode arrays. Front Neural Circuits. 6, 80 (2012).
- Gagliano, G., et al. Non-linear frequency dependence of neurovascular coupling in the cerebellar cortex implies vasodilation-vasoconstriction competition. Cells. 11 (6), 1047 (2022).
- Goodchild, S. J., et al. Molecular pharmacology of selective Na(V)1.6 and dual Na(V)1.6/Na(V)1.2 channel inhibitors that suppress excitatory neuronal activity ex vivo. ACS Chem Neurosci. 15 (6), 1169-1184 (2024).
- Hu, X., Khanzada, S., Klutsch, D., Calegari, F., Amin, H. Implementation of biohybrid olfactory bulb on a high-density CMOS-chip to reveal large-scale spatiotemporal circuit information. Biosens Bioelectron. 198, 113834 (2022).
- Kim, S., et al. Alteration of neural network and hippocampal slice activation through exosomes derived from 5XFAD nasal lavage fluid. Int J Mol Sci. 24 (18), 14064 (2023).
- Mahadevan, A., Codadu, N. K., Parrish, R. R. Xenon LFP analysis platform is a novel graphical user interface for analysis of local field potential from large-scale MEA recordings. Front Neurosci. 16, 904931 (2022).
- Medrihan, L., Ferrea, E., Greco, B., Baldelli, P., Benfenati, F. Asynchronous GABA release is a key determinant of tonic inhibition and controls neuronal excitability: A study in the synapsin II-/- mouse. Cereb Cortex. 25 (10), 3356-3368 (2015).
- Monteverdi, A., Di Domenico, D., D’Angelo, E., Mapelli, L. Anisotropy and frequency dependence of signal propagation in the cerebellar circuit revealed by high-density multielectrode array recordings. Biomedicines. 11 (5), 1475 (2023).
- Obien, M. E. J., Hierlemann, A., Frey, U. Accurate signal-source localization in brain slices by means of high-density microelectrode arrays. Sci Rep. 9 (1), 788 (2019).
- Thouta, S., et al. Pharmacological determination of the fractional block of Nav channels required to impair neuronal excitability and ex vivo seizures. Front Cell Neurosci. 16, 964691 (2022).
- Tognolina, M., Monteverdi, A., D’Angelo, E. Discovering microcircuit secrets with multi-spot imaging and electrophysiological recordings: The example of cerebellar network dynamics. Front Cell Neurosci. 16, 805670 (2022).
- Hierlemann, A., Frey, U., Hafizovic, S., Heer, F. Growing cells atop microelectronic chips: Interfacing electrogenic cells in vitro with CMOS-based microelectrode arrays. Proceedings of the IEEE. 99 (2), 252-284 (2011).
- Maccione, A., et al. Experimental investigation on spontaneously active hippocampal cultures recorded by means of high-density MEAs: Analysis of the spatial resolution effects. Front Neuroeng. 3, 4 (2010).
- van Vliet, E., et al. Electrophysiological recording of re-aggregating brain cell cultures on multi-electrode arrays to detect acute neurotoxic effects. Neurotoxicology. 28 (6), 1136-1146 (2007).
- Emery, B. A., et al. Large-scale multimodal recordings on a high-density neurochip: Olfactory bulb and hippocampal networks. Annu Int Conf IEEE Eng Med Biol Soc. 2022, 3111-3114 (2022).
- Veleanu, M., et al. Modified climbing fiber/Purkinje cell synaptic connectivity in the cerebellum of the neonatal phencyclidine model of schizophrenia. Proc Natl Acad Sci U S A. 119 (21), e2122544119 (2022).
- Giansante, G., et al. Neuronal network activity and connectivity are impaired in a conditional knockout mouse model with PCDH19 mosaic expression. Mol Psychiatry. , (2023).
- Dossi, E., Blauwblomme, T., Nabbout, R., Huberfeld, G., Rouach, N. Multi-electrode array recordings of human epileptic postoperative cortical tissue. J Vis Exp. (92), e51870 (2014).
- Hajos, N., et al. Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur J Neurosci. 29 (2), 319-327 (2009).
- Hill, M. R., Greenfield, S. A. The membrane chamber: a new type of in vitro recording chamber. J Neurosci Methods. 195 (1), 15-23 (2011).
- Raimondo, J. V., et al. Methodological standards for in vitro models of epilepsy and epileptic seizures. A TASK1-WG4 report of the AES/ILAE Translational Task Force of the ILAE. Epilepsia. 58 (Suppl 4), 40-52 (2017).
- Hales, C. M., Rolston, J. D., Potter, S. M. How to culture, record and stimulate neuronal networks on micro-electrode arrays (MEAs). J Vis Exp. (39), 2056 (2010).
- Lin, C. H., Lee, J. K., LaBarge, M. A. Fabrication and use of microenvironment microarrays (MEArrays). J Vis Exp. (68), 4152 (2012).
- Panuccio, G., Colombi, I., Chiappalone, M. Recording and modulation of epileptiform activity in rodent brain slices coupled to microelectrode arrays. J Vis Exp. 135, 57548 (2018).
- Patel, C., Muthuswamy, J. High efficiency, site-specific transfection of adherent cells with siRNA using microelectrode arrays (MEA). J Vis Exp. 67, e4415 (2012).
- Ting, J. T., Daigle, T. L., Chen, Q., Feng, G. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol Biol. 1183, 221-242 (2014).
- Papouin, T., Haydon, P. G. Obtaining acute brain slices. Bio Protoc. 8 (2), e2699 (2018).
- Ting, J. T., et al. Preparation of acute brain slices using an optimized N-Methyl-D-glucamine protective recovery method. J Vis Exp. 132, 53825 (2018).
- Van Hoeymissen, E., Philippaert, K., Vennekens, R., Vriens, J., Held, K. Horizontal hippocampal slices of the mouse brain. J Vis Exp. (163), 61753 (2020).
- . 3Brain Available from: https://www.3brain.com/ (2022)
- Bridges, D. C., Tovar, K. R., Wu, B., Hansma, P. K., Kosik, K. S. MEA Viewer: A high-performance interactive application for visualizing electrophysiological data. PLoS One. 13 (2), e0192477 (2018).
- Hawrylycz, M., et al. Inferring cortical function in the mouse visual system through large-scale systems neuroscience. Proc Natl Acad Sci U S A. 113 (27), 7337-7344 (2016).
- Maccione, A., et al. Microelectronics, bioinformatics and neurocomputation for massive neuronal recordings in brain circuits with large scale multielectrode array probes. Brain Res Bull. 119 (Pt B), 118-126 (2015).
- . 3Brain Available from: https://www.3brain.com/products/software/brainwave4 (2022)
- Mahadevan, A. . Xenon LFP Analysis. , (2022).
- Mahadevan, A. . xenon-lfp-analysis github. , (2022).
- Codadu, N. K., et al. Divergent paths to seizure-like events. Physiol Rep. 7 (19), e14226 (2019).
- Kirsch, G. E., Drewe, J. A. Gating-dependent mechanism of 4-aminopyridine block in two related potassium channels. J Gen Physiol. 102 (5), 797-816 (1993).
- Levesque, M., Salami, P., Behr, C., Avoli, M. Temporal lobe epileptiform activity following systemic administration of 4-aminopyridine in rats. Epilepsia. 54 (4), 596-604 (2013).
- Myers, T. L., Gonzalez, O. C., Stein, J. B., Bazhenov, M. Characterizing concentration-dependent neural dynamics of 4-Aminopyridine-induced epileptiform activity. Epilepsy J. 4 (2), 128 (2018).
- Perreault, P., Avoli, M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol. 65 (4), 771-785 (1991).
- Rutecki, P. A., Lebeda, F. J., Johnston, D. 4-Aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. J Neurophysiol. 57 (6), 1911-1924 (1987).
- Chen, Y., Chad, J. E., Cannon, R. C., Wheal, H. V. Reduced Mg2+ blockade of synaptically activated N-methyl-D-aspartate receptor-channels in CA1 pyramidal neurons in kainic acid-lesioned rat hippocampus. Neuroscience. 88 (3), 727-739 (1999).
- Fujiwara-Tsukamoto, Y., Isomura, Y., Takada, M. Comparable GABAergic mechanisms of hippocampal seizure-like activity in posttetanic and low-Mg2+ conditions. J Neurophysiol. 95 (3), 2013-2019 (2006).
- Swartzwelder, H. S., Anderson, W. W., Wilson, W. A. Mechanism of electrographic seizure generation in the hippocampal slice in Mg2+-free medium: the role of GABAa inhibition. Epilepsy Res. 2 (4), 239-245 (1988).
- Trevelyan, A. J., Graham, R. T., Parrish, R. R., Codadu, N. K. Synergistic positive feedback mechanisms underlying seizure initiation. Epilepsy Curr. 23 (1), 38-43 (2023).
- Croning, M. D., Haddad, G. G. Comparison of brain slice chamber designs for investigations of oxygen deprivation in vitro. J Neurosci Methods. 81 (1-2), 103-111 (1998).
- Hajos, N., Mody, I. Establishing a physiological environment for visualized in vitro brain slice recordings by increasing oxygen supply and modifying aCSF content. J Neurosci Methods. 183 (2), 107-113 (2009).
- Huang, Y., Williams, J. C., Johnson, S. M. Brain slice on a chip: opportunities and challenges of applying microfluidic technology to intact tissues. Lab Chip. 12 (12), 2103-2117 (2012).
- Andrew, R. D., et al. The critical role of spreading depolarizations in early brain injury: Consensus and contention. Neurocrit Care. 37 (Suppl 1), 83-101 (2022).
- Devonshire, I. M., Dommett, E. J., Grandy, T. H., Halliday, A. C., Greenfield, S. A. Environmental enrichment differentially modifies specific components of sensory-evoked activity in rat barrel cortex as revealed by simultaneous electrophysiological recordings and optical imaging in vivo. Neuroscience. 170 (2), 662-669 (2010).
- Parrish, R. R., Codadu, N. K., Mackenzie-Gray Scott, C., Trevelyan, A. J. Feedforward inhibition ahead of ictal wavefronts is provided by both parvalbumin- and somatostatin-expressing interneurons. J Physiol. 597 (8), 2297-2314 (2019).
- Wang, H., Jing, M., Li, Y. Lighting up the brain: genetically encoded fluorescent sensors for imaging neurotransmitters and neuromodulators. Curr Opin Neurobiol. 50, 171-178 (2018).
- Yaksi, E., Jamali, A., Diaz Verdugo, C., Jurisch-Yaksi, N. Past, present and future of zebrafish in epilepsy research. FEBS J. 288 (24), 7243-7255 (2021).
- He, M. F., et al. Ex vivo calcium imaging for drosophila model of epilepsy. J Vis Exp. 200, 65825 (2023).
- Driscoll, N., et al. Multimodal in vivo recording using transparent graphene microelectrodes illuminates spatiotemporal seizure dynamics at the microscale. Commun Biol. 4 (1), 136 (2021).
- Parrish, R. R., Grady, J., Codadu, N. K., Trevelyan, A. J., Racca, C. Simultaneous profiling of activity patterns in multiple neuronal subclasses. J Neurosci Methods. 303, 16-29 (2018).
- Valderhaug, V. D., et al. Criticality as a measure of developing proteinopathy in engineered human neural networks. bioRxiv. , (2020).
- Carleo, G., Lee, Y. -. S., Secondo, A., Miceli, F., Taglialatela, M. Multi-electrode array (MEASs) to investigate pathogenetic disease mechanisms and pharmacological properties in iPSC-derived neurons modelling neuropsychiatric diseases. , 667-672 (2022).
- Ruz, I. D., Schultz, S. R. Localising and classifying neurons from high density MEA recordings. J Neurosci Methods. 233, 115-128 (2014).
- Franke, F., Natora, M., Boucsein, C., Munk, M. H. J., Obermayer, K. An online spike detection and spike classification algorithm capable of instantaneous resolution of overlapping spikes. J Comput Neurosci. 29 (1-2), 127-148 (2010).
- Vollgraf, R., Obermayer, K. Improved optimal linear filters for the discrimination of multichannel waveform templates for spike-sorting applications. IEEE Signal Processing Letters. 13 (3), 121-124 (2006).
- Muller, J., et al. High-resolution CMOS MEA platform to study neurons at subcellular, cellular, and network levels. Lab Chip. 15 (13), 2767-2780 (2015).
- Mapelli, L., et al. implementation, and functional validation of a new generation of microneedle 3D high-density CMOS multi-electrode array for brain tissue and spheroids. bioRxiv. , (2022).
- Reddy, D. S., Kuruba, R. Experimental models of status epilepticus and neuronal injury for evaluation of therapeutic interventions. Int J Mol Sci. 14 (9), 18284-18318 (2013).
- Parrish, R. R., Trevelyan, A. J. Stress-testing the brain to understand its breaking points. J Physiol. 596 (11), 2033-2034 (2018).

