Esophageal Heat Transfer for Patient Temperature Control and Targeted Temperature Management
Summary
This study presents a novel method to provide efficient patient temperature control for cooling or warming patients. A single use, triple lumen device is placed into the esophagus, analogous to a standard orogastric tube, and connects to existing heat exchange units to perform automatic patient temperature management.
Abstract
Controlling patient temperature is important for a wide variety of clinical conditions. Cooling to normal or below normal body temperature is often performed for neuroprotection after ischemic insult (e.g. hemorrhagic stroke, subarachnoid hemorrhage, cardiac arrest, or other hypoxic injury). Cooling from febrile states treats fever and reduces the negative effects of hyperthermia on injured neurons. Patients are warmed in the operating room to prevent inadvertent perioperative hypothermia, which is known to cause increased blood loss, wound infections, and myocardial injury, while also prolonging recovery time. There are many reported approaches for temperature management, including improvised methods that repurpose standard supplies (e.g., ice, chilled saline, fans, blankets) but more sophisticated technologies designed for temperature management are typically more successful in delivering an optimized protocol. Over the last decade, advanced technologies have developed around two heat transfer methods: surface devices (water blankets, forced-air warmers) or intravascular devices (sterile catheters requiring vascular placement). Recently, a novel device became available that is placed in the esophagus, analogous to a standard orogastric tube, that provides efficient heat transfer through the patient's core. The device connects to existing heat exchange units to allow automatic patient temperature management via a servo mechanism, using patient temperature from standard temperature sensors (rectal, Foley, or other core temperature sensors) as the input variable. This approach eliminates vascular placement complications (deep venous thrombosis, central line associated bloodstream infection), reduces obstruction to patient access, and causes less shivering when compared to surface approaches. Published data have also shown a high degree of accuracy and maintenance of target temperature using the esophageal approach to temperature management. Therefore, the purpose of this method is to provide a low-risk alternative method for controlling patient temperature in critical care settings.
Introduction
There is significant need for patient temperature management when treating a wide range of conditions, including cardiac arrest, refractory or recurrent fever, neurogenic fever, and major surgery. In the United States, a half-million cardiac arrests annually originate either in the hospital (for example, in patients who are undergoing care for general medical or surgical conditions)1 or out of the hospital (for example, at home or in public venues, who are then brought to the emergency department)2. In both scenarios, patient outcomes are significantly improved when active temperature management is administered3 and targeted temperature management (TTM) has been a standard of care for cardiac arrest since 2005. Over 5 million patients are admitted to intensive care units annually in the U.S4 . Of these, fever develops in up to 45% of non-neurologically injured patients5 and up to 70% of neurologically injured patients6. Fever control in the intensive care unit is associated with improved outcomes and reduced risk of death, because elevated temperature increases metabolic demands, worsens cerebral ischemia, and increases neuronal loss7. At least 10 million surgeries performed annually in the U.S. require active patient warming to prevent inadvertent perioperative hypothermia8. In the operating room, patients undergoing surgery should maintain a body temperature above 36 °C to avoid a large number of adverse effects. Unplanned decreases in body temperature before, during, or after surgery increase blood loss, infections, and hospital length of stay, which adds $7,000 or more per patient to hospitalization costs9,10,11,12.
Despite great clinical need, the most widely administered temperature management protocols demonstrate inadequate performance or introduce significant risk to the patient. Surface devices (such as water blankets, conduction mattresses, and forced-air covers) are cumbersome, have limited heat transfer capacity, and must be removed to allow access to the patient for patient care and procedures. Intravascular devices are invasive, difficult to place, and predispose patients to infections and blood clots. Existing approaches to prevent inadvertent perioperative hypothermia fail to maintain normothermia up to 70% of the time12,13,14,15,16 and a retrospective analysis of post-cardiac arrest cooling found that overall, 30% of patients failed to reach target temperature within 6 h17.
The esophageal approach to patient temperature management offers substantial advantages to existing technologies18. The esophageal temperature management device maintains the functionality of the gastric tube typically placed in the critical care and surgical patient populations. It allows continuous gastric suction and decompression of gas and liquids while adding the ability to control a patient's temperature safely and efficiently by leveraging the favorable heat exchange environment of the esophagus (Figure 1). Temperature modulation is achieved by connecting the esophageal temperature management device to any of several external heat exchangers (also called chillers) that use water as the coolant. Several vendors produce compatible heat exchangers that are available in hospitals for powering existing temperature control products (most often water blankets). Nurses, nurse practitioners, or physicians typically place an esophageal temperature management device, but it can also be inserted by any provider trained to place a standard orogastric tube. The esophageal temperature management device does not restrict access to the patient, need not be sterile, avoids the risk of needle stick injuries among providers, and avoids the risks of skin complications, bloodstream infections, and blood clots in the patient. Therefore, the purpose of this method is to provide a low-risk alternative method for controlling patient temperature in critical care and operating room settings.
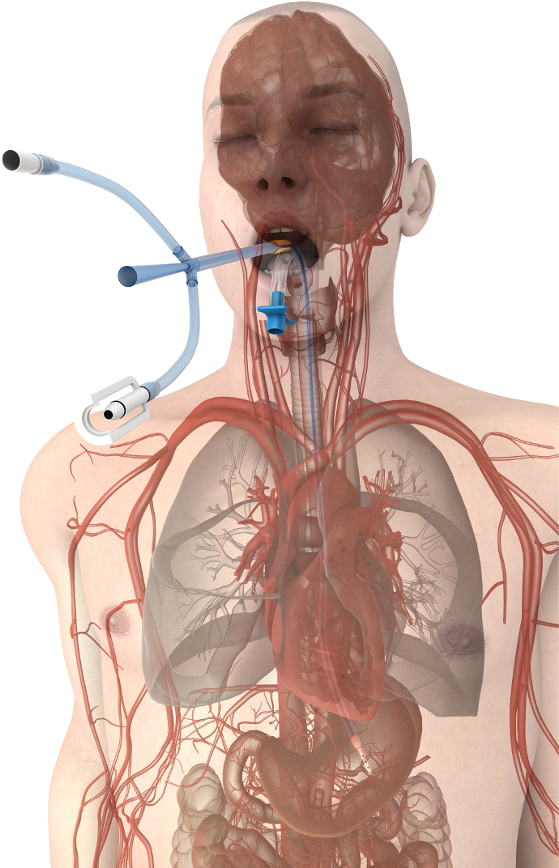
Figure 1. Esophageal temperature management device placement. Device proximity to the great vessels and heart promote efficient heat transfer at the patient's core. Please click here to view a larger version of this figure.
Protocol
Representative Results
Discussion
Modification and troubleshooting required for this protocol is generally limited to that featured above, and involves the typical monitoring of patient care employed in critical care settings. Modulating core temperature is critical to patient outcomes in a growing range of clinical scenarios. These include the intentional induction of hypothermia from normothermia, rewarming from inadvertent or intentional hypothermia, and actively maintaining normal body temperature (i.e., normothermia) during conditions in which inadvertent hypothermia are common, such as in the operating room. As specific clinical examples, patients who suffer ischemia-reperfusion injury, such as occurs during cardiac arrest, benefit from cooling (generally to temperatures below normal body temperature) followed by gentle rewarming and fever prevention for up to 3 days after resuscitation28,29,30. Neonates suffering from hypoxic ischemic encephalopathy obtain improved outcomes if cooled below normal body temperature31. The cooling of kidney donors after neurologic death, prior to organ transplant, has been shown to reduce the rate of delayed graft function32. Controlling fevers in septic shock patients may reduce vasopressor requirements and contribute to reducing early mortality33. Maintaining normothermia in patients undergoing surgical procedures reduces surgical wound infections, myocardial complications, blood loss, and transfusion requirements, while shortening length of stay and reducing the likelihood of death10,11,16.
Limitations of the technique include challenges encountered in managing critical care patients. While targeted temperature management promotes good outcomes, the most common temperature modulation techniques introduce risks to patients and logistical challenges to providers (including difficulties in placement, bloodstream infections, blood clots, skin damage, and cost). Esophageal temperature management is designed to overcome these shortcomings34,35,36. When appropriately managed, the esophageal temperature management device does not come into contact with the vasculature (as do the intravascular temperature modulation devices) or skin (as do the surface temperature modulation devices), thereby avoiding blood clots, bloodstream infection, and skin degradation. The device can be placed quickly by a variety of healthcare providers, typically in a matter of minutes21,37. The insertion technique mimics standard orogastric tube placement, which minimizes workflow disruptions that might delay therapy initiation. Using a core approach also appears to pose significantly less shivering burden than surface approaches27,38,39,40 . This has the advantage of reducing sedative and anti-shivering medication costs, which then shortens the patient length of stay via quicker awakening from the lower degree of sedation required. These features, considered in concert with the clinical performance described above, support esophageal temperature management as a viable option for providers in the emergency department, intensive care unit, and operating room. A growing set of data published on the device likewise supports this new approach21,22,23,24,27,41.
Critical steps within the protocol include initiating flow within the device prior to insertion, providing adequate lubrication to the device in order to assure easy placement, performing gastric suctioning and decompression to allow maximum contact between device and patient, and addressing any patient shivering that may develop. Following this protocol will provide optimum results and allow a high degree of performance and safety in the care of this important patient population.
Disclosures
The authors have nothing to disclose.
Acknowledgements
None.
Materials
| EnsoETM | Attune Medical | ECD01 | Device compatible with Gaymar/Stryker Medi-Therm III and Stryker Altrix Precision Temperature Management System |
| EnsoETM | Attune Medical | ECD02 | Device compatible with Cincinnati SubZero Blanketrol II and Cincinnati SubZero Blanketrol III |
| Gaymar/Stryker Medi-Therm III | Stryker | n/a | Compatible heater-cooler with the ECD01 |
| Cincinnati SubZero Blanketrol II | Gentherm | n/a | Compatible heater-cooler with the ECD02 |
| Cincinnati SubZero Blanketrol III | Gentherm | n/a | Compatible heater-cooler with the ECD02 |
| Stryker Altrix Precision Temperature Management System | Stryker | n/a | Compatible heater-cooler with the ECD01 |
| Water-soluble lubricant | Various | n/a | Standard water-soluble lubricant used to ease insertion of tubes, catheters, and digits |
| Securement device | Various | n/a | E.g., Guard360 by PrimeGuard Medical |
References
- Chan, P. S., Krumholz, H. M., Nichol, G., Nallamothu, B. K. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 358 (1), 9-17 (2008).
- Callans, D. J. Out-of-Hospital Cardiac Arrest — The Solution Is Shocking. N Engl J Med. 351 (7), 632-634 (2004).
- Kochanek, P. M., Jackson, T. C. The Brain and Hypothermia-From Aristotle to Targeted Temperature Management. Crit Care Med. 45 (2), 305-310 (2017).
- Laupland, K. B., et al. Occurrence and outcome of fever in critically ill adults. Crit Care Med. 36 (5), 1531-1535 (2008).
- Diringer, M. N., Reaven, N. L., Funk, S. E., Uman, G. C. Elevated body temperature independently contributes to increased length of stay in neurologic intensive care unit patients. Critical Care Medicine. 32 (7), 1489-1495 (2004).
- Laupland, K. B. Fever in the critically ill medical patient. Critical care medicine. 37 (Suppl 7), S273-S278 (2009).
- Mathias, J. M. Hospitals to report on normothermia. OR manager. 25 (9), 22-24 (2009).
- Rajagopalan, S., Mascha, E., Na, J., Sessler, D. I. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 108 (1), 71-77 (2008).
- Kurz, A., Sessler, D. I., Lenhardt, R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 334 (19), 1209-1215 (1996).
- Sessler, D. I. New surgical thermal management guidelines. Lancet. 374 (9695), 1049-1050 (2009).
- Young, V. L., Watson, M. E. Prevention of perioperative hypothermia in plastic surgery. Aesthet Surg J. 26 (5), 551-571 (2006).
- Hedrick, T. L., et al. Efficacy of protocol implementation on incidence of wound infection in colorectal operations. J Am Coll Surg. 205 (3), 432-438 (2007).
- Forbes, S. S., et al. Implementation of evidence-based practices for surgical site infection prophylaxis: results of a pre- and postintervention study. J Am Coll Surg. 207 (3), 336-341 (2008).
- Sun, Z., et al. Intraoperative core temperature patterns, transfusion requirement, and hospital duration in patients warmed with forced air. Anesthesiology. 122 (2), 276-285 (2015).
- Leary, M., et al. The association of body mass index with time to target temperature and outcomes following post-arrest targeted temperature management. Resuscitation. 85 (2), 244-247 (2014).
- Naiman, M., Shanley, P., Garrett, F., Kulstad, E. Evaluation of advanced cooling therapy’s esophageal cooling device for core temperature control. Expert Rev Med Devices. 13 (5), 423-433 (2016).
- Naiman, M., Markota, A., Hegazy, A. F., Dingley, J., Kulstad, E. Temperature Management in Critical Care and Burn Patients using an Esophageal Heat Transfer Device. Military Medicine. , (2017).
- Hegazy, A. F., Lapierre, D. M., Butler, R., Martin, J., Althenayan, E. The esophageal cooling device: A new temperature control tool in the intensivist’s arsenal. Heart Lung. , (2017).
- Hegazy, A. F., Lapierre, D. M., Butler, R., Althenayan, E. Temperature control in critically ill patients with a novel esophageal cooling device: a case series. BMC Anesthesiol. 15, 152 (2015).
- Bukovnik, N., Markota, A., Velnar, T., Rebol, J., Sinkovic, A. Therapeutic hypothermia and inhalation anesthesia in a patient with severe pneumococcal meningitis and secondary cardiac arrest. Am J Emerg Med. 35 (4), 665.e665-665.e666 (2017).
- Markota, A., Fluher, J., Kit, B., Balazic, P., Sinkovic, A. The introduction of an esophageal heat transfer device into a therapeutic hypothermia protocol: A prospective evaluation. Am J Emerg Med. 34 (4), 741-745 (2016).
- Markota, A., Kit, B., Fluher, J., Sinkovic, A. Use of an oesophageal heat transfer device in therapeutic hypothermia. Resuscitation. 89, e1-e2 (2015).
- Schroeder, D. C., et al. Oesophageal heat exchangers with a diameter of 11mm or 14.7mm are equally effective and safe for targeted temperature management. PLoS One. 12 (3), e0173229 (2017).
- Williams, D., et al. Use of an Esophageal Heat Exchanger to Maintain Core Temperature during Burn Excisions and to Attenuate Pyrexia on the Burns Intensive Care Unit. Case Reports in Anesthesiology. 2016, 6 (2016).
- Khan, I., et al. . 14th Annual Neurocritical Care Society Meeting. , (2016).
- HACA. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 346 (8), 549-556 (2002).
- Bernard, S. A., et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 346 (8), 557-563 (2002).
- Callaway, C. W., et al. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 132 (18 Suppl 2), S465-S482 (2015).
- Wyckoff, M. H., et al. Part 13: Neonatal Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 132 (18 Suppl 2), S543-S560 (2015).
- Niemann, C. U., et al. Therapeutic Hypothermia in Deceased Organ Donors and Kidney-Graft Function. N Engl J Med. 373 (5), 405-414 (2015).
- Schortgen, F., et al. Fever control using external cooling in septic shock: a randomized controlled trial. Am J Respir Crit Care Med. 185 (10), 1088-1095 (2012).
- Reccius, A., Mercado, P., Vargas, P., Canals, C., Montes, J. Inferior Vena Cava Thrombosis Related to Hypothermia Catheter: Report of 20 Consecutive Cases. Neurocrit Care. 23 (1), 72-77 (2015).
- Maze, R., et al. Endovascular cooling catheter related thrombosis in patients undergoing therapeutic hypothermia for out of hospital cardiac arrest. Resuscitation. 85 (10), 1354-1358 (2014).
- Simosa, H. F., Petersen, D. J., Agarwal, S. K., Burke, P. A., Hirsch, E. F. Increased risk of deep venous thrombosis with endovascular cooling in patients with traumatic head injury. Am Surg. 73 (5), 461-464 (2007).
- Kulstad, E., et al. Induction, maintenance, and reversal of therapeutic hypothermia with an esophageal heat transfer device. Resuscitation. 84 (11), 1619-1624 (2013).
- van Zanten, A. R., Polderman, K. H. Blowing hot and cold? Skin counter warming to prevent shivering during therapeutic cooling. Crit Care Med. 37 (6), 2106-2108 (2009).
- Tommasi, E., et al. Cooling techniques in mild hypothermia after cardiac arrest. J Cardiovasc Med. , (2014).
- Diringer, M. N. Treatment of fever in the neurologic intensive care unit with a catheter-based heat exchange system. Crit Care Med. 32 (2), 559-564 (2004).
- Hegazy, A. F., Lapierre, D. M., Butler, R., Martin, J., Althenayan, E. The esophageal cooling device: A new temperature control tool in the intensivist’s arsenal. Heart Lung. 46 (3), 143-148 (2017).

