Small-scale Propagation of Human iPSCs in Serum-free Conditions for Routine Immunocytochemical Characterization
Summary
Regular characterization of induced pluripotent stem cells (iPSCs), to ascertain maintenance of their pluripotent state, is an important step before these cells are used for other applications. Here we describe a method for the small-scale propagation of human iPSCs specifically designed to enable their easy and routine characterization via immunocytochemistry.
Abstract
There is great interest in utilizing human induced pluripotent stem cells (hiPSCs) for disease modeling and cell therapeutics due to their patient specificity and characteristic stemness. However, the pluripotency of iPSCs, which is essential to their functionality, must be confirmed before these cells can be used in such applications. While a rigorous characterization of iPSCs, through different cellular and functional assays is necessary to establish their pluripotency, routine assessment of pluripotency maintenance can be achieved more simply and effectively through immunocytochemical techniques. Here, we present a systematic protocol for culturing hiPSCs, in a scaled-down manner, to particularly facilitate the verification of their pluripotent state using immunocytochemistry. More specifically, this methodology encompasses an efficient and cost-effective means of growing iPSCs in serum-free conditions and plating them on small chamber slides or glass coverslips ideal for immunocytochemistry.
Introduction
Reprogramming human adult somatic cells into induced pluripotent stem cells (iPSCs) provides a way to obtain a potentially unlimited supply of patient-specific cells to study disease1,2. Recapitulating a disease phenotype in vitro would make it plausible to examine cellular and molecular mechanisms associated with disease, and enhance drug discovery and personalized medicine3. In addition, human iPSCs (hiPSCs) offer the possibility of deriving specific cell types which can be used as a unique resource to replace dead or dysfunctional cells and restore function in the context of several disorders4,5.
An important prerequisite to using iPSCs in the above applications is ensuring that their pluripotent and undifferentiated state is maintained during expansion in culture. Typically, techniques such as flow cytometry, western blotting, polymerase chain reaction and functional assays, which require large quantities of cells and specialized equipment, are used for the detailed analysis of iPSC pluripotency6,7,8,9,10. However, routine assessment of the iPSCs' undifferentiated state might effectively be achieved through the limited propagation of these cells specifically for immunocytochemistry (ICC), thus involving reduced time and resources.
Recent advances allow for the growth of iPSCs in defined serum-free conditions, which is a significant improvement over conventional culture systems that require murine fibroblast feeder layers and serum containing media. However, the current literature does not include clear stepwise protocols that describe how to transition iPSCs from feeder layer to feeder-free systems.
In this context, the present protocol systematically details how hiPSCs grown on irradiated mouse embryonic fibroblast (iMEF) feeder layers can be (1) adapted to propagate in serum-free medium, and (2) cultured on a small-scale to specifically support robust immunocytochemical analysis. Overall, this methodology represents a timely and cost-effective procedure for propagating human iPSCs in serum-free conditions for confirming their pluripotency on a routine basis using immunocytochemistry.
Protocol
hiPSCs were derived from human dermal fibroblasts isolated from 4 mm skin punch biopsies and reprogrammed in house via Sendai virus-mediated reprogramming11. The University of Arizona Institutional Review Board approved all procedures for subject recruitment and biopsy collection.
1. Preparation of Extracellular Matrix Coated Surface for iPSC Culture
- One day prior to the confluence of hiPSC cultures growing on iMEFs, prepare extracellular matrix (Matrigel) coated plates.
- Slow thaw an aliquot of the extracellular matrix (lot dependent, follow instructions on the specification sheet) on ice in 4 °C for 2 h.
- In a biosafety hood, using cold pipet tips (stored at -20 °C), add 1 aliquot of extracellular matrix to cold Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12/HEPES) according to the lot specific dilution factor.
- Dispense ~ 600 µL of extracellular matrix per well of each new cell culture treated 12-well plate needed.
- Incubate the plate at room temperature for several hours. Here, use a 3 h incubation time.
NOTE: Plates can be used immediately or stored at 4 °C for up to two weeks.
2. Transfer of hiPSCs Grown on iMEF Feeder Cells onto Extracellular Matrix for Propagation
- On the day of passaging, remove the matrix coated plate from 4 °C and allow the plate to acclimate to room temperature for 1 h in the tissue culture hood.
- Aspirate the medium from 1 well of a 6-well plate of hiPSC culture growing on iMEFs and replace with 500 µL collagenase dissociation reagent (1 mg/mL in basal culture medium).
- Incubate at 37 °C for ~ 10 – 30 min until the edges of the iPSC colonies appear to lift slightly.
NOTE: Incubation time is line dependent and will vary. Monitor dissociation under a microscope. - Carefully aspirate the dissociation reagent and wash gently 2 times with phosphate buffered saline (PBS, pH 7.4).
- Add 1 mL of room temperature mTeSR1 complete medium with 10 ng/mL Rho-associated protein kinase inhibitor (Y-27632 ROCK inhibitor) to the well.
NOTE: Using a 50:50 mixture of iMEF-conditioned medium and mTeSR1 during this initial passage is suggested and can be beneficial for the survival of the hiPSCs during this media transition. - Using a small phase contrast microscope placed partially inside the hood, manually score and pick 1 – 2 hiPSC colonies (~ 700 – 1,000 µm in diameter) using a syringe and needle (25 G, 1½ in). Push off the scored pieces of the colony into the medium with a P200 pipet tip.
- Aspirate and dispose of the matrix from the new plate, being careful not to disturb the coating.
- Transfer 1 mL of the cell suspension from the old well containing the scored iPSCs into the new matrix coated well.
- Place the plate in the incubator at 37 °C, 5% CO2. Rock the plate in several quick, back-and-forth, and side-to-side movements to evenly spread out the cells. Do not disturb the plate for 24 h.
- Perform daily full media changes with mTeSR1 complete medium (addition of Y-27632 ROCK Inhibitor is not needed after the initial plating in step 2.5).
3. Small-scale Passaging of hiPSCs on Matrix-coated Plates
NOTE: Generally, after the initial transfer of the hiPSCs to matrix coated plates, the cells should be passaged once more in a similar manner to ensure all iMEFs have been eliminated and cells have adapted to feeder-free conditions.
- One day prior to iPSC confluency prepare freshly coated matrix plates as discussed in section 1.
- On the day of passage, remove the matrix coated plate from 4 °C incubation and allow the plate to acclimate to room temperature for 1 h in the tissue culture hood.
- Aspirate the medium from 1 well of a 6-well plate of hiPSCs (originally transitioned from feeder to matrix) and replace with 500 µL cell dissociation buffer.
NOTE: The commercial cell dissociation buffer used here (see Materials Table) is more suitable for use with mTeSR1 medium compared to collagenase. - Incubate at room temperature for 3 – 7 min until the edges of the iPSC colonies appear to lift slightly. As mentioned before, the incubation time is line dependent and variable. Therefore, monitor the cellular dissociation under a microscope to determine the optimal time.
- Carefully aspirate the dissociation buffer and wash gently 2 times with PBS.
- Add 500 µL of room temperature mTeSR1 complete medium with 10 ng/mL Y-27632 ROCK inhibitor to the well.
- Instead of scoring colonies as in Step 2.6, push the desired number of colonies into the media using a P200 tip and gently triturate the cell suspension 2 times against one corner of the well to break the hiPSC colonies into clumps of ~ 50 – 200 µm in size. For example, for a 12-well plate, pick 1 – 2 colonies to transfer into each 1 new well. Transfer the cell suspension from the old well into a single new well of a 12-well plate.
- Rock the plate in several quick, back-and-forth, and side-to-side movements to evenly spread out the cells, and let the plate rest for 24 h in a 37 °C, 5% CO2 incubator.
- Perform daily full media changes with mTeSR1 complete medium. As mentioned before, it is not necessary to add Y-27632 ROCK Inhibitor after the initial plating.
NOTE: Unused iPSC colonies from Step 3.7 may be easily cryopreserved at this point.
4. Preparation of Chamber Slides and Coverslips for Seeding of hiPSCs for Immunocytochemistry
NOTE: The following protocol utilizes 4-well plastic chamber slides and 12 mm glass coverslips with appropriate volumes of media in the multi-wells to support cost-effective immunocytochemistry. However, media volumes can be increased if the use of larger well and coverslip sizes is desired.
- One day prior to hiPSC confluence, prepare chamber slides freshly coated with extracellular matrix as discussed in section 1. Add enough matrix solution (~ 300 – 500 µL per well of a 4-well chamber slide) to fully coat each well without any concern of evaporation. Alternatively, after placing 1 cleaned and sterilized coverslip into each well of a 24-well plate, coat with 500 µL extracellular matrix and make sure the coverslip is not floating on top of the solution.
- On the day of passage, remove the coated chamber slides/coverslips from 4 °C and allow acclimation to room temperature for 1 h in the tissue culture hood.
- Aspirate the medium from 1 well of a 12-well plate of hiPSCs and replace with 500 µL dissociation buffer.
- Incubate at room temperature for ~ 3 – 7 min until the edges of the iPSC colonies appear to lift slightly.
- Carefully aspirate the dissociation buffer and add 1 mL of room temperature mTeSR1 complete medium with 10 ng/mL Y-27632 ROCK Inhibitor to the well.
- Using a small phase contrast microscope placed inside the tissue culture hood, manually pick 1 colony/new well of a 4-well chamber slide or 1 new coverslip to be plated (~ 1,000 – 1,500 µm in diameter) using a P200 pipet tip by pushing it off the plate surface into the medium.
- Aspirate the matrix from the new chamber slide/coverslip, being careful not to disturb the coating.
- Gently triturate the cell suspension 2 times against one corner of the well to break the hiPSC colonies into clumps of ~ 50 – 200 µm in size. Transfer 250 µL of the cell suspension from the old well into each new well of a 4-well chamber slide/coverslip.
- Bring the volume of each new well up to 500 µL with mTeSR1 containing 10 ng/mL Y-27632 ROCK Inhibitor.
- Place the chamber slide/coverslips in the incubator at 37 °C, 5% CO2.
- After 24 h, perform daily full media changes with mTeSR1 complete medium. Again as mentioned previously, the addition of Y-27632 ROCK Inhibitor is not necessary after the initial plating.
5. Preparation of iPSCs for Immunocytochemistry
- Once confluent, preserve the hiPSC colonies via fixation by removing the media from the wells and adding pre-warmed CAUTION 4% paraformaldehyde (~ 300 µL/well of a 4-well chamber slide/coverslip). Incubate at room temperature for 20 min.
- Discard the paraformaldehyde appropriately according to institutional biohazard and chemical safety guidelines.
- Wash the cells 3 times for 5 min each on a bench-top shaker with PBS.
NOTE: Cells on chamber slides/coverslips can be stored with ample PBS at 4 °C indefinitely at this stage. Alternatively, they can be immediately used for ICC.
6. Immunostaining of hiPSCs with Pluripotency Markers
NOTE: Immunostain hiPSCs with standard pluripotency antibodies. As an example, here cells are double-stained with antibodies targeting one 1 surface and 1 intracellular antigen in a sequential manner as follows: Anti-Stage-Specific Embryonic Antigen-4 (SSEA4) with anti-Octamer-binding protein 4 (OCT-4) and anti-Tra-1-60 with anti-SRY related HMG BOX gene 2 (SOX2) (Please see the Materials Table for antibody details).
- Aspirate the PBS from the wells and add in 500 µL/well Blocking Solution without Triton-X-100. Incubate for 1 h at room temperature.
- Prepare a pre-determined concentration (here, use 1:200) of the surface antibodies, either SSEA4 or Tra-1-60, by diluting in Blocking Solution without Triton X-100.
- For chamber slides, prepare 300 µL/well of a 4-well chamber slide by mixing 298.5 µL of Blocking Solution above and 1.5 µL of anti-SSEA4 or Tra-1-60 primary antibody.
- For coverslips, prepare 40 µL/coverslip by mixing 199 µL of Blocking Solution and 1 µL of anti-SSEA4 or Tra-1-60 primary antibody.
- Aspirate the Blocking Solution from the chamber slide and dispense the primary antibody into appropriate wells. For coverslips, place a piece of parafilm firmly inside a Petri dish and dispense 40 µL of the primary antibody on top as a droplet. Using a bent syringe needle and forceps, carefully lift the coverslip out of the culture well and place it cell side down onto the drop of antibody on the parafilm. Incubate overnight at 4 °C.
- The next morning, wash the cells 3 times, 5 – 10 min each, by removing the antibody from the chamber slide and adding in PBS. For coverslips, lift each one off the parafilm and place it back in a well of a 24-well plate, cell side up. Carefully add PBS.
- Prepare appropriate secondary antibodies at 1:500 in the Blocking Solution.
- For chamber slides, prepare 300 µL/well of a 4-well chamber slide by mixing 499 µL of Blocking Solution and 1 µL of goat anti-mouse IgG3 Alexa Fluor 488 (for SSEA4) or 1 µL of goat anti-mouse IgM Alexa Fluor 488 (for Tra-1-60).
- For coverslips, prepare 40 µL/coverslip by mixing 499 µL of Blocking Solution and 1 µL of goat anti-mouse IgG3 Alexa Fluor 488 (for SSEA4) or 1 µL of goat anti-mouse IgM Alexa Fluor 488 (for Tra-1-60).
- Aspirate the PBS from the chamber slide and dispense the secondary antibody into appropriate wells. For coverslips, following Step 6.2, invert the coverslips onto the antibody solution placed on parafilm at room temperature for 2 h.
NOTE: If using fluorescent tagged secondary antibodies, the cells must be protected from light. Place the chamber slides and the coverslips in a dark environment during incubations and washes. - Wash the cells 3 times for 5 – 10 min each by removing the antibody from the chamber slide and adding in PBS. For coverslips, lift each one carefully from the parafilm and place back in a well of a 24-well plate, cell side up. Gently add PBS during washes.
- Prior to probing for the second antigen, intracellular OCT-4 or SOX2 in this case, incubate again in Blocking Solution made with permeabilization reagent (Triton-X-100) for 1 h at room temperature.
- To immunostain for OCT-4 or SOX2, simply follow the methodology described in Steps 6.1-6.6 for applying the primary and secondary antibodies. For the purposes of this protocol, use OCT-4 and SOX2 at a concentration of 1:200 and the secondary antibody goat anti-rabbit IgG Alexa Fluor 594 at 1:500.
- After the abovementioned primary and secondary antibody treatments, wash the cells 3 times for 5 – 10 min each with PBS and counterstain with 4',6'-diamidino-2-phenylindole, dihydrochloride (DAPI) following standard protocols if nuclear visualization is desired.
7. Preparation for Viewing
- For chamber slides, carefully remove the upper chamber from the slide, following the manufacturer's instructions. Then, rinse in distilled water, and add mounting medium and a coverglass to enable microscopy.
- For coverslips, invert the coverslips on to a drop of mounting medium placed on a glass microscope slide.
- Image under a standard or confocal fluorescence microscope.
Representative Results
This protocol provides a step-wise description of how human iPSCs can be transferred from feeder layer to feeder-free conditions, and subsequently propagated in a limited manner to specifically enable cost-effective immunocytochemistry for confirming pluripotency maintenance. Figure 1 shows a schematic representation of this protocol. Figure 2A shows hiPSC colonies growing on iMEFs in 6-well plates. These colonies exhibit typical morphology with defined borders and dense phase bright centers. As shown in Figure 2B, after the transfer of iPSCs to feeder-free conditions in 12-well plates, colony morphology appears somewhat chaotic with less distinct edges. Also, some iMEFs may remain in the culture at this stage. Figure 2C shows that after an additional passage in the feeder-free system, iPSC colonies display a classic monolayer morphology with a high nucleus to cytoplasm ratio. At this stage, it is seen that iMEFs have been virtually eliminated from the culture.
Colonies growing under feeder-free conditions are mechanically picked and plated onto small multi-well chamber slides or glass coverslips, and probed for specific pluripotency antigens via immunocytochemistry. Figure 3 shows representative colonies that are immunopositive for SSEA4 or Tra-1-60 (3B, 3F, surface pluripotency antigens) and Oct-4 or Sox2 (3 A, 3E, intracellular pluripotency antigens).
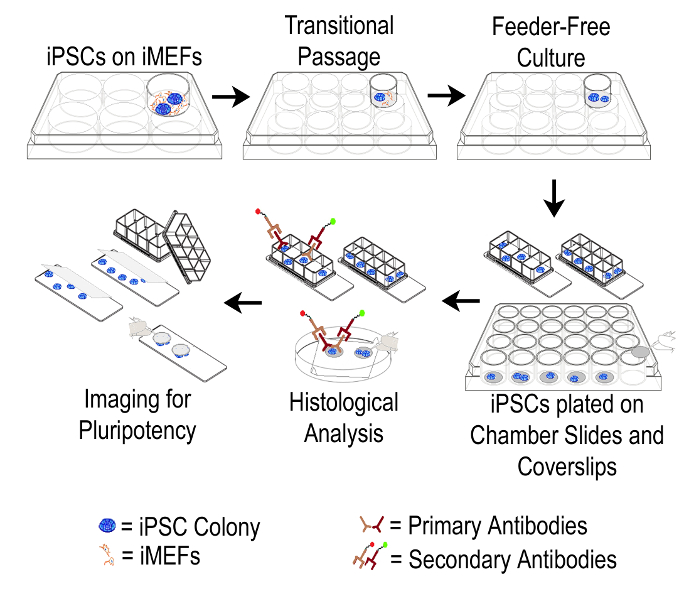
Figure 1: Schematic Representation of the Protocol. Overall schema of the described method used to transition human iPSCs from iMEF feeders to feeder-free culture and subsequent probing of cells with pluripotency markers. iMEF: irradiated Mouse Embryonic Fibroblast; iPSC: Induced Pluripotent Stem Cell. Please click here to view a larger version of this figure.
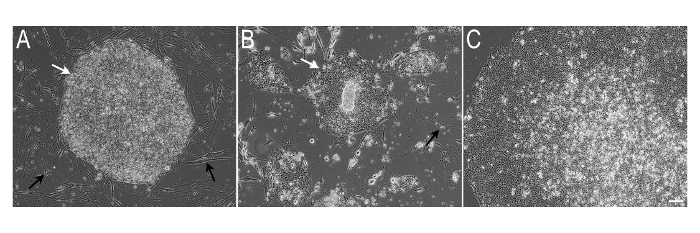
Figure 2. Representative Images of iPSC Colonies Transitioning from iMEF Feeders to Feeder-free Culture Conditions. A) Dense iPSC colony (white arrow) grown on iMEF feeder cells (black arrow). B) After the initial passage on to a matrix-coated 12-well plate in serum-free medium, a few iMEFs may still be observed in culture (black arrow). C) Typical morphology of a monolayer iPSC colony grown feeder-free. No iMEFs remain in culture at this stage. Scale Bar (A-C) = 100 µm. Please click here to view a larger version of this figure.
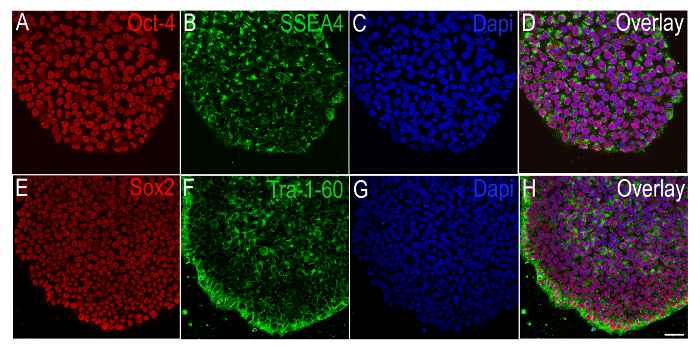
Figure 3. Immunocytochemical Characterization of Human iPSCs Plated in Small Well Plates. Representative immunofluorescence images of iPSCs, obtained via a confocal microscope, showing the positive expression of pluripotency markers A) Oct-4 (red) and B) SSEA4 (green) with C) the nuclear stain DAPI (blue), and E) Sox2 (red) and F) Tra-1-60 (green) with G) DAPI (blue). D and H show the overlay images relating to A-C and E-G. Scale bar (A-H) = 100 µm. Please click here to view a larger version of this figure.
| hiPSC Media for maintenance on Feeders | ||
| Component | Stock Concentration | Final Concentration |
| DMEM-F12/HEPES | 100% | 80% |
| Knockout Serum Replacement | 100% | 20% |
| L-Glutamine | 200 mM | 1 mM |
| MEM-NEAA | 10 mM | 0.1 mM |
| 2-mercaptoethanol | 55 mM | 0.1 mM |
| Recombinant Human FGF-Basic | 10 µg/mL | 10 ng/mL |
| Y-27632 ROCK Inhibitor | 10 µM | |
| Matrigel coating for Feeder-Free cultures | ||
| Component | Amount | |
| Matrigel hESC-qualified Matrix | Dilution Factor 269 µL | |
| DMEM-F12/HEPES | 25 mL | |
| Note: Dilution Factor is lot dependent and must be ascertained from certificate of analysis | ||
| mTeSR1 complete media for Feeder-Free cultures | ||
| Component | Amount | |
| mTeSR1 Basal Medium | 400 mL | |
| mTeSR1 5x Supplement | 100 mL | |
| Note: Once mixed, mTeSR1 complete media may be frozen in aliquots and used until component expiration date | ||
| Note: mTeSR1 complete media must be warmed at room temperature only. Do not place in water bath | ||
Table 1: iPSC Culture Media Recipes and Plate Coatings.
| 4% Paraformaldehyde fixative | |
| Component | Amount |
| 0.1 M PO4 Buffer | 2 L |
| Paraformaldehyde, prill | 80 g |
| ICC Blocking Solution without Triton-X-100 | |
| Component | Amount |
| 1x Phosphate Buffered Saline | 49 mL |
| Normal Goat Serum | 1 mL |
| Bovine Serum Albumin | 0.5 g |
| ICC Blocking Solution with Triton-X-100 | |
| Component | Amount |
| 1x Phosphate Buffered Saline | 49 mL |
| Normal Goat Serum | 1 mL |
| Bovine Serum Albumin | 0.5 g |
| Triton-X-100 | 200 µL |
Table 2: Fixative and Immunocytochemistry Recipes.
Discussion
The systematic protocol presented here offers a timesaving and cost-effective method, in the form of a scaled-down culture technique, specifically designed to support effective pluripotency analysis via immunocytochemistry.
The main advantages of the described methodology are as follows. Traditionally more than 3 to 4 passages are needed to transition iPSCs from feeder layers to feeder-free culture conditions in order to eliminate residual iMEFs remaining after the use of bulk dissociation techniques and for typical monolayer morphology to appear12,13. In contrast, the method presented here involves a shortened timeline, which allows for the relatively quick transfer of iPSCs to serum-free conditions via the manual picking of iPSC colonies. The iPSCs are grown in limited amounts in small chamber slides or coverslips conducive for immunocytochemistry. In fact, the volume of culture medium needed per well is just 0.5 mL and the volume of diluted antibody solution required for a single coverslip is as nominal as 40 µL or 300 µL per chamber well, when using this protocol.
We find that the addition of equal parts iMEF conditioned media to the mTeSR1 during the initial passage (Step 2.5) is an important step that aids in the survival and maintenance of the iPSC's pluripotent morphology. In addition, if cell adherence is not optimal after passaging (Steps 2.2, 3.3 and 4.3), troubleshooting can be performed by further optimizing the dissociation times used.
Finally, a limitation of the presented small-scale method, when compared to other techniques such as flow cytometry also used to verify pluripotency, is that it does not allow for the continued propagation of analyzed cells for downstream applications. The usefulness of our method arises from its specific design which supports the cost-effective and efficient culture of the hiPSCs for confirming pluripotency via routine immunocytochemistry.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Funding Sources: The University of Arizona, The Jim Himelic Foundation, and the Arizona Center for the Biology of Complex Diseases.
Materials
| DMEM-F12/HEPES | Life Technologies | 11330032 | |
| Knockout Serum Replacement | Life Technologies | 10828028 | |
| L-Glutamine | Life Technologies | 25030081 | |
| MEM-NEAA | Life Technologies | 11140050 | |
| 2-mercaptoethanol | Life Technologies | 21985023 | |
| Recombinant Human FGF-Basic | Cell Sciences | CRF001B | |
| Y-27632 ROCK Inhibitor | R&D | 1254 | |
| Collagenase Type IV | Life Technologies | 17104019 | |
| Matrigel hESC-qualified Matrix | Corning | 354277 | |
| mTeSR1 Basal Medium | StemCell Technologies | 05850 | |
| mTeSR1 5X Supplement | StemCell Technologies | 05850 | |
| Gentle Cell Dissociation Buffer | StemCell Technologies | 07174 | |
| 0.1M PO4 Buffer | In-House | n/a | |
| Paraformaldeyde, prill | Electron Microscopy Sciences | 19202 | |
| 1X Phoshate Buffered Saline | n/a | n/a | |
| Normal Goat Serum | Life Technologies | 16210072 | |
| Bovine Serum Albumin | Sigma-Aldrich | A2153 | |
| Triton-X-100 | Sigma-Aldrich | X100 | |
| Oct-4A (C30A3) Rabbit mAb, Sox2 (D6D9) Rabbit mAb, SSEA4 (MC813) Mouse mAb, TRA-1-60(S) (TRA-1-60(S)) Mouse mAb |
Cell Signaling Cell Signaling Cell Signaling Cell Signaling |
2840 3579 4755 4746 |
Alternatively, a combination of 6 pluripotency primary antibodies can be purchased together as a kit in Catalog #9656 |
| Goat anti-Ms IgM Alexa Fluor 488 | Life Technologies | A21042 | |
| Goat anti-Ms IgG3 Alexa Fluor 488 | Life Technologies | A21151 | |
| Goat anti-Rb IgG Alexa Fluor 594 | Life Technologies | A11037 | |
| Multiwell Cell Culture Plates | Fisher Scientific | 0720080/0720081 | Available in 6, 12, 24, 48, 96 well sizes |
| Chamber Slides | Fisher Scientific | 12 565 21 | Available in Glass or Permanox Plastic in 1, 2, 4, 8, 16 well sizes |
| Coverglass for growth | Fisher Scientific | 12 545 82 | Available in 12, 15, 18, 22 and 25mm sizes |
References
- Takahashi, K., Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126, 663-676 (2006).
- Park, I. H., et al. Disease-specific induced pluripotent stem cells. Cell. 134, 877-886 (2008).
- Hallett, P. J., et al. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease. Cell stem cell. 16, 269-274 (2015).
- Haston, K. M., Finkbeiner, S. Clinical Trials in a Dish: The Potential of Pluripotent Stem Cells to Develop Therapies for Neurodegenerative Diseases. Annu rev pharm toxicol. 56, 489-510 (2016).
- Zhang, L., et al. Derivation and high engraftment of patient-specific cardiomyocyte sheet using induced pluripotent stem cells generated from adult cardiac fibroblast. Circ heart fail. 8, 156-166 (2015).
- Byrne, J. A., Nguyen, H. N., Reijo Pera, R. A. Enhanced generation of induced pluripotent stem cells from a subpopulation of human fibroblasts. PloS one. 4, 7118 (2009).
- Sivapatham, R., Zeng, X. Generation and Characterization of Patient-Specific Induced Pluripotent Stem Cell for Disease Modeling. Methods mol bio. 1353, 25-44 (2016).
- Park, I. H., et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 451, 141-146 (2008).
- Ruff, D., Lieu, P. T. Profiling stem cells using quantitative PCR protein assays. Methods mol bio. 997, 225-236 (2013).
- Pripuzova, N. S., et al. Development of a protein marker panel for characterization of human induced pluripotent stem cells (hiPSCs) using global quantitative proteome analysis. Stem cell res. 14, 323-338 (2015).
- Fusaki, N., Ban, H., Nishiyama, A., Saeki, K., Hasegawa, M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc jpn acad ser B phys biol sci. 85, 348-362 (2009).
- Bigdeli, N., et al. Adaptation of human embryonic stem cells to feeder-free and matrix-free culture conditions directly on plastic surfaces. J Biotechnol. 133, 146-153 (2008).
- Stover, A. E., Schwartz, P. H. Adaptation of human pluripotent stem cells to feeder-free conditions in chemically defined medium with enzymatic single-cell passaging. Methods mol bio. 767, 137-146 (2011).

