Radiotracer Administration for High Temporal Resolution Positron Emission Tomography of the Human Brain: Application to FDG-fPET
Summary
This manuscript describes two radiotracer administration protocols for FDG-PET (constant infusion and bolus plus infusion) and compares them to bolus administration. Temporal resolutions of 16 s are achievable using these protocols.
Abstract
Functional positron emission tomography (fPET) provides a method to track molecular targets in the human brain. With a radioactively-labelled glucose analogue, 18F-fluordeoxyglucose (FDG-fPET), it is now possible to measure the dynamics of glucose metabolism with temporal resolutions approaching those of functional magnetic resonance imaging (fMRI). This direct measure of glucose uptake has enormous potential for understanding normal and abnormal brain function and probing the effects of metabolic and neurodegenerative diseases. Further, new advances in hybrid MR-PET hardware make it possible to capture fluctuations in glucose and blood oxygenation simultaneously using fMRI and FDG-fPET.
The temporal resolution and signal-to-noise of the FDG-fPET images is critically dependent upon the administration of the radiotracer. This work presents two alternative continuous infusion protocols and compares them to a traditional bolus approach. It presents a method for acquiring blood samples, time-locking PET, MRI, experimental stimulus, and administering the non-traditional tracer delivery. Using a visual stimulus, the protocol results show cortical maps of the glucose-response to external stimuli on an individual level with a temporal resolution of 16 s.
Introduction
Positron emission tomography (PET) is a powerful molecular imaging technique that is widely used in both clinical and research settings (see Heurling et al.1 for a recent comprehensive review). The molecular targets that can be imaged using PET are only limited by the availability of radiotracers, and numerous tracers have been developed to image neural metabolism receptors, proteins, and enzymes2,3. In neuroscience, one of the most used radiotracers is 18F-fluorodeoxyglucose (FDG-PET), which measures glucose uptake, usually interpreted as an index of cerebral glucose metabolism. The human brain requires a constant and reliable supply of glucose to satisfy its energy requirements4,5, and 70-80% of cerebral glucose metabolism is used by neurons during synaptic transmission6. Changes to cerebral glucose metabolism are thought to initiate and contribute to numerous conditions, including psychiatric, neurodegenerative, and ischemic conditions7,8,9. Furthermore, as FDG uptake is proportional to synaptic activity10,11,12, it is considered a more direct and less confounded index of neuronal activity compared to the more widely used blood oxygenation level-dependent functional magnetic resonance imaging (BOLD-fMRI) response. BOLD-fMRI is an indirect index of neural activity and measures changes in deoxygenated hemoglobin that occur following a cascade of neurovascular changes following neuronal activity.
Most FDG-PET studies of the human brain acquire static images of cerebral glucose uptake. The participant rests quietly for 10 min with their eyes open in a darkened room. The full radiotracer dose is administered as a bolus over a period of seconds, and the participant then rests for a further 30 min. Following the uptake period, participants are placed in the center of the PET scanner, and a PET image that reflects the cumulative FDG distribution over the course of the uptake and scanning periods is acquired. Thus, the neuronal activity indexed by the PET image represents the cumulative average of all cognitive activity over uptake and scan periods and is not specific to cognitive activity during the scan. This method has provided great insight into the cerebral metabolism of the brain and neuronal function. However, the temporal resolution is equal to the scan duration (often ~45 min, effectively yielding a static measurement of glucose uptake; this compares unfavourably to neuronal response during cognitive processes and common experiments in neuroimaging. Due to the limited temporal resolution, the method provides a non-specific index of glucose uptake (i.e., not locked to a task or cognitive process) and cannot provide measures of within-subject variability, which can lead to erroneous scientific conclusions due to Simpson's Paradox13. Simpson’s Paradox is a scenario, where brain-behavior relationships calculated across-subjects are not necessarily indicative of the same relationships tested within-subjects. Furthermore, recent attempts to apply functional connectivity measures to FDG-PET can only measure across-subjects connectivity. Thus, differences in connectivity can only be compared between groups and cannot be calculated for individual subjects. While it is debatable what exactly across-subject connectivity measures14, it is clear that measures calculated across-but not within-subjects cannot be used as a biomarker for disease states or used to examine the source of individual variation.
In the past five years, the development and wider accessibility of clinical-grade simultaneous MRI-PET scanners has sparked renewed research interest in FDG-PET imaging2 in cognitive neuroscience. With these developments, researchers have focused on improving the temporal resolution of FDG-PET to approach the standards of BOLD-fMRI (~0.5−2.5 s). Note that the spatial resolution of BOLD-fMRI can approach submillimeter resolutions but the spatial resolution of FDG-PET is fundamentally limited to around 0.54 mm full width at half maximum (FWHM) due to the positron range15. Dynamic FDG-PET acquisitions, which are often used clinically, use the bolus administration method and reconstruct the list-mode data into bins. The bolus dynamic FDG-PET method offers a temporal resolution of around 100 s (e.g., Tomasi et al.16). This is clearly much better compared to static FDG-PET imaging but is not comparable to BOLD-fMRI. Additionally, the window in which brain function may be examined is limited, because the blood plasma concentration of FDG diminishes soon after the bolus is administered.
To expand this experimental window, a handful of studies17,18,19,20,21 have adapted the radiotracer infusion method previously proposed by Carson22,23. In this method, sometimes described as 'functional FDG-PET' (FDG-fPET, analogous to BOLD-fMRI), the radiotracer is administered as a constant infusion over the course of the entire PET scan (~90 min). The goal of the infusion protocol is to maintain a constant plasma supply of FDG to track dynamic changes in glucose uptake across time. In a proof-of-concept study, Villien et al.21 used a constant infusion protocol and simultaneous MRI/FDG-fPET to show dynamic changes in glucose uptake in response to checkerboard stimulation with a temporal resolution of 60 s. Subsequent studies have used this method to show task-locked FDG-fPET (i.e., time-locked to an external stimulus19) and task-related FDG-fPET (i.e., not time-locked to an external stimulus17,18) glucose uptake. Using these methods, FDG-fPET temporal resolutions of 60 s have been obtained, which is a substantial improvement over bolus methods. Preliminary data show that the infusion method can provide temporal resolutions of 20−60 s19.
Despite the promising results from the constant infusion method, the plasma radioactivity curves of these studies show that the infusion method is not sufficient to reach a steady-state within the timeframe of a 90 min scan19,21. In addition to the constant infusion procedure, Carson22 also proposed a hybrid bolus/infusion procedure, where the goal is to quickly reach equilibrium at the beginning of the scan, and then sustain plasma radioactivity levels at equilibrium for the duration of the scan. Rischka et al.20 recently applied this technique using a 20% bolus plus 80% infusion. As expected, the arterial input function quickly rose above baseline levels and was sustained at a higher rate for a longer time, compared to results using an infusion-only procedure19,21.
This paper describes the acquisition protocols for acquiring high temporal resolution FDG-fPET scans using infusion-only and bolus/infusion radiotracer administration. These protocols have been developed for use in a simultaneous MRI-PET environment with a 90−95 min acquisition time19. In the protocol, blood samples are taken to quantify plasma serum radioactivity for subsequent quantification of PET images. While the protocol's focus is the application of infusion methods for functional neuroimaging using BOLD-fMRI/FDG-fPET, these methods can be applied to any FDG-fPET study regardless of whether simultaneous MRI, BOLD-fMRI, computed tomography (CT), or other neuroimages are acquired. Figure 1 shows the flowchart of procedures in this protocol.
Protocol
This protocol has been reviewed and approved by the Monash University Human Research Ethics Committee (approval number CF16/1108 – 2016000590) in accordance with the Australian National Statement on Ethical Conduct in Human Research24. Procedures were developed under the guidance of an accredited Medical Physicist, Nuclear Medicine Technologist, and clinical radiographer. Researchers should refer to their local experts and guidelines for the administration of ionizing radiation in humans.
1. Required equipment and personnel
- See the Table of Materials for the scanner room, radiochemistry lab, and general materials. A commercial supplier was used for the radiotracer.
- In the simultaneous MRI-PET environment, use four personnel: a radiographer (RG) to run the scan, a nuclear medicine technologist (NMT) to oversee the administration of the radiotracer and acquisition of blood samples, a laboratory assistant (LA) to spin blood, and a research assistant (RA) responsible to oversee the experimental design and stimulus presentation.
2. Preparation
- Tracer dose preparation by the NMT
- Calculate the infusion volume that will be administered over the course of the scan. In this protocol, the rate of infusion is 0.01 mL/s over 95 min. So, in a 95 min scan, participants receive 0.01 mL/s x 60 s x 95 min = 57 mL.
- Calculate the tracer dose that will be diluted into the administered saline solution. In this protocol, a total dose of 260 MBq is administered to the participant over 95 min. This dose was chosen to limit radiation exposure to 4.9 mSv, to keep within the 'low level risk' categorization according to Australian Radiation Protection and Nuclear Safety Agency (ARPANSA) guidelines for exposure of humans to ionizing radiation25. Decay correct 260 MBq from the mid-infusion point (47.5 min) back to T0. Using Equation 1, solve for A0
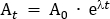
Where At is the radioactivity (MBq) at the mid-timepoint of the infusion, A0 is the initial radioactivity, and λ is the radioactive decay constant specific to the tracer. For FDG, the value of is λ ≈ 0.693/T1/2. T1/2 is the half-life of 18F (110 min).
NOTE: In this example, At = 260 MBq, λ = 0.693/110, and t = -47.5, so A0 = 350.942 MBq. - Calculate the required radiotracer dose for the 100 mL saline bag that will be used to administer the dose to the participant. The required radiotracer for the saline bag is diluted up to a total volume of 5 mL and drawn up in a 5 mL syringe. Therefore, for the 100 mL saline bag, the dilution factor is the volume of saline (100 mL) in addition to the 5 mL volume of the syringe with radiotracer. This total volume of 105 mL is divided by the infusion volume of 57 mL (i.e., 105 mL/57 mL = 1.842). So, the total radioactivity in a volume of 5 mL required for addition to the 100 mL bag is A0 x the dilution factor (i.e., 350.942 MBq x 1.842 = 646.44 MBq). Aseptically add the radiotracer to the saline bag.
NOTE: It is important to note that the calculated activity of 646.44 MBq that is added to the saline bag is the activity required at the commencement of the infusion. Generally, the doses for this protocol are prepared between 15 min to 1 h before administration. Therefore, it is important to factor in the decay of the radioisotope. Equation 1 in 2.1.2. can be used to account for this, where time (t) is the total number of minutes from the preparation of the dose to when the activity will be administered, At = 646.44 MBq, by solving for A0. - Prepare the priming dose. Withdraw 20 mL from the bag into a syringe and cap it. Calibrate this 20 mL syringe and label. The syringe is calibrated as a reference check to ensure that the radioactivity has evenly dispersed within the saline bag.
- Prepare the dose. Using a 50 mL syringe, withdraw 60 mL from the bag and cap with a red Combi stopper. This syringe is not calibrated, as the concentration of the radioactivity is known from the time it was added to the saline bag (step 2.1.3). Store both syringes in the radiochemistry lab until ready to scan.
NOTE: It is possible to draw a 60 mL volume in a 50 mL syringe, because Terumo syringes are marked to 20% above the labelled volume (i.e., a 50 mL syringe is marked to 60 mL). - Prepare the reference dose. Fill a 500 mL volumetric flask with approximately 480 mL of distilled water. Draw up 10 MBq of 18F-FDG into a syringe, decay-corrected to the scan start time (using Equation 1) and add it to the flask. Top the volume up to the 500 mL mark with more distilled water and mix thoroughly. Affix labels pre- and post-calibration for the syringe.
- Scanner room preparation by the NMT
- Once the participant is positioned in the scanner, there is very little room to manipulate or salvage the line for infusion or blood samples if blockage occurs. Prepare the scanner room to minimize the chance of line blockage.
- Ensure that all blood-collection equipment is within easy reach of the collection site. Place underpads at the end of the cannula and on any surface that will hold blood containers. Place bins for regular waste and biohazardous waste within easy reach of the blood collection site.
- Infusion pump preparation by the NMT
- Set up the infusion pump in the scanner room on the side that will be connected to the participant. Build lead bricks around the base of the pump and place the lead shield in front of the pump. Connect the tubing for the infusion pump that delivers the infusion to the participant and ensure the correct infusion rate has been entered. For this protocol, the rate is 0.01 mL/s.
- Prime the tubing before it is connected to the participant's cannula. Connect the 20 mL priming dose to the infusion pump. On the end of the tubing that will be connected to the participant, attach a three-way tap and an empty 20 mL syringe. Ensure that the tap is positioned to allow the 18F-FDG solution to flow from the priming dose through the tubing and collect only into the empty syringe.
- Preset the infusion pump to prime a volume of 15 mL. Select the Prime button on the pump and follow the prompts to prime the line.
- Attach the 50 mL dose syringe to the infusion pump in place of the priming dose. The 15 mL primed dose on the three-way tap can remain there until the participant is ready to be connected to the pump.
- Participant preparation by the NMT, RA, and RG
- Advise participants to fast for 6 h, and to consume only water (approximately two glasses), prior to the scan.
- Have the RA conduct the consent procedures and acquire additional measures (e.g., demographic surveys, cognitive batteries, etc.). Have the NMT and RG conduct the safety screens, the NMT review safety for PET scanning (e.g., exclusion for pregnancy, diabetes, chemotherapy or radiotherapy in the previous 8 weeks, and known allergies), and the RG review participant safety for MRI scanning (e.g., exclusion for pregnancy, medical or non-medical metallic implants, non-removable dental implants, claustrophobia).
- Cannulate the participant.
- Use two cannulas: one for dose administration and the other for blood sampling. The most appropriate cannula varies across participants, but the most suitable vein should be reserved for blood collection. A 22 G cannula is the preferred minimum size. Collect a 10 mL baseline blood sample while cannulating. Disconnect all saline flushes under pressure to maintain patency of the line.
- Test the participant's blood sugar level and other baseline blood measures (e.g., hemoglobin) from the baseline sample.
- Participant positioning in the scanner by the RG and NMT
- Have the RG position the participant in the scanner bore. For long scans, it is imperative to ensure comfort in order to reduce the risk of the participant dropping out and motion artefact due to discomfort. The participant should be covered with a disposable blanket to maintain a comfortable body temperature.
- Have the NMT flush the cannula to ensure it is patent with minimal resistance before connecting the infusion line. Once connected, the tubing can be lightly taped near the wrist. Instruct the participant to keep their arm straightened. Use supports such as foam or cushions for comfort. Have the NMT also check the cannula that will be used for plasma samples to ensure that it is able to withdraw blood with minimal resistance. It may be necessary to connect an extension tube primed with normal saline to make the cannula more accessible while the participant is in the scanner. If this is required, it should be checked for leakages.
- Once the subject is in the scanner bore, have the NMT check that they have suitable access to both cannulas.
- Have the NMT notify the RG and RA if there are any issues with the blood collection cannula, infusion cannula, or the infusion pump (e.g., occlusion, battery, extravasation) at any time during the scan.
3. Scan the participant
- Starting the scan with the NMT, RG, and RA
- At the start of the scan, situate the NMT in the scanner room to monitor the infusion equipment. Ensure the NMT is wearing hearing protection and using the barrier shield to minimize radiation exposure from the dose where possible.
- As the RG performs the localizer scan to ensure that the participant is in the correct position, check the details for the PET acquisition (e.g., scan duration, list-mode data collection, correct isotope).
- Design the protocol so that the PET acquisition will commence with the first MRI sequence. The RG prepares and starts the MRI sequence. The start time of the 95 min PET acquisition is time-locked to the start of the MRI sequence. If required, the NMT should deliver the bolus at the time of PET acquisition (Figure 1).
- Start the infusion pump. The RG should signal the NMT (e.g., via a thumbs-up sign) to start the pump 30 s after the start of the PET acquisition. This protocol starts the infusion pump 30 s after the scan start time to provide a safety buffer in case of scan failure. This also ensures that the first image taken during the PET scan indexes the brain prior to radiotracer administration for complete time activity curve data collection. Have the NMT observe the pump to ensure it has started to infuse the 18F-FDG and that there is no immediate occlusion of the line.
- Have the RA initiate any external stimulus at the agreed upon time (i.e., at the start of a functional run/experimental block) and calculate the times for blood samples. An example record form is shown in Supplement 1. Have the RA calculate the predicted time of each blood sample and provide copies to the NMT and lab assistant (LA). Have the RA ensure that the NMT takes the blood samples at approximately the correct time, and monitors equipment (e.g., infusion pump, stimulus) for any signs of errors.
- Take blood samples at regular time intervals
- Have the NMT and RA take one sample every 10 min. There are usually 10 samples in total, not including the baseline sample.
- If acquiring MRI scans simultaneously with PET scans, have the NMT wear hearing protection when entering the scanner room.
- Have the NMT wear gloves and swab the tip of the cannula clean. While the cannula site dries, open a 5 mL and a 10 mL syringe, vacutainer, and a 10 mL saline flush.
- Using the 5 mL syringe, withdraw 4-5 mL of fresh blood and discard the syringe in the biohazard waste.
- Using the 10 mL syringe, withdraw up to 10 mL of blood. The volume may be limited by how easily the blood can be withdrawn. It is important to minimize any resistance subsequently causing damage to the red blood cells that can hemolyze. At the midcollection point, have the NMT signal to the RA, who will mark this time on the record form (Supplement 1) as the 'actual' time of sample.
- Connect the 10 mL syringe to the vacutainer and then deposit the blood into the relevant blood tube.
- Quickly flush the cannula with 10 mL of saline, disconnected under pressure, to minimize any chance of line clotting.
- Immediately take the blood sample to the radiochemistry lab for analysis.
- Spinning the blood by the LA
- Have the LA get all the equipment ready (Table 1) and be wear gloves. Have three racks set out for the samples: one for blood tubes, one for pipetting the sample, and one for filled pipetted samples (pre- and post-counting).
- Have the LA regularly change gloves throughout the procedure, especially when handling the counting tube. If the LA has any radioactive plasma contamination on their gloves, it can be transferred to the counting tube and spuriously increase the number of recorded counts of the sample.
- The blood sample can be placed in the centrifuge as the availability of staffing resources permits, because the time that the blood sample was taken, and the time it was counted was noted. Spin all samples at a relative centrifugal force of 724 x g. The centrifuge settings used for this protocol are 2,000 rpm for 5 min with the acceleration and deceleration curves set to eight.
- Once the sample has been spun, place the tube in the pipetting rack. Remove the tube cap to not disturb sample separation. Place a labelled counting tube in the rack. The label should correspond to the blood tube.
- Ensure the tip is securely fastened to the pipette. Have a tissue ready for any drips. Steadily pipette 1,000 µL of plasma from the blood tube, transfer to the counting tube, and replace the lids on the counting tube and blood tube.
- Place the counting tube into the well counter and count for 4 min. Record the counting start time on the record sheet ('measurement time') for every sample. This is required for subsequent corrections to the PET acquisition start time. At later time points during the scan, have the LA perform each step in rapid succession to avoid a backlog of samples.
- Dispose of any blood product waste in biohazard bags.
- Have the LA get all the equipment ready (Table 1) and be wear gloves. Have three racks set out for the samples: one for blood tubes, one for pipetting the sample, and one for filled pipetted samples (pre- and post-counting).
Representative Results
Study-specific methods
Here, study-specific details for the representative results are reported. These details are not critical to the procedure and will vary across studies.
Participants and task design
Participants (n = 3, Table 2) underwent a simultaneous BOLD-fMRI/FDG-fPET study. As this manuscript focuses on the PET acquisition protocol, MRI results are not reported. Participants received 260 MBq of 18F-FDG over the course of a 95 min scan. Participant 1 received the full dose as a bolus at the start of the scan. Participant 2 received the dose in an infusion-only protocol. Participant 3 received the same dose with a hybrid 50% bolus plus 50% infusion. For both infusion-only and bolus/infusion protocols, infusion duration was 50 min.
The task was presented in an embedded block design (Figure 2)19. This design was previously shown to provide simultaneous contrast for task-evoked BOLD-fMRI and FDG-fPET data. Briefly, the task alternated between 640 s flashing checkerboard blocks and 320 s rest blocks. This slow alternation provides FDG-fPET contrast. These timing parameters were entered into the first-level general linear models during the analysis. Within the 640 s checkerboard blocks, checkerboard and rest periods alternated at a rate of 20 s on/20 s off. This fast alternation, which is suited to BOLD-fMRI, will hopefully be detectible with FDG-fPET with future analysis and reconstruction advances. In this protocol, rest periods were with eyes open, fixated on a cross centrally presented on the screen.
Image acquisition and processing
MR and PET images were acquired on a Siemens 3T Biograph mMR. PET data were acquired in list mode. The MRI and PET scans were acquired in the following order (details provided only for images relevant to the current manuscript): (i) T1-weighted 3D MPRAGE (TA = 7.01 min, TR = 1,640 ms, TE = 2.34 ms, flip angle = 8°, FOV = 256 x 256 mm2, voxel size = 1 x 1 x 1 mm3, 176 slices, sagittal acquisition; (ii) T2-weighted FLAIR (TA = 5.52 min); (iii) QSM (TA = 6.86 min); (iv) gradient field map TA = 1.03 min; (v) MR attenuation correction Dixon (TA = 0.39 min, TR = 4.1 ms, TEin phase = 2.5 ms, TEout phase = 1.3 ms, flip angle = 10°); (vi) T2*-weighted echo-planar images (EPIs) (TA = 90.09 min), P-A phase correction (TA = 0.36 min); (vii) UTE (TA = 1.96 min). The onset of the PET acquisition was locked to the onset of the T2* EPIs.
T1-weighted structural images were neck-cropped using FSL-robustfov26, bias corrected using N427, and brain extracted using ANTs28,29 with OASIS-20 templates30,31. T1-weighted images were non-linearly normalized to a 2 mm MNI template using ANTs32 with the default parameter set defined by antsRegistrationSyN.sh.
This manuscript examined dynamic FDG-fPET results with bin size 16 s. All data were reconstructed offline using Siemens Syngo E11p and corrected for attenuation using pseudoCT33. The ordinary Poisson ordered subset expectation maximization (OP-OSEM) algorithm with point spread function (PSF) modelling34 was used with three iterations, 21 subsets, and 344 x 344 x 127 (voxel size: 2.09 x 2.09 x 2.03 mm3) reconstruction matrix size. A 5-mm 3D Gaussian post-filtering was applied to the final reconstructed images.
Spatial realignment was performed on the dynamic FDG-fPET images using FSL MCFLIRT35. A mean FDG-PET image was derived from the entire dynamic timeseries and rigidly normalized to the individual's high-resolution T1-weighted image using advanced normalization tools (ANT)32. The dynamic FDG-fPET images were then normalized to MNI space using the rigid transform in combination with the non-linear T1 to MNI warp.
First-level general linear models were estimated using SPM12 (Wellcome Centre for Human Neuroimaging) with the event time-course (checkerboard on, fixation) modelled as the effect of interest. Average uptake across a control region, the frontopolar cortex (left and right FP1/236), was included as a covariate. The model did not include global normalization, high-pass filter, convolution with the hemodynamic response, autoregressive model, or masking threshold. An explicit mask of the visual cortex in hOC1−5 (left and right hOC1,2,3d,3v,4d,4la4lp,4v,537,38,39; SPM Anatomy Toolbox v 2.2b40,41,42) was included in the model to restrict the model estimation to regions of interest (ROI). In the clinical environment, multiple regions are analyzed using brain atlases. T contrasts were used to estimate parameter maps of the individual-level activity, liberally thresholded at p = 0.1 (uncorrected), k = 50 voxels. The results for each individual are also shown at multiple thresholds in Supplement 2.
Plasma radioactivity concentration results
The plasma radioactivity concentration curve for each participant is given in Figure 3. The largest peak plasma radioactivity concentration (3.67 kBq/mL) was obtained using the bolus method. Visual inspection of Figure 3 shows that the peak occurs within the first 10 min of the protocol, and the concentration decreases thereafter. Note that protocols that use arterial or automated sampling at a rate of less than 1 min will likely find a peak plasma concentration within the first minute. The delay here is because the first blood sample was taken at 5 min post-bolus. By the end of the recording period, the plasma radioactivity was 35% of the peak (1.28 kBq/mL). The infusion-only protocol reached maximum (2.22 kBq/mL) at 50 min, the end of the infusion period. By the end of the recording period, the concentration was sustained at 68% of its peak (1.52 kBq/mL). Like the bolus-only protocol, the bolus/infusion protocol reached its peak plasma radioactivity concentration (2.77 kBq/mL) within the first 5 min. By the end of the recording period, bolus/infusion concentration was at 53% of the peak (1.49 kBq/mL).
Qualitatively, plasma radioactivity levels were sustained for the longest duration in the bolus/infusion protocol. Both infusion-only and bolus/infusion protocols show an apparent reduction in radioactivity when the infusion period ends (50 min). Visually comparing bolus-only and bolus/infusion protocols, plasma radioactivity was smaller in bolus-only vs. bolus/infusion by 40 min post-injection. Critically, the plasma radioactivity was minimally varied for a period of approximately 40 min in the bolus/infusion protocol. In contrast, neither the infusion-only nor bolus-only protocol exhibit a qualitatively sustained period of consistent activity.
PET Signal Results
Individual-level parameter maps from the general linear model, PET signal and GLM fitted response, and errors are shown in Figure 4. Parameter maps are also shown at different statistical thresholds in Supplement 2.
Figure 4ii shows the PET signal across the scan period (i.e., across stimulation and rest periods) in the bilateral visual cortex (hOC1−5) and in the control region (frontal pole, FP1/2) for the three administration protocols. Qualitatively, the bolus/infusion participant showed clearer differences between the ROIs, compared to bolus-only and infusion-only participants. For the bolus/infusion protocol, the frontopolar ROI showed the highest image intensity, with the lowest for hOC4. For the bolus-only participant, there was a similar trend, with hOC5 and FP1/2 showing the highest intensity, with hOC4 showing the lowest. For the infusion-only participant, the FP1/2 and right hOC5 showed the highest intensity, with little difference between the remaining ROIs.
Visual inspection of Figure 4ii suggests that in the bolus-only protocol, there is a sharp increase in signal following the bolus. The slope of the uptake is relatively fast in the next 20−30 min, but the rate of uptake decreases in the remainder of the measurement period. In the bolus/infusion protocol, there is a sharp increase in uptake at the start of the scan that is of smaller magnitude than in the bolus-only protocol, and the uptake continues at a comparatively faster rate for the duration of the scan. By the end of the recording period, the bolus/infusion protocol shows a larger uptake than the bolus-only protocol. By comparison, the infusion-only protocol shows low signal for the first 40 min of the scan, and the peak uptake is substantially lower than the bolus-only or bolus/infusion protocol. Uptake is fastest in the first ~50 min of the scan and slows for the remainder of the recording period.
Parameter Maps and Fitted Response Results
Figure 4i shows the individual-level T maps for the three administration protocols. Figure 4iii shows the general linear model fitted response and error at the peak voxel for each subject. Note that for the infusion-only protocol (Figure 4Biii), the scale is larger than for the bolus-only and bolus/infusion protocols. Furthermore, for the infusion-only protocol, the signal during the first rest block was close to zero, as very little of the tracer had been administered during that time, and the general linear model estimation failed when considering this block. Thus, the general linear model was estimated for this participant starting with the first task block, and the fitted response is shown from the start of the first checkerboard period.
To visualize the task effects across time, the time course data for each subject was extracted (first eigenvariate) and the inverse of the coefficient of variation (mean/standard deviation) was calculated for each block. The inverse of the coefficient of variation approximates the signal-to-noise ratio. As can be seen from Figure 5, the signal increased roughly linearly across the recording period for the three protocols. The slope of the line was highest for the infusion-only protocol (m = 2.794), intermediate for the bolus-only (1.377) and smallest for the bolus/infusion protocol (1.159).
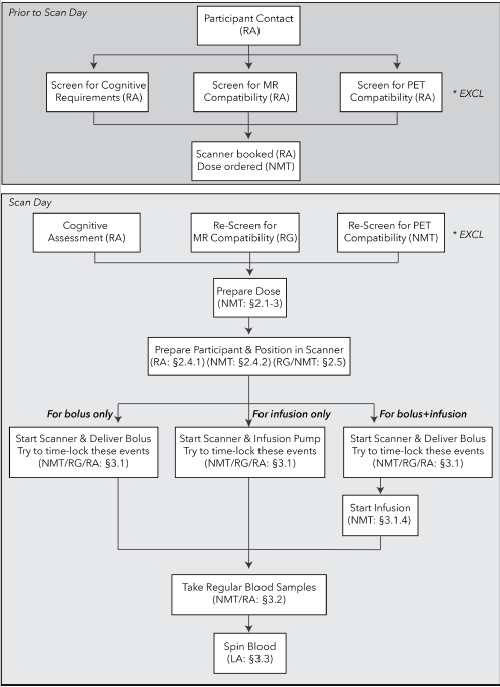
Figure 1: Flowchart of procedures for FDG-fPET experiments. Top: procedures for prescreening of participants prior to study recruitment. Bottom: procedures for the bolus-only (left), infusion-only (center), and bolus/infusion (right) protocols. The staff member responsible for each procedure is listed in parentheses. Section identifiers refer to the sections in the text where the procedure is described. *EXCL indicates timepoints when participants may be excluded, either for MR or PET scanning incompatibility, or not meeting study entry requirements (e.g., cognitive and psychological requirements). NMT = Nuclear Medicine Technologist, RA = Research Assistant, RG = Radiographer, LA = Lab Assistant. Please click here to view a larger version of this figure.
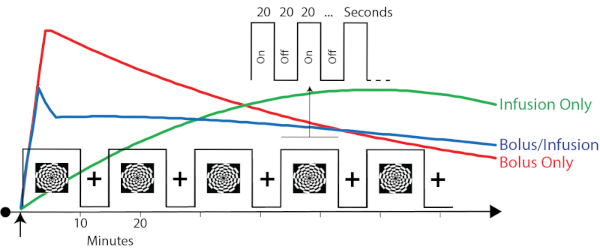
Figure 2: Timing parameters and the predicted plasma radioactivity from the three protocols. Red, green, and blue traces represent the hypothesized plasma radioactivity curves for the bolus, infusion, and bolus/infusion protocols, respectively. Note that these traces are for illustrative purposes only. See Figure 3 for obtained plasma radioactivity curves. The timing parameters are superimposed to show the relative timing of the task relative to the expected plasma radioactivity. The embedded block design (Jamadar et al. 201919) has a slow alternation (10/5 min) between checkerboard stimulation and eyes-open rest. Embedded within the 'on' blocks is a fast alternating (20 s) on/off design. The slow alternation provides FDG-fPET contrast. The fast alternation provides BOLD-fMRI contrast. Please click here to view a larger version of this figure.
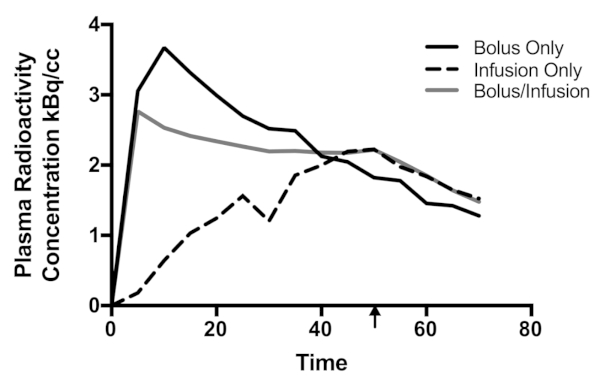
Figure 3: Plasma radioactivity curves for the three participants. Decay was corrected to the time the blood was sampled. The arrow indicates the cessation of the infusion for the infusion-only and bolus/infusion protocols. Time is in minutes. Please click here to view a larger version of this figure.
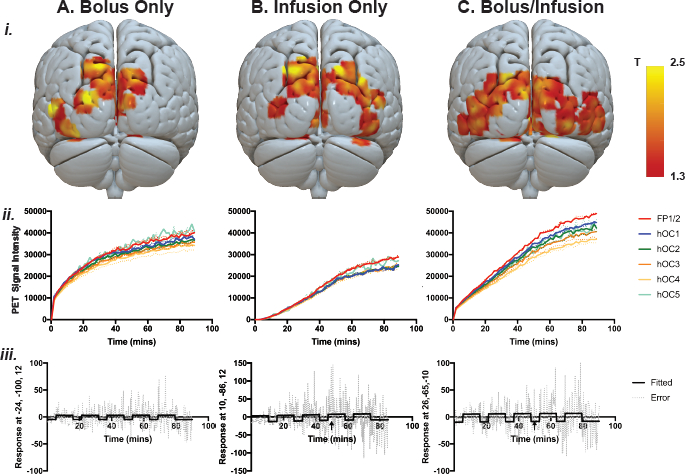
Figure 4: Individual-level parameter maps from the general linear model, PET signal, and GLM fitted response and error. (i) Individual-level statistical parameter (T) maps for each of the three subjects, thresholded at p (uncorrected) < 0.1, k = 50 voxels. (ii) PET signal across the visual cortex in regions of interest: five occipital (left and right hOC1, hOC2, average hOC3d/3v, average 4d/4la/4lp/4v, hOC5) and frontal (left and right average FP1/2) control areas. Note that the left regions are shown in solid lines, right regions shown in dotted lines. (iii) Model fit and error across time for the peak of activity in each subject. Arrow shows the end of the infusion period. (Aiii) bolus-only peak activity MNI coordinate (-24, -100, 12), T = 4.07; infusion-only (Biii) peak activity MNI coordinate (10, -86, 12), T = 4.25; bolus/infusion peak activity coordinate (26, -65, -10), T = 5.17. Note that for the infusion-only protocol, the model could not be estimated for the first rest period due to a very low signal. Also note the larger scale for the infusion-only protocol compared to the bolus-only and bolus/infusion protocols. Please click here to view a larger version of this figure.
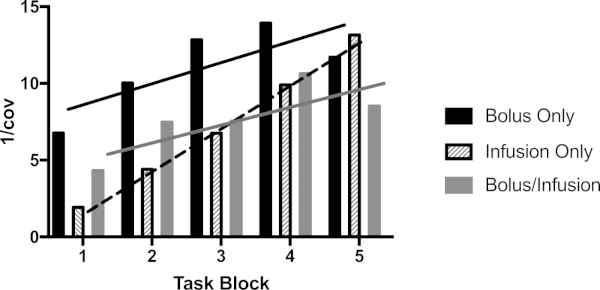
Figure 5: Signal to noise ratio across the recording period. The plot shows the inverse of the coefficient of variation (mean/SD) of the first eigenvariate of the activity within the peak voxel in each checkerboard block. SD = standard deviation. Please click here to view a larger version of this figure.
| Participant 1 | Participant 2 | Participant 3 | |
| Administration protocol | bolus only | infusion only | bolus/infusion |
| Age (years) | 18 | 19 | 19 |
| Sex | F | M | F |
| Handedness | R | R | R |
| Years of Education | 12 | 14 | 14 |
| Current Axis I Psychiatric illness | None | None | None |
| History of Cardiovascular Disease | None | None | None |
| Regular Medication | None | None | None |
Table 1: Demographic information for the three participants.
Supplement 1: Example participant record form. In this protocol, the RA is responsible for recording the time of bolus and infusion start and calculating the time of blood samples. The RA then provides copies of this form to the NMT and LA. During the experiment, the RA records the times that the samples were taken for subsequent decay correction. The LA records the time of measurement and the measurement values in the Notes section. Please click here to view this file (Right click to download).
Supplement 2: Variability in statistical parameter maps with different statistical thresholds. Results are presented in slices at a range of thresholds from p = 1.0 to FWE p < 0.05. Please click here to view this file (Right click to download).
Discussion
FDG-PET is a powerful imaging technology that measures glucose uptake, an index of cerebral glucose metabolism. To date, most neuroscience studies using FDG-PET use a traditional bolus administration approach, with a static image resolution that represents the integral of all metabolic activity over the course of the scan2. This manuscript describes two alternative radiotracer administration protocols: the infusion-only (e.g., Villien et al., Jamadar et al.19,21) and the hybrid bolus/infusion (e.g., Rischka et al.20) protocols. The three protocols demonstrated a temporal resolution of 16 s, time-locked to a stimulus, at the individual-level.
The critical point in the method is the start of the scanning protocol. At this point, the beginning of the PET acquisition must be time-locked to the beginning of the BOLD-fMRI sequence (if using simultaneous MR-PET), as well as the start of the stimulus presentation. Stimulus onsets and durations must be able to be locked to the onset of the scan for the first-level models. In the bolus-only protocol, the bolus should be delivered at the beginning of the PET acquisition to capture the peak signal (Figure 4). In the infusion-only protocol, the beginning of the infusion should be locked to the PET acquisition, to ensure accurate modelling of the uptake at the first-level. In the bolus/infusion protocol, the bolus should be time-locked to the PET acquisition, with the infusion starting at a known, short period, after the bolus. In order for the procedures to flow correctly within this short time period, each of the staff members (NMT, RG, RA) should be adequately prepared prior to the start of the scan (Figure 1). 'Dress rehearsals' are recommended to choreograph the timing of this critical stage.
To date, approximately 60 subjects have been tested using one of these protocols in our lab (the largest number using the infusion-only protocol). There are two common causes of subject attrition or acquisition failure. (1) Researchers are unable to cannulate the participant due to difficulty finding veins. To address this, all participants must drink at least two glasses of water before the scan. If only one cannula can be achieved, blood sampling is omitted for that participant. (2) Participants are unable to complete the scan. Unlike MRI, the PET acquisition cannot be paused and restarted. The most common causes of in-scan participant withdrawal are due to toilet breaks and difficulty with thermal regulation. Participants have reported that the requirement to consume water before the scan increases the need to urinate. Thus, all participants are required to do so prior to scanning. Participants have also reported that the infusion of the tracer leaves them feeling very cold, and shivering is triggered in some people. Previous studies have shown that ambient temperature can influence artefactual activity in FDG-PET scans46. This issue is addressed by using a disposable quilt for all participants during the scan.
Results are shown at the individual subject level for the three administration protocols. As expected, the blood plasma radioactivity concentration (Figure 3) had the largest peak for the bolus-only protocol, but the most sustained radioactivity was obtained in the bolus/infusion protocol. The plasma concentration was lowest for the infusion-only protocol. For both the infusion-only and bolus/infusion protocols, the concentration decreased at the time the infusion ceased. PET signal across the ROIs (Figure 4Bii) showed the largest signal in the bolus/infusion protocol. This participant also showed the clearest differentiation between the ROIs. Qualitatively, the PET signal was weakest in the infusion-only protocol. It is possible that the infusion-only protocol would yield better results in a longer experiment (>50 min). However, this would likely increase the rate of participant attrition. In the first-level general linear models, model error was much greater in the infusion-only protocol compared to the bolus-only and bolus/infusion protocols (Figure 4iii). Signal-to-noise during the task periods (Figure 5) suggested that the most stable signal across the recording period was obtained using the bolus/infusion protocol. Further studies are required to determine if these effects are sustained in a larger sample.
fPET is a relatively new method (first published by Villien et al.21), and the data are relatively complex to acquire compared to traditional neuroimaging approaches like static PET and MRI/fMRI. Thus, there is substantial room for improvement for the data acquisition protocols. This study presents the acquisition protocol for three tracer administration protocols (bolus-only, infusion-only, and bolus plus infusion) and the representative results from individual subjects for each method. In this group, no arterial sampling was performed due to the invasiveness of the procedure and the requirement for an MD on site. Our image analyses therefore do not benefit from the quantitative information provided by arterial sampling. Note that Hahn et al.17 found excellent agreement between arterial and venous sampling for determining cortical cerebral metabolic rate of glucose (CMRGlc) for constant infusion FDG-fPET. Other published works43,44,45 discuss arterial, venous, and image-derived input functions for PET in detail.
Manual blood sampling, whether arterial or venous, requires staff to enter the scanner room while scanning is underway. Most scanners have an RF interlock for the scanner room, which enables staff to access the room during scanning without causing electromagnetic interference artefacts in the MR images. However, staff entering the room during the scan may increase the radiation exposure to staff, cause participant discomfort, and increase participant movement and disengagement from cognitive tasks. These factors encourage the collection of as few samples as necessary. Taking samples every 5−10 min while the dose is administered is sufficient to observe the low-frequency blood dynamics expected from the three protocols examined here. However, this sampling rate limits the ability to quantify high-frequency temporal characteristics, particularly the exact size and shape of the peak following bolus administration. Where such characteristics are of importance, the use of automated blood sampling equipment may be beneficial.
Lastly, traditional PET modelling methods were developed for static imaging (e.g., kinetic, Patlak). More work is required to update the mathematical models for application to fPET data.
In summary, this manuscript presents alternative methods of FDG radiotracer administration for high temporal resolution FDG-PET, with a resolution of 16 s. This temporal resolution compares favorably to current standards in the literature. Hahn et al., Jamadar et al., and Villien et al.17,18,19,21 report FDG-fPET with 1 min resolution, and Rischka et al.20 achieved stable FDG-fPET results with a frame duration of 12 s using 20/80% bolus plus infusion. The bolus/infusion protocol presented here appears to provide the most stable signal for the longest period of time compared to the bolus-only and infusion-only protocols.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Jamadar is supported by an Australian Council for Research (ARC) Discovery Early Career Researcher Award (DECRA DE150100406). Jamadar, Ward, and Egan are supported by the ARC Centre of Excellence for Integrative Brain Function (CE114100007). Chen and Li are supported by funding from the Reignwood Cultural Foundation.
Jamadar, Ward, Carey, and McIntyre designed the protocol. Carey, McIntyre, Sasan, and Fallon collected the data. Jamadar, Ward, Parkes, and Sasan analyzed the data. Jamadar, Ward, Carey, and McIntyre wrote the first draft of the manuscript. All authors have reviewed and approved the final version.
Materials
| Blood Collection Equipment | |||
| –12-15 vacutainers | Becton Dickinson, NJ USA | 364880 | Remain in sterile packaging until required to put blood in tube |
| –12-15 10mL LH blood collecting tubes | Becton Dickinson | 367526 | Marked with the sample number (e.g., S1, S2…) and subsequently marked with the sample time (e.g., time 0 + x min [T0+x]) |
| –2-15 10mL Terumo syringe | Terumo Tokyo, Japan | SS+10L | These are drawn up on the day of the study and capped with the ampoule that contained the saline |
| — pre-drawn 0.9% saline flushes | Pfizer, NY, USA | 61039117 | |
| –12-15 5mL Terumo syringes | Terumo Tokyo, Japan | SS+05S | Remain in sterile packaging until ready to withdraw a blood sample |
| Safety & Waste Equipment | All objects arranged on a plastic chair inside the scanner room on the same side as the arm from which the blood samples will be taken. Biohazard and non-biohazard waste bags to be used. Gloves and waste bags to be easily accessible when preparing the radioactivity in the dispensing area and when pipetting the plasma samples. Biohazard and non-biohazard waste bags to be used. All waste generated is checked with the Geiger counter to ensure that radioactive contaminated waste is stored until it is safe to be disposed of according to Australian Radiation Protection and Nuclear Safety Agency (APRANSA) guidelines for Radiation protection series No.6 (2017). | ||
| — Gloves | Westlab, VIC, Australia | 663-219 | |
| — waste bags | Austar Packaging, VIC, Australia | YIW6090 | |
| –cello underpads ‘blueys’ Underpads 5 Ply | Halyard Health, NSW, Australia | 2765A | |
| –Blue Sharpie pen | Sharpie, TN, USA | S30063 | |
| Dose Syringes | Remain in sterile packaging until ready for use. All syringes used in this facility have an additional 20% volume capacity above the stated volume on the packaging. This is important for the 50mL syringe where the total capacity of 60mL is used | ||
| –5mL | Terumo Tokyo, Japan | SS+05S | |
| — 20mL | Terumo Tokyo, Japan | SS+20L | |
| –50mL | Terumo Tokyo, Japan | SS*50LE | |
| –1 Terumo 18-gauge needle | Terumo Tokyo, Japan | NN+1838R | Remain in sterile packaging until ready to inject [18F]FDG into the saline bag |
| –100mL 0.9% saline bag | Baxter Pharmaceutical, IL, USA | AHB1307 | Remain in sterile packaging until ready to inject [18F]FDG |
| Radiochemistry Lab Supplies | |||
| –Heraeus Megafuge 16 centrifuge; Rotor Bioshield 720 | ThermoScientific MA, USA | 75004230 | Relative Centrifugal Force = 724 Our settings are 2000RPM for 5mins. Acceleration and deceleration curves set to 8 |
| –Single well counter | Laboratory Technologies, Inc. IL, USA | 630-365-1000 | Complete daily quality control (includes background count) and protocol set to 18F and 4mins. Cross calibration is performed between the well counter, dose calibrator and scanner on a bi-monthly basis. |
| –Pipette | ISG Xacto, Vienna, Austria | LI10434 | We use a 100-1000 μL set to 1000μL. It is calibrated annually. |
| –12-15 plasma counting tubes | Techno PLAS; SA Australia | P10316SU | Marked in the same manner as the LH blood tubes |
| –12-15 pipette tips | Expell Capp, Denmark | 5130140-1 | |
| –3 test tube racks | Generic | Checked with a Geiger counter to ensure there is no radiation contamination on them | |
| –500mL volumetric flask and distilled water | Generic | Need approximately 500mL of distilled water to prepare the reference for gamma counting | |
| –Synchronised clocks in scanner room, console and radiochemistry lab | Generic | Synchronisation checks are routinely completed in the facility on a weekly basis | |
| –Haemoglobin Monitor | EKF Diagnostic Cardiff, UK Haemo Control. | 3000-0810-6801 | Manufacturer recommended quality control performed before testing on participant’s blood sample. |
| –Glucometre | Roche Accu-Chek | 6870252001 | Accu-Chek Performa is used to measure participant blood sugar levels in mmol/L. Quality control is performed daily using high and low concentration solution control test. |
| Cannulating Equipment | Check expiry dates and train NMT to prepare aseptically for cannulation. | ||
| –Regulation tourniquet | CBC Classic Kimetec GmBH | K5020 | |
| –20, 22 and 24 gauge cannulas | Braun, Melsungen Germany | 4251644-03; 4251628-03; 4251601-03 | |
| –tegaderm dressings | 3M, MN USA | 1624W | |
| –alcohol and chlorhexidine swabs | Reynard Health Supplies, NSW Australia | RHS408 | |
| –0.9% saline 10mL ampoules; for flushes | Pfizer, NY, USA | 61039117 | |
| –10mL syringes | Terumo Tokyo, Japan | SS+10L | |
| –3-way tap | Becton Dickinson Connecta | 394600 | |
| –IV bung | Safsite Braun PA USA | 415068 | |
| –Optional extension tube, microbore extension set | M Devices, Denmark | IV054000 | |
| Scanner Room Equipment | |||
| –Siemens Biograph 3T mMR | Siemens, Erlangen, Germany | ||
| –Portable lead barrier shield | Gammasonics | Custom-built | MR-conditional lead barrier shield. Positioned at the 2000 Gauss line with the castors locked to provide additional shielding of the radioactivity connected to the infusion pump. |
| –Infusion pump BodyGuard 323 MR-conditional infusion pump | Caesarea Medical Electronics | 300-040XP | MR-compatible. This model is cleared for use on 1.5 and 3T scanners at 2000 Gauss with castors locked. |
| –Infusion pump tubing | Caesarea Medical Electronics | 100-163X2YNKS | Tubing is administration set with an anti-siphon valve and male luer lock (REF 100-163X2YNKS). |
| –Lead bricks | Custom built | Tested for ferromagnetic translational force | |
| Other Equipment | |||
| –Syringe shields | Biodex, NY USA | Custom-built | There is a 5mL tungsten syringe shield that is MR-safe, as well as a 50mL lead shield that has been tested for ferromagnetic attraction prior to use in the MR-PET scanner. It is used to transport the radioactive dose from the radiochemistry lab into the scanner to minimise radiation exposure to the NMT. |
| –Geiger counter Model 26-1 Integrated Frisker | Ludlum Measurements, Inc. TX USA | 48-4007 | This is calibrated annually and used to monitor potential contamination and waste. It is not taken into the MR-PET scanner. |
References
- Heurling, K., et al. Quantitative positron emission tomography in brain research. Brain Research. 1670, 220-234 (2017).
- Chen, Z., et al. From simultaneous to synergistic MR-PET brain imaging: A review of hybrid MR-PET imaging methodologies. Human Brain Mapping. 39 (12), 5126-5144 (2018).
- Jones, T., Rabiner, E. A. The development, past achievements, and future directions of brain PET. Journal of Cerebral Blood Flow & Metabolism. 32 (7), 1426-1454 (2012).
- Kety, S. S. . Metabolism of the nervous system. , 221-237 (1957).
- Sokoloff, L. The metabolism of the central nervous system in vivo. Handbook of Physiology, section I, neurophysiology. 3, 1843-1864 (1960).
- Harris, J. J., Jolivet, R., Attwell, D. Synaptic energy use and supply. Neuron. 75 (5), 762-777 (2012).
- Mosconi, L., et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. European Journal of Nuclear Medicine and Molecular Imaging. 36 (5), 811-822 (2009).
- Pagano, G., Niccolini, F., Politis, M. Current status of PET imaging in Huntington’s disease. European Journal of Nuclear Medicine and Molecular Imaging. 43 (6), 1171-1182 (2016).
- Petit-Taboue, M., Landeau, B., Desson, J., Desgranges, B., Baron, J. Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage. 7 (3), 176-184 (1998).
- Chugani, H. T., Phelps, M. E., Mazziotta, J. C. Positron emission tomography study of human brain functional development. Annals of Neurology. 22 (4), 487-497 (1987).
- Phelps, M. E., Mazziotta, J. C. Positron emission tomography: human brain function and biochemistry. Science. 228 (4701), 799-809 (1985).
- Zimmer, E. R., et al. [18 F] FDG PET signal is driven by astroglial glutamate transport. Nature Neuroscience. 20 (3), 393 (2017).
- Roberts, R. P., Hach, S., Tippett, L. J., Addis, D. R. The Simpson’s paradox and fMRI: Similarities and differences between functional connectivity measures derived from within-subject and across-subject correlations. Neuroimage. 135, 1-15 (2016).
- Horwitz, B. The elusive concept of brain connectivity. Neuroimage. 19 (2), 466-470 (2003).
- Moses, W. W. Fundamental limits of spatial resolution in PET. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 648, S236-S240 (2011).
- Tomasi, D. G., et al. Dynamic brain glucose metabolism identifies anti-correlated cortical-cerebellar networks at rest. Journal of Cerebral Blood Flow & Metabolism. 37 (12), 3659-3670 (2017).
- Hahn, A., et al. Quantification of task specific glucose metabolism with constant infusion of 18F-FDG. Journal of Nuclear Medicine. 57 (12), 1933-1940 (2016).
- Hahn, A., et al. Task-relevant brain networks identified with simultaneous PET/MR imaging of metabolism and connectivity. Brain Structure and Function. 223 (3), 1369-1378 (2018).
- Jamadar, S. D., et al. Simultaneous task-based BOLD-fMRI and [18-F] FDG functional PET for measurement of neuronal metabolism in the human visual cortex. Neuroimage. 189, 258-266 (2019).
- Rischka, L., et al. Reduced task durations in functional PET imaging with [18F] FDG approaching that of functional MRI. Neuroimage. 181, 323-330 (2018).
- Villien, M., et al. Dynamic functional imaging of brain glucose utilization using fPET-FDG. Neuroimage. 100, 192-199 (2014).
- Carson, R. E. PET physiological measurements using constant infusion. Nuclear Medicine and Biology. 27 (7), 657-660 (2000).
- Carson, R. E., et al. Comparison of bolus and infusion methods for receptor quantitation: application to [18F] cyclofoxy and positron emission tomography. Journal of Cerebral Blood Flow & Metabolism. 13 (1), 24-42 (1993).
- National Health and Medical Research Council. . National statement on ethical conduct in human research. , (2007).
- Australian Radiation Protection and Nuclear Safety Agency. . Code of practice for the exposure of humans to ionizing radiation for research purposes. , (2005).
- Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W., Smith, S. M. FSL. Neuroimage. 62 (2), 782-790 (2012).
- Tustison, N. J., et al. N4ITK: improved N3 bias correction. IEEE Transactions on Medical Imaging. 29 (6), 1310 (2010).
- Avants, B., Klein, A., Tustison, N., Woo, J., Gee, J. C. . 16th Annual Meeting for the Organization of Human Brain Mapping. , (2010).
- Avants, B. B., Epstein, C. L., Grossman, M., Gee, J. C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis. 12 (1), 26-41 (2008).
- Klein, A., et al. Mindboggling morphometry of human brains. PLoS Computational Biology. 13 (2), e1005350 (2017).
- Tustison, N. J., et al. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage. 99, 166-179 (2014).
- Avants, B. B., et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 54 (3), 2033-2044 (2011).
- Burgos, N., et al. Attenuation correction synthesis for hybrid PET-MR scanners: application to brain studies. IEEE Transactions on Medical Imaging. 33 (12), 2332-2341 (2014).
- Panin, V. Y., Kehren, F., Michel, C., Casey, M. Fully 3-D PET reconstruction with system matrix derived from point source measurements. IEEE Transactions on Medical Imaging. 25 (7), 907-921 (2006).
- Jenkinson, M., Bannister, P., Brady, M., Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 17 (2), 825-841 (2002).
- Bludau, S., et al. Cytoarchitecture, probability maps and functions of the human frontal pole. Neuroimage. 93, 260-275 (2014).
- Amunts, K., Malikovic, A., Mohlberg, H., Schormann, T., Zilles, K. Brodmann’s areas 17 and 18 brought into stereotaxic space-where and how variable?. Neuroimage. 11 (1), 66-84 (2000).
- Malikovic, A., et al. Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT+: a probabilistic, stereotaxic map of area hOc5. Cerebral Cortex. 17 (3), 562-574 (2006).
- Wilms, M., et al. Human V5/MT+: comparison of functional and cytoarchitectonic data. Anatomy and Embryology. 210 (5-6), 485-495 (2005).
- Eickhoff, S. B., Heim, S., Zilles, K., Amunts, K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 32 (2), 570-582 (2006).
- Eickhoff, S. B., et al. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 36 (3), 511-521 (2007).
- Eickhoff, S. B., et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 25 (4), 1325-1335 (2005).
- Everett, B. A., et al. Safety of radial arterial catheterization in PET research subjects. Journal of Nuclear Medicine. 50 (10), 1742-1742 (2009).
- Takagi, S., et al. Quantitative PET cerebral glucose metabolism estimates using a single non-arterialized venous-blood sample. Annals of Nuclear Medicine. 18 (4), 297-302 (2004).
- Zanotti-Fregonara, P., Chen, K., Liow, J. S., Fujita, M., Innis, R. B. Image-derived input function for brain PET studies: many challenges and few opportunities. Journal of Cerebral Blood Flow & Metabolism. 31 (10), 1986-1998 (2011).
- O’Loughlin, S., Currie, G. M., Trifonovic, M., Kiat, H. Ambient temperature and cardiac accumulation of 18F-FDG. Journal of Nuclear Medicine Technology. 42 (3), 188-193 (2014).

