Using Chicken Embryo as a Powerful Tool in Assessment of Developmental Cardiotoxicities
Summary
Chicken embryos, as a classical developmental model, are used in our lab to assess developmental cardiotoxicities following exposure to various environmental contaminants. Exposure methods and morphological/functional assessment methods established are described in this manuscript.
Abstract
Chicken embryos are a classical model in developmental studies. During the development of chicken embryos, the time window of heart development is well-defined, and it is relatively easy to achieve precise and timely exposure via multiple methods. Moreover, the process of heart development in chicken embryos is similar to mammals, also resulting in a four-chambered heart, making it a valuable alternative model in the assessment of developmental cardiotoxicities. In our lab, the chicken embryo model is routinely used in the assessment of developmental cardiotoxicities following exposure to various environmental pollutants, including per- and polyfluoroalkyl substances (PFAS), particulate matter (PMs), diesel exhaust (DE) and nano materials. The exposure time can be freely selected based on the need, from the beginning of development (embryonic day 0, ED0) all the way to the day prior to hatch. The major exposure methods include air-cell injection, direct microinjection, and air-cell inhalation (originally developed in our lab), and the currently available endpoints include cardiac function (electrocardiography), morphology (histological assessments) and molecular biological assessments (immunohistochemistry, qRT-PCR, western blotting, etc.). Of course, the chicken embryo model has its own limitations, such as limited availability of antibodies. Nevertheless, with more laboratories starting to utilize this model, it can be used to make significant contributions to the study of developmental cardiotoxicities.
Introduction
The chicken embryo is a classic developmental model, which has been used for over two hundred years1. The chicken embryo model has various advantages compared to traditional models. First of all, as early as over 70 years ago, the normal development of the chicken embryo had been illustrated very clearly in the Hamburger-Hamilton staging guide2, in which a total of 46 stages during chicken embryo development were defined with precise time and morphological characteristics, facilitating detections of abnormal development. Additionally, the chicken embryo model has other features such as being relatively low-cost and redundant in quantity, relatively accurate exposure-dose controls, an independent, closed system within the shell and easy manipulation of the developing embryo, all of which guarantees its potential to be used as a powerful toxicological assessment model.
In cardiotoxicity, the chicken embryo features a four chambered heart, similar to mammalian hearts but with thicker walls, allowing easier morphological assessments. Additionally, the chicken embryo allows for developmental inhalation exposure, which is not possible in mammalian models: during the later stage of development, the chicken embryo will transition from internal respiration to external respiration (getting oxygen via the lung); the latter requires that the embryo penetrates the air cell membrane with the beak, and starts to breathe air3, making the air cell a mini-inhalation chamber. Utilizing this phenomenon, the toxicological effects of gas contaminants on the heart (and other organs) may be assessed without the need of dedicated inhalation chamber instruments.
In this manuscript, several exposure/endpoint assessment methods are described, all of which serve to make the chicken embryo a powerful tool in the assessment of development cardiotoxicity following exposure to environmental contaminants.
Protocol
All procedures described were approved by the Institutional Animal Care and Use Committee (IACUC) of Qingdao University. In our lab, the eggs were incubated in two incubators. Eggs were held upright in the incubator and randomly placed on the shelves. The incubation conditions for the eggs were as follows: incubation temperature started at 37.9 °C, and gradually decreased to 37.1 °C as incubation proceeded; the humidity started at 50% and gradually increased to 70%.
1. Exposure methods
NOTE: Exposure of environmental contaminants to chicken embryos may be achieved in several ways. In this section, three routinely used methods are described in detail.
- Air cell injection (Figure 1)
NOTE: This is the classical exposure method to chicken embryos4,5,6, suitable for a wide range of materials, and may be performed at a very wide time window, from the beginning of development (embryonic day zero, ED0) all the way to the day prior to hatch (ED20). Sunflower oil is used as the vehicle. Previous studies have shown that no significant changes in mortality, hatchability, or body weight have been observed between untreated embryos and embryos injected with sunflower oil7.- Prepare the following necessary reagents/tools: 75% ethanol, tissue paper, metal probe (can substitute with any sharp metal needle/stick/awl), melted paraffin, brush, povidone iodide solution, pipette, pipette tips, candling lamp, dosing mixture. Prepare the dosing mixture with sunflower oil (recommended)4. To use other diluents, perform vehicle control (versus untreated embryos).
- Clean the surface of the eggs with povidone iodide solution (commercially available povidone iodide solution 1:5 diluted with deionized water), and dip-dry the egg shell with tissue paper without scrubbing. Scrubbing will break the protective layer coating the outside of the shell.
- Candle the eggs in a dark room and mark the air cell with pencil. Exclude eggs with cracks on the shell. Exclude eggs with air cells on the side instead of blunt tip, as those are highly unlikely to hatch normally.
- Sanitize the air cell area with 75% ethanol, and then drill a small hole at the center of air cell area with the metal probe. Do not stick the probe deep into the air cell or the inner membrane may be damaged, instead, only break the shell with the tip of the probe. If the hole is not large enough to fit in a fine pipette tip, break the shell again at the vicinity of existing hole, until the hole is large enough to allow insertion of 10 µL pipette tip.
- Vortex the dosing mixture vigorously, and immediately draw solution to pipette tip. The recommended injection volume is 1 µL per 10 gram of egg (e.g., 5 µL injection volume for a 50-gram egg) as larger injection volumes may create hypoxic or anoxic conditions for the developing embryo. Calculate the concentration of the dosing solution for desired mg/egg kg dose.
- Insert the pipette tip into the hole, with the tip touching the inner membrane. Slowly eject the dosing mixture, hold for at least ten seconds (allow the viscous oil to be fully dispensed), and then remove the tip.
- Seal the hole with a brush and a drop of melted paraffin. Be careful not to drip melted paraffin onto the inner membrane.
- Once sealed, place the eggs in the incubator until they reach the desired embryonic stage. On already developing embryos, perform the whole process as soon as possible to prevent potential embryo loss due to low environmental temperatures.
- Microinjection (Figure 2)
NOTE: This is a more direct exposure method, resulting in definitive exposure to the substance-of-interest, and especially suitable for compounds with a short duration of action (e.g., lentivirus), since the classical air cell injection requires time for the compounds to penetrate the inner membrane. This method may also be tried if satisfying results could not be achieved by air cell injection. This method is best suitable for early embryos (up to ED2), but also can be performed on older embryos (with higher risk of embryo loss).- Prepare the following necessary reagents/tools: 75% ethanol, povidone iodide solution, micro-injector (5 µL), metal probe (can substitute with any sharp metal needle/stick/awl should work), fine forceps, tape. Prepare the dosing mixture with sterile saline, which also serves as an injection control without affecting hatchability significantly. Ensure the sterility of the saline, as a contaminated injection will dramatically increase mortality.
- Clean the eggs as described in 1.1.2.
- Candle the eggs as described in 1.1.3.
- Sanitize the air cell area with 75% ethanol, and then drill a small hole at the center of air cell area with the metal probe. Do not stick the probe deep into the air cell or the inner membrane may be damaged, instead, only break the shell with the tip of the probe. Then use the fine forceps to carefully enlarge the hole until the diameter is approximately 2 mm, allowing visual confirmation of the inner membrane.
- Load the solution into microinjector (maximum injection volume: 0.5 µL/10 g egg. (e.g., 2.5 µL for a 50 g egg) and carefully insert the needle through the hole into the inner membrane for approximately 2-3 mm. Gently dispense the solution and remove the needle. Keep the needle as perpendicular to the membrane as possible.
- Seal the hole with a small piece of tape. Completely cover the hole to prevent embryo dehydration and death during subsequent incubation. Nevertheless, avoid pieces of tape that are too large to prevent hypoxia.
- Once sealed, place the eggs in the incubator until they reach the desired embryonic stage. On already developing embryos, perform the whole process as soon as possible to prevent potential embryo loss due to low environmental temperature.
- Air cell inhalation (Figure 3)
NOTE: This is a novel inhalation method taking advantage of the air cell, from which the late-stage chicken embryo will start to breathe air. It is suitable for gas or aerosol exposures and may achieve very early-in-life inhalation exposures, and fill the lungs with the target gas/aerosol when they open for the first time in life.- Prepare the following necessary reagents/tools: Sampling bag (PVF bag, for storage of gas/aerosol sample before exposure), catheter needle, syringe, metal probe (can substitute with any sharp metal needle/stick/awl should work), tape, fume hood.
- Clean the eggs as described in 1.1.2 and candle them as described in 1.1.3 (it is not necessary to mark air cell prior to incubation), and then incubate eggs without treatment until ED17.
- Candle the eggs at ED17 to mark the air cell area.
- At ED18, take an egg out from the incubator, sanitize the air cell area with 75% ethanol, and then carefully drill two small holes on two sides of the air cell. One is for injection of gas/aerosol, the other is for expelling of air. Carefully control the size of the holes so that the size of the injection hole is just enough for the catheter needle to be inserted, while the diameter of the expelling hole is a bit larger (approximately 1 mm).
- Gently inject 10 mL of target gas/aerosol from the injection hole with a syringe attached to the catheter needle. Inject air for an inhalation control group, which should have no significant differences to a negative control group8. Apply pressure against the catheter needle (with appropriate amount of pressure the elastic needle can be pushed against the shell) to minimize the leakage from injection hole. Seal both holes immediately with tape afterwards, and return the egg to incubator.
NOTE: This procedure should be performed in a fume hood to prevent inhalation of gas/aerosol by the operator. - Repeat the described procedure after one hour to further ensure the whole air cell is filled up with target gas/aerosol (optional).
- Repeat the described procedure at ED19 again (optional). Repeating the exposure helps to ensure consistent exposure until hatch. Record the hatch time for an approximate estimation of exposure duration.
- Once desired exposures have been performed and sealed, place the eggs in the incubator for hatching. Minimize the time the egg spends outside of the incubator to prevent death from low environmental temperature.
2. Endpoint assessment methods
NOTE: Following exposure of contaminant-of-interest to the developing embryo, several toxicity parameters can be evaluated, including cardiotoxicity. In this section, two frequently used specific methods are described in detail.
- Electrocardiography (Figure 4)
NOTE: It is impossible to do non-invasive electrocardiography in hatchling chickens due to the presence of feathers. Thus, subcutaneous implantation of electrodes is essential, requiring anesthesia. The dose used in the lab is 33 mg/kg pentobarbital via intraperitoneal injection (some chickens may require up to 50% dose increase). This method will result in stable electrocardiography in over 90% of animals, allowing for analyzation of heart rate.- Prepare the following necessary reagents/tools: 1% (10 mg/mL) pentobarbital solution in saline, syringe, electrical balance, heater (if necessary), an electrocardiography instrument attached (e.g., BL-420E+) with two-channel metal needle electrodes.
- Weigh the chickens with a balance and calculate the necessary volume of pentobarbital solution and inject the chickens. For a chicken that weighs 30 g, 0.1 mL of pentobarbital solution is needed. Make sure that the injection is done on the lateral side of the abdomen, as the yolk is located at the middle and injection there may not be effective.
- Wait until the injected chickens are anesthetized (hold the chicken in hand, if the neck has no tension and the head can be swung freely, the anesthetization is sufficient). Place chickens on the operation table (heaters are necessary if room temperature is below 20 °C).
- Insert two needle electrodes from two sides of the abdomen, subcutaneously. Make sure that the needles do not enter the abdominal cavity by lifting the skin a little bit and inserting needle from there. Once inserted, carefully push the needle forward until it reaches the side of chest cavity. Make sure that the needle does not go deep into the body or stick out from the skin.
- Make the measurements with the electrocardiography instrument. Other similar instruments capable of electrocardiography can be used.
- If the chickens are to be sacrificed, perform euthanasia after the electrocardiography since they are already under anesthesia. If chickens are to survive, place them back into their cages and warm until waking up. Returning them to the incubator is another option.
- Histomorphometry (Figure 5)
NOTE: A specific method is developed to assess the right ventricular wall thickness in transverse sections of the heart. Morphological assessment of right ventricle dimension via echocardiography is not 100% accurate due to the irregular shape of right ventricle, and this method may serve as a good supplement in the morphological assessments for the right ventricle.- Prepare the following necessary reagents/tools: 4% phosphate buffered formaldehyde, sharp blade, phosphate buffered saline, paper towel, electrical balance, small scissors, general histological processing agents (graded ethanol, xylene, paraffin).
- Once animals are sacrificed, use water to wet the feathers. This is to minimize the potential contamination due to flying feathers while opening up the chest.
- Open the chest cavity carefully without damaging the heart. Use small scissors to cut the vasculature and gently remove the heart from the chest cavity. Leave a small piece (approximately 1-2 mm) of vasculature attached to the heart as this may be convenient for subsequent handling of the heart without damaging the heart.
- Once removed, rinse the heart in cold phosphate buffered saline to remove blood and relax the muscle. Then dip dry the heart on paper towel before weighing for accurate weight reading. Put the heart into 10x volume fixative (4% phosphate buffered formaldehyde) for 24 hours at room temperature. Fixed tissues may be subsequently processed to paraffin blocks, or be stored at 4 °C for years (not recommended if immunohistochemistry is planned).
- Before embedding, cut the tissues at approximately 60% length of the heart counting from the apex (Figure 5A), for easier subsequent processing. A microtome blade is recommended for a quick and vertical clean cut. For chickens older than one day, make another cut at approximately 25-30% length from the apex for easier paraffin penetration and to allow the tissue to fit in tissue cassettes.
- Process the tissues with the following conditions (adjust as needed): 70% ethanol for 1 h, 80% ethanol for 1 h, 95% ethanol for 1 h x2, 100% ethanol for 30 min x2, xylene for 5 min x2, paraffin (melting point 52-54 °C) for 1.5 h, paraffin (melting point 62-64 °C) for 1 h, and then embed the tissues in a 3:1 mixture of paraffin (melting point 62-64 °C) and paraffin (melting point 52-54 °C).
- Section the tissue at 6 µm thickness. Carefully maintain identical relative positions of the cross-sections by confirming the presence and size of an anatomical landmark (septomarginal trabecula) in the right ventricle. Confirm a landmark with moderate length on each section (Figure 5B, arrow).
- Make two electronic rulers with Logo programmer: Ruler 1 is a straight line with 7 radius measure lines attached to the middle point, with 22.5° in between two adjacent measure lines. Ruler 2 is just two perpendicular lines in a T shape (Figure 5B).
- Measure with two software programs: Adobe Photoshop and ImageJ.
- In Photoshop, resize ruler 1 (NO reshaping) to place the two ends of the ruler on the two ends of free right ventricular wall, so that the seven measure lines on ruler 1 will each meet the inner right ventricular wall. Then use ruler 2 to make perpendicular measurements from the inner to external ventricular wall (Figure 5B).
- Use ImageJ to make the seven measurements for each heart.
- Depending on specific needs, analyze the seven measurements or average for one representative right ventricular wall thickness. Normalize to whole heart weight for specific ventricular wall thickness changes.
Representative Results
Exposure results
Air cell injection
Air cell injection can effectively expose developing chicken embryos to various agents, which may be subsequently detected in the collected samples (serum, tissue, etc.) of embryos/hatchling chickens. Here is an example, in which perfluorooctanoic acid (PFOA) was air-cell injected, and serum PFOA concentrations were then determined with Ultra-performance liquid chromatography-mass spectrometry. The serum concentrations corresponded with the injected doses, indicating the effectiveness of this procedure (Figure 6).
Microinjection
Microinjection may expose the developing embryos to agents that may not effectively penetrate the inner membrane, or with a short duration of action, such as lentivirus. Here is an example, in which lentivirus was injected at embryonic day two with this method and then significant green fluorescence was observed in the heart of embryonic day 15 embryos, indicating the effectiveness of lentivirus transfection (Figure 7).
Air cell infusion
Air cell infusion is a novel method, which may work very well for small amount of gas/aerosol inhalation exposure during the initiation stage of external respiration. Here is an example, in which diesel exhaust was infused into air cell at embryonic day 18 and 19, resulting in significant fibrotic changes in the cardiac as well as pulmonary tissues (Figure 8).
Endpoint assessment results
Electrocardiography results
Due to the limitation of two electrodes, only 3 channels of electrocardiography may be shown. But they are sufficient to distinguish r waves, thus they may be used for functional assessments. In a real-life example, electrocardiography of chickens exposed to diesel exhaust indicated significantly shortened R-R interval, indicating functional changes (Figure 9).
Histopathology results
Our method of right ventricular wall thickness assessment was successfully used in several studies5, 7,8,9,10,11,12. In one of our previous studies, diesel exhaust exposure resulted in thickened right ventricular wall (Figure 10).
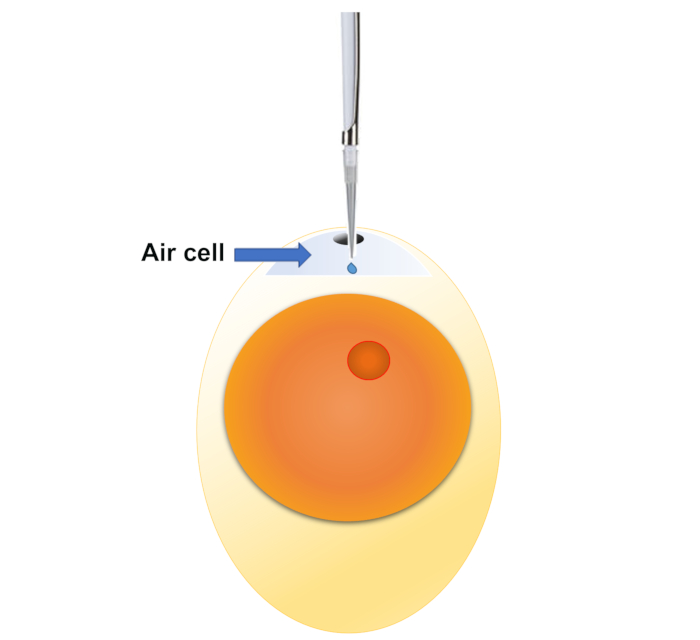
Figure 1: Demonstration of air cell injection. An undeveloped fertile egg is shown in the picture, but embryos at all different stages may be exposed with this method. Please click here to view a larger version of this figure.
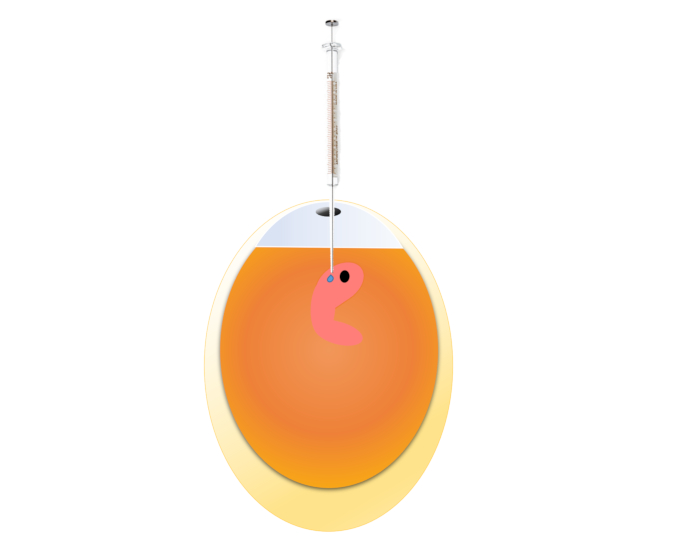
Figure 2: Demonstration of microinjection. An early embryo is shown in the picture, which is the preferred exposure time point for this method, but other time points may also be tried. Please click here to view a larger version of this figure.
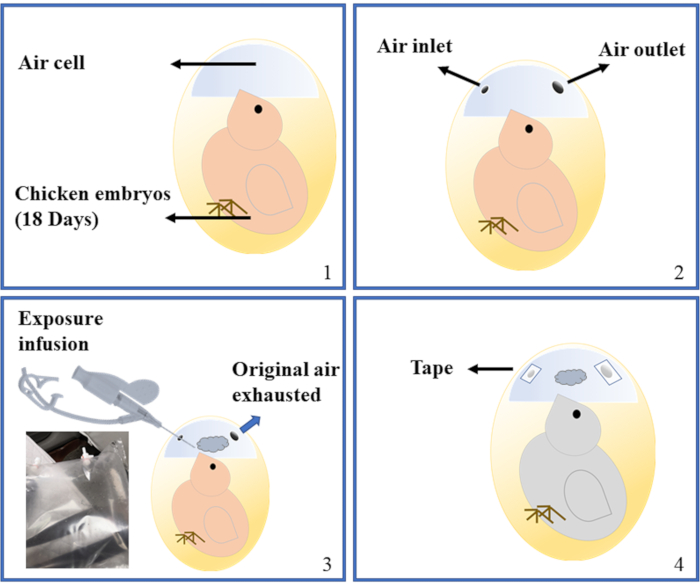
Figure 3: Demonstration of air cell infusion. A late-stage embryo undergoing internal pipping is shown in the picture, which is the preferred exposure time point for this method. Four stages of the operation were shown. 1: Intact embryo. 2: Two holes have been made. 3: Infusion is being performed. The PVF sampling bag is also shown at the bottom left. 4: Infusion is finished, holes sealed with tape. Please click here to view a larger version of this figure.
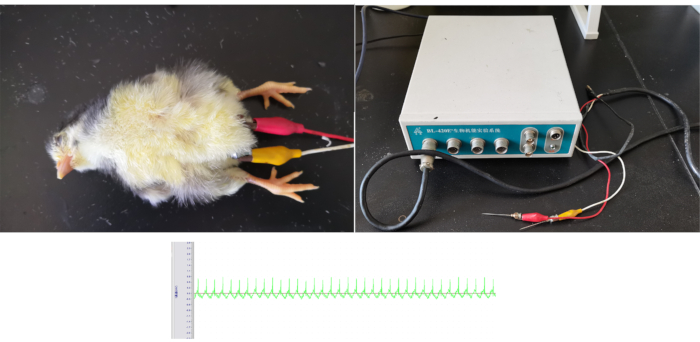
Figure 4: Demonstration of electrocardiography. Top left panel showed how a hatchling chicken was anesthetized and undergoing electrocardiography measurement. Top right panel shows the electrocardiography instrument with the electrodes attached. Bottom panel shows a representative electrocardiography acquired from the chickens. Please click here to view a larger version of this figure.
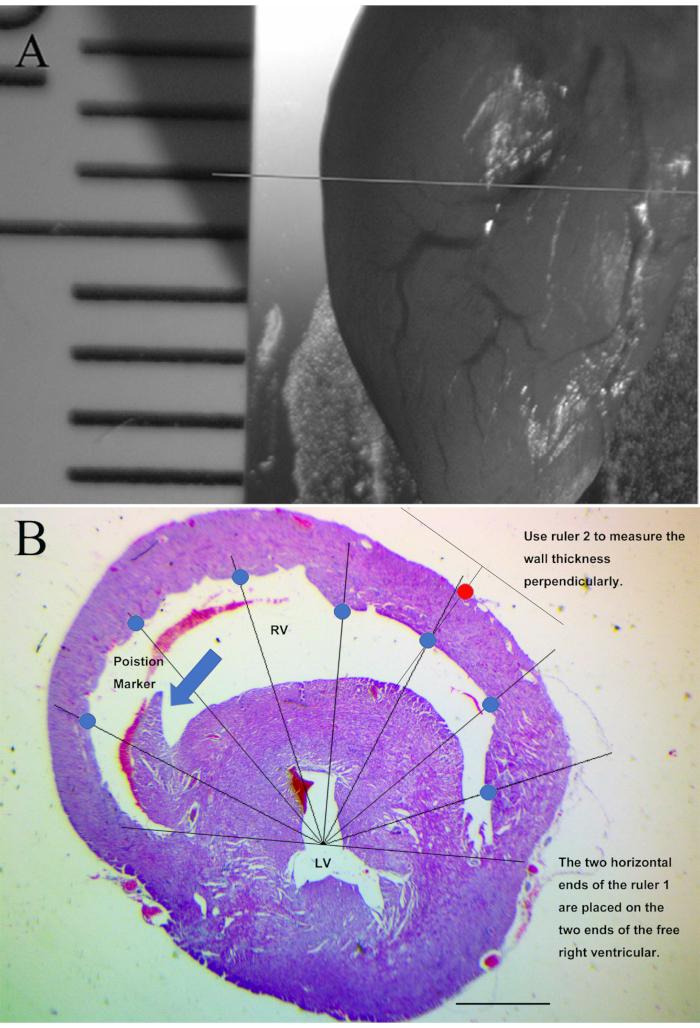
Figure 5: Demonstration of histopathological assessment of right ventricular wall thickness (Hematoxylin and eosin staining). (A) Demonstration of the cutting position of chicken hearts prior to embedding. (B) Demonstration of the right ventricular wall thickness measurement. Scale bars represent 1000 µm. Blue circles demonstrate the seven measurement points on the inner right ventricular wall. Red circle demonstrates a measurement point on the outer right ventricular wall. Arrow demonstrates the anatomic landmark for the appropriate cross-section position. This figure has been modified from Jiang et al. Toxicology. 293 (1-3), 97-106 (2012)7. Please click here to view a larger version of this figure.
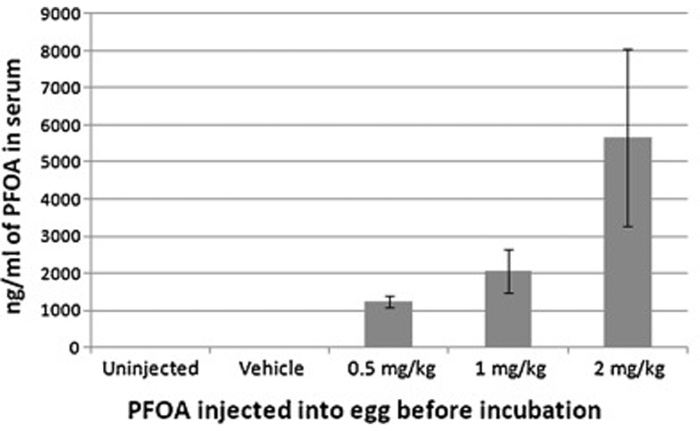
Figure 6: Serum concentration of perfluorooctanoic acid from hatchling chickens following air cell injection with 0, 0.5, 1 or 2 mg/egg kg perfluorooctanoic acid prior to incubation. The resulting serum concentrations corresponded with the injected doses, indicating the effectiveness of air cell injection. This figure has been modified from Jiang et al. Toxicology. 293 (1-3), 97-106 (2012)7. Please click here to view a larger version of this figure.

Figure 7: Demonstration of lentivirus transfection efficacy following microinjection exposure (Direct observation following cryo-sectioning). Left panels showed light field images, while right panels showed green fluorescence for the same tissue sections. Embryonic day two chicken embryos were injected with lentivirus or control, and then incubated until embryonic day 15. The hearts were frozen-sectioned and directly visualized under fluorescent microscope. (A) Control group, little green fluorescence was present. (B) Lentivirus exposed group, significant green fluorescence was observed, indicating the effectiveness of lentivirus transfection following microinjection. Scale bars represent 125 µm. This figure has been modified from Zhao et al. Environmental Toxicology and Pharmacology. 56, 136-144 (2017)11. Please click here to view a larger version of this figure.
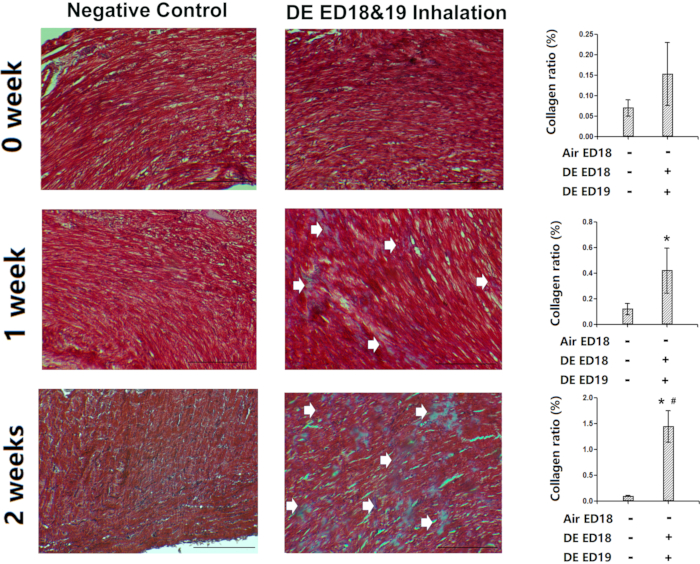
Figure 8: Demonstration of the effectiveness of air cell infusion. Chicken embryos were infused with diesel exhaust at embryonic day 18 and 19, and then the hatched chickens were kept for 0, 1 or 2 weeks and then sacrificed. The heart tissues were assessed with Masson Trichrome staining for fibrotic lesions. Arrows showed the fibrotic lesions (blue staining). *: statistically different from control (P<0.05 from analysis of variance and least significant difference tests). Scale bars represent 150 µm. This figure has been modified from Jiang et al. Environmental Pollution. 264, 114718 (2020)8. Please click here to view a larger version of this figure.
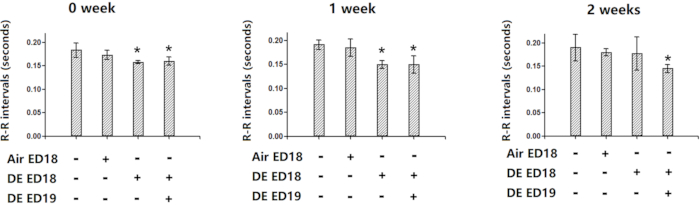
Figure 9: Demonstration of the effectiveness of electrocardiography. Chicken embryos were infused with diesel exhaust at embryonic day 18 and 19, and then the hatched chickens were kept for 0, 1 or 2 weeks and then electrocardiography was performed. Significantly shortened R-R intervals were observed in the chickens exposed to diesel exhaust via air cell infusion, indicating the effectiveness of the method. *: statistically different from control (P<0.05 from analysis of variance and least significant difference tests). This figure has been modified from Jiang et al. Environmental Pollution. 264, 114718 (20208. Please click here to view a larger version of this figure.
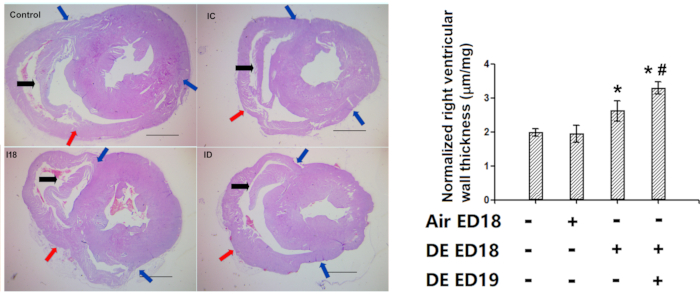
Figure 10: Demonstration of the effectiveness of right ventricular wall thickness measurement (Hematoxylin and eosin staining). Chicken embryos were infused with diesel exhaust at embryonic day 18 and 19, and then the hatched chickens were kept for 1 week, and then histological assessment of the right ventricular wall thickness was performed. A: Representative pictures of the heart cross-sections. Note the presence of anatomical marker in all the right ventricles (In older chickens, the marker tends to be a bit longer at desired position, which does not affect the accuracy of measurements). B: Quantification of the right ventricular wall thickness, which were firstly converted to actual length with standard slides, and then normalized with whole heart weight thus were represented in the form of um/ug. Blue arrows: two ends of the free right ventricular wall. Red arrows: the middle points of the right ventricular wall. Black arrows: anatomical marker. *: statistically different from control (P<0.05 from analysis of variance and least significant difference tests). Scale bars represent 1000 µm. This figure has been modified from Jiang et al. Environmental Pollution. 264, 114718 (2020)8. Please click here to view a larger version of this figure.
Discussion
The chicken embryo has been a classical model in developmental studies for 200 years1. Our methods presented in this manuscript have been used in the assessment of several environmental contaminants, including perfluorooctanoic acid, particulate matter, and diesel exhaust with success5, 7,8,9,10,11,12. With these methods, developmental cardiotoxicity was indicated cost-effectively and clearly. Furthermore, it is not difficult to expose chicken embryos with other compounds-of-interest and assess potential developmental cardiotoxicity.
The air-cell injection method is a classical method used previously in many studies13,14,15, which is convenient and effective. Compared to other developmental exposure methods, such as rodent models16,17,18, it features direct exposure into a closed system, which greatly reduces the variabilities due to maternal effects and varied excretion. Microinjection is an enhancement of the air-cell injection method, ensuring definitive exposure on or in the vicinity of developing early embryo, which may achieve similar effects as in utero injections in rodent models19,20. Comparing to in utero injections, our method allows visual confirmation of the injection with relatively easy manipulation steps, and accurate injection is easily achieved by controlling for the egg weight, which is not possible in the in utero injection, where the actual quantity and weight of embryos are not easily acquired. The infusion method is mainly for assessment of inhaled agents on the pulmonary system, but cardiotoxicity and pulmonary toxicity often co-occur. This method takes advantage of the air cell, into which a small amount of gas or aerosol are infused, allowing continuous inhalation of gas/aerosol without the need of specific inhalation chambers. Counterpart rodent models need to use relatively large amounts of gas/aerosol and large, expensive inhalation instruments21,22.
The two routinely tested endpoints in our lab, electrocardiography and histomorphometrical assessment of right ventricular wall thickness, represent functional and morphological changes following toxicant exposure, respectively. The assessment of right ventricular wall thickness has specific advantages in getting a comprehensive understanding of the right ventricular wall, as the traditional echocardiography-based assessment on right ventricle is usually challenging and not very accurate, due to the asymmetrical and complex crescent shape of right ventricle23. Our method may help to overcome this inaccuracy by supplementing with additional information about the right ventricular wall thickness at a representative position. Currently it is all manual, in the future, the measurements may be made automatically and the number of measurement points may be increased considerably, further improving the accuracy of this method.
Chicken embryo-based developmental models have several advantages in toxicological studies, such as the ability to deliver a relatively accurate exposure dose, an independent exposure system within the shell, and easy manipulation of the developing embryo. With respect to cardiotoxicity, chickens have relatively large hearts and thick ventricular walls, allowing easy histomorphometrical assessments. There are some shortcomings, such as availability of antibodies/primers and extra cage space requirements comparing to rodents if rearing chickens after hatch. Nevertheless, the chicken embryo is still a good alternative toxicological model to be used for potential developmental cardiotoxicity assessments.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 91643203, 91543208, 81502835).
Materials
| 4% phosphate buffered formaldehydefixative | Biosharp, Hefei, China | REF: BL539A | |
| 75% ethanol | Guoyao,Shanghai,China | CAS:64-17-5 | |
| Biosignaling monitor BL-420E+ | Taimeng, Chengdu, China | BL-420E+ | |
| Candling lamp | Zhenwei, Dezhou, China | WZ-001 | |
| Disposable syringe | Zhiyu, Jiangsu, China | ||
| Egg incubator | Keyu,Dezhou, China | KFX | |
| Electrical balance | OHAUS, Shanghai, China | AR 224CN | |
| Electro-thermal incubator | Shenxian, Shanghai, China | DHP-9022 | |
| Ethanol absolute | Guoyao,Shanghai,China | CAS:64-17-5 | |
| Fertile chicken egg | Jianuo, Jining, China | ||
| Hematoxylin and Eosin Staining Kit | Beyotime, Bejing, China | C0105 | |
| Histology paraffin | Aladdin, Shanghai, China | P100928-500g | Melt point 52~54°C |
| Histology paraffin | Aladdin, Shanghai, China | P100936-500g | Melt point 62~64°C |
| IV catheter | KDL, Zhejiang, China | The catheters have to be soft, plastic ones. | |
| Lentivirus | Genechem, Shanghai, China | The lentivirus were individually designed/synthesized by Genechem. | |
| Masson's trichrome staining kit | Solarbio, Beijing, China | G1340 | |
| Metal probe | Jinuotai, Beijing, China | ||
| Microinjector (5 uL) | Anting,Shanghai, China | ||
| Microscope | CAIKON, Shanghai, China | XSP-500 | |
| Microtome | Leica, Germany | HistoCore BIOCUT | |
| Microtome blade | Leica,Germany | Leica 819 | |
| Pentobarbitual sodium | Yitai Technology Co. Ltd., Wuhan, China | CAS: 57-33-0 | |
| Pipetter(10ul) | Sartorius, Germany | ||
| Povidone iodide | Longyuquan, Taian, China | ||
| Scissor | Anqisheng,Suzhou, China | ||
| Sterile saline | Kelun,Chengdu, China | ||
| Sunflower oil | Mighty Jiage, Jiangsu, China | Any commerical sunflower oil for human consumption should work | |
| Tape | M&G, Shanghai, China | ||
| Tedlar PVF Bag (5L) | Delin, Dalian, China | ||
| Vortex mixer | SCILOGEX, Rocky Hill, CT, US | MX-F | |
| Xylene | Guoyao,Shanghai,China | CAS:1330-20-7 |
References
- Kain, K. H., et al. The chick embryo as an expanding experimental model for cancer and cardiovascular research. Developmental Dynamics. 243 (2), 216-228 (2014).
- Menna, T. M., Mortola, J. P. Effects of posture on the respiratory mechanics of the chick embryo. Journal of Experimental Zoology. 293 (5), 450-455 (2002).
- Hamburger, V., Hamilton, H. L. A series of normal stages in the development of the chick embryo. Journal of Morphology. 88 (1), 49-92 (1951).
- Yamamoto, F. Y., Neto, F. F., Freitas, P. F., Oliveira Ribeiro, C. A., Ortolani-Machado, C. F. Cadmium effects on early development of chick embryos. Environmental Toxicology and Pharmacology. 34 (2), 548-555 (2012).
- Lv, N., et al. The roles of bone morphogenetic protein 2 in perfluorooctanoic acid induced developmental cardiotoxicity and l-carnitine mediated protection. Toxicology and Applied Pharmacology. 352, 68-76 (2018).
- Kmecick, M., Vieira da Costa, M. C., Oliveria Ribeiro, C. A., Ortolani-Machado, C. F. Morphological evidence of neurotoxic effects in chicken embryos after exposure to perfluorooctanoic acid (PFOA) and inorganic cadmium. Toxicology. 4227, 152286 (2019).
- Jiang, Q., Lust, R. M., Strynar, M. J., Dagnino, S., DeWitt, J. C. Perflurooctanoic acid induces developmental cardiotoxicity in chicken embryos and hatchlings. Toxicology. 293 (1-3), 97-106 (2012).
- Jiang, Q., et al. In ovo very early-in-life exposure to diesel exhaust induced cardiopulmonary toxicity in a hatchling chick model. Environmental Pollution. 264, 114718 (2020).
- Jiang, Q., Lust, R. M., DeWitt, J. C. Perfluorooctanoic acid induced-developmental cardiotoxicity: are peroxisome proliferator activated receptor alpha (PPARalpha) and bone morphorgenic protein 2 (BMP2) pathways involved. Journal of Toxicology and Environmental Health, Part A. 76 (11), 635-650 (2013).
- Jiang, Q., et al. Changes in the levels of l-carnitine, acetyl-l-carnitine and propionyl-l-carnitine are involved in perfluorooctanoic acid induced developmental cardiotoxicity in chicken embryo. Environmental Toxicology and Pharmacology. 48, 116-124 (2016).
- Zhao, M., et al. The role of PPAR alpha in perfluorooctanoic acid induced developmental cardiotoxicity and l-carnitine mediated protection-Results of in ovo gene silencing. Environmental Toxicology and Pharmacology. 56, 136-144 (2017).
- Jiang, Q., et al. Particulate Matter 2.5 Induced Developmental Cardiotoxicity in Chicken Embryo and Hatchling. Front Pharmacol. 11, 841 (2020).
- Molina, E. D., et al. Effects of air cell injection of perfluorooctane sulfonate before incubation on development of the white leghorn chicken (Gallus domesticus) embryo. Environmental Toxicology and Chemistry. 25 (1), 227-232 (2006).
- Crump, D., Chiu, S., Williams, K. L. Bis-(3-allyl-4-hydroxyphenyl) sulfone decreases embryonic viability and alters hepatic mRNA expression at two distinct developmental stages in chicken embryos exposed via egg injection. Environmental Toxicology and Chemistry. 37 (2), 530-537 (2018).
- Franci, C. D., et al. Potency of polycyclic aromatic hydrocarbons in chicken and Japanese quail embryos. Environmental Toxicology and Chemistry. 37 (6), 1556-1564 (2018).
- Rand, M. D., et al. Developmental exposure to methylmercury and resultant muscle mercury accumulation and adult motor deficits in mice. Neurotoxicology. 81, 1-10 (2020).
- Tanaka, T., Suzuki, T., Inomata, A., Moriyasu, T. Combined effects of maternal exposure to fungicides on behavioral development in F1 -generation mice: 2. Fixed-dose study of thiabendazole. Birth Defects Research. , (2020).
- Kofman, O., Lan, A., Raykin, E., Zega, K., Brodski, C. Developmental and social deficits and enhanced sensitivity to prenatal chlorpyrifos in PON1-/- mouse pups and adults. PLoS One. 15 (9), 0239738 (2020).
- Kischel, A., Audouard, C., Fawal, M. A., Davy, A. Ephrin-B2 paces neuronal production in the developing neocortex. BMC Developmental Biology. 20 (1), 12 (2020).
- Okolo, F., Zhang, G., Rhodes, J., Gittes, G. K., Potoka, D. A. Intra-Amniotic Sildenafil Treatment Promotes Lung Growth and Attenuates Vascular Remodeling in an Experimental Model of Congenital Diaphragmatic Hernia. Fetal Diagnosis and Therapy. , 1-13 (2020).
- Vyslouzil, J., et al. Subchronic continuous inhalation exposure to zinc oxide nanoparticles induces pulmonary cell response in mice. Journal of Trace Elements in Medicine and Biology. 61, 126511 (2020).
- Wahle, T., et al. Evaluation of neurological effects of cerium dioxide nanoparticles doped with different amounts of zirconium following inhalation exposure in mouse models of Alzheimer’s and vascular disease. Neurochemistry International. 138, 104755 (2020).
- Tanabe, K. Three-Dimensional Echocardiography- Role in Clinical Practice and Future Directions. Circ J. 84 (7), 1047-1054 (2020).

