Fingerprinting Cardiolipin in Leukocytes by Mass Spectrometry for a Rapid Diagnosis of Barth Syndrome
Summary
This protocol shows how to obtain a mass spectrometric “fingerprint” of leukocyte cardiolipin for the diagnosis of Barth syndrome. The assessment of elevated monolysocardiolipin to cardiolipin ratio discriminates patients with Barth syndrome from control heart failure patients with 100% sensitivity and specificity.
Abstract
Cardiolipin (CL), a dimeric phospholipid carrying four fatty acid chains in its structure, is the lipid marker of mitochondria, wherein it plays a crucial role in the functioning of the inner membrane. Its metabolite monolysocardiolipin (MLCL) is physiologically nearly absent in the lipid extract of animal cells and its appearance is the hallmark of the Barth syndrome (BTHS), a rare and often misdiagnosed genetic disease that causes severe cardiomyopathy in infancy. The method described here generates a “cardiolipin fingerprint” and allows a simple assay of the relative levels of CL and MLCL species in cellular lipid profiles. In the case of leukocytes, only 1 mL of blood is required to measure the MLCL/CL ratio via matrix-assisted laser desorption ionization – time-of-flight/mass spectrometry (MALDI-TOF/MS) just within 2 h from blood withdrawal. The assay is straightforward and can be easily integrated into the routine work of a clinical biochemistry laboratory to screen for BTHS. The test shows 100% sensitivity and specificity for BTHS, making it a suitable diagnostic test.
Introduction
Barth syndrome (BTHS) is a rare X-linked disease characterized by early-onset cardiomyopathy, skeletal muscle myopathy, growth delay, neutropenia, variable mitochondrial respiratory chain dysfunction, and abnormal mitochondrial structure1,2,3,4,5. BTHS has a prevalence of one case per million males with currently less than 250 known cases worldwide, though it is widely accepted that the disease is underdiagnosed2,6. BTHS results from loss-of-function mutations of the Tafazzin (TAFAZZIN) gene localized to chromosome Xq28.127,8 causing deficient remodeling of the mitochondrial phospholipid cardiolipin (CL), a process that normally leads to a highly symmetric and unsaturated acyl composition9,10. CL has been considered the signature lipid of mitochondria, where it is an important constituent of the inner membrane, vital for oxidative phosphorylation (i.e., mitochondrial energy metabolism), supercomplex formation, protein import, and involved in mitochondrial dynamics, mitophagy, and apoptosis11,12,13,14,15,16. Upon TAFAZZIN loss-of-function, CL remodeling fails and specific phospholipid abnormalities arise in mitochondria of BTHS patients: mature CL level (CLm) is decreased, while increased levels of monolysocardiolipin (MLCL) and altered CL acyl composition (i.e., immature CL species, CLi) occur. This brings to a dramatic increase of the MLCL/CL ratio17.
Diagnosis of BTHS is often difficult, as the disorder presents extremely variable clinical and biochemical features and may differ between affected individuals from the same family and within a patient over time3,5. Many BTHS boys show a very high level of urinary excretion of 3-methylglutaconic acid (3-MGCA), but the urine level may be normal or only mildly increased in patients over time3. However, increased 3-MGCA is a feature of various other mitochondrial and non-mitochondrial disorders, such as 3-methylglutaconyl-CoA hydratase deficiency (AUH defect), 3-methylglutaconic aciduria, dystonia-deafness, encephalopathy, Leigh-like (MEGDEL) syndrome, Costeff syndrome, and dilated cardiomyopathy with ataxia (DCMA) syndrome18,19. Hence, the poor specificity of 3-MCGA as a marker for BTHS and the enormous variability in patients render the biochemical diagnosis ambiguous.
Moreover, over 120 different TAFAZZIN mutations have been described causing the disorder5 and, therefore, a genetic diagnosis can be complicated, slow, and expensive. Moreover, molecular analysis of the TAFAZZIN gene can lead to false-negative results in the presence of mutations in noncoding or regulating sequences3. BTHS can be unambiguously tested by determining the relative amounts and distribution of (monolyso-)CL species and confirmed by TAFAZZIN gene sequencing or vice versa.
A practical test for diagnosis is the measurement of the MLCL/CL ratio by High-Performance Liquid Chromatography (HPLC) and Electro Spray Ionization / Mass spectrometry (ESI/MS) analysis in blood spot20,21. Measuring CL level alone is not adequate for diagnosis as some patients have near-normal levels of CL but altered MLCL/CL ratio. Therefore, measurement of MLCL/CL ratio has 100% sensitivity and specificity for BTHS diagnosis21. Another validated method based on HPLC and ESI/MS analysis has been set up on leukocytes22, but the complex chromatographic techniques for separation of lipids previously extracted and the expensiveness of the instruments restrict this analysis to a few clinical laboratories. All these factors, together with the lack of a straightforward diagnostic test, have contributed to the under-diagnosis of the condition.
MALDI-TOF/MS is a further valid tool in lipid analysis23,24.This analytical technique can be used to directly obtain lipid profiles of various biological samples, thus skipping extraction and separation steps25,26,27,28,29, including in tissue sections for MS Imaging applications30. Given this advantage, a simple and fast method to diagnose BTHS by profiling mitochondrial CL in intact leukocytes with MALDI-TOF/MS was developed28. Leukocyte isolation from only 1 mL of whole blood by erythrocyte sedimentation and lysis is straightforward and does not require special equipment or reagents. Furthermore, a fast lipid "mini-extraction" protocol applicable to minute amounts of leukocytes was described to warrant the successful acquisition of spectra having cleaner MS signals with a higher signal-to-noise ratio (S/N) than in those obtained from intact leukocytes28. This further step takes little time and allows for analyses to be reproducible even when carried out on MS instruments with poor sensitivity. In summary, the analytical method described here requires minimal sample preparation because time-consuming and labor-intensive chromatographic lipid separation can be skipped, thereby speeding up the test.
Protocol
Blood samples of healthy donors and heart failure patients were collected at the Policlinic Hospital of Bari (Italy), while samples of BTHS patients were obtained by the National Health Service UK BTHS clinic at Bristol Royal Hospital for Children (UK). Written informed consent of healthy donors, patients, and parents (where appropriate) and approvals by the respective ethics committees were obtained.
NOTE: If not used immediately, blood (in K-EDTA gel tube) can be stored at 4 °C for up to 24-48 h.
1. Isolation of leukocytes by dextran sedimentation of red blood cells (RBCs)
- Put blood samples (in K-EDTA) on an orbital shaker for 10 min to mix blood.
- To 0.9 mL of whole blood in a 1.5 mL tube, add 0.1 mL of 20% dextran solution (molecular mass > 100 kDa, in 0.9% NaCl). Pipette and disperse the suspension gently 20 times while avoiding air bubbles that would otherwise retain RBCs at the top of the tube. Let RBCs sediment for about 1 h at room temperature.
- Using a syringe, collect and transfer the yellow supernatant to a 15 mL tube, and then centrifuge at 400 x g for 15 min at room temperature (no brake, swing-bucket rotor).
- Discard the supernatant and resuspend the pellet containing mainly leukocytes and residual RBCs in 0.6 mL of ice-cold bi-distilled water (ddH2O).
- After ~15 s, add 0.2 mL of 0.6 M KCl to the cell suspension to restore the correct osmolarity. The short osmotic shock will lyse RBC and leave leukocytes intact. Adjust the final volume to 2.5 mL with 1x PBS.
- Centrifuge the suspension at 400 x g for 15 min at room temperature (no brake, swing-bucket rotor). Discard the supernatant and wash the leukocyte pellet again with 2.5 mL of 1x PBS.
- Centrifuge as in step 1.6 and discard the supernatant. Resuspend the pellet containing leukocytes in 200 µL of sterilized ddH2O.
NOTE: In the authors' experience, this corresponds to a protein content ranging from 200 µg to 400 µg. - Freeze the suspension at -80 °C or directly perform lipid extraction as follows.
2. "Mini-extraction" of lipids from isolated leukocytes
- Transfer 20 µL of leukocytes suspension (about 20-40 µg proteins) to a 1.5 mL tube and spin at 16,000 x g for 30 s.
- Discard the supernatant, add 10 µL of CHCl3 to the remaining pellet, and pipette repeatedly to promote lipid extraction.
- Finally, add 10 µL of 9-aminoacridine (9-AA) matrix solution (10 mg/mL 9-AA in 2-propanol/acetonitrile, 60:40 v/v) to the pellet in CHCl3. Pipette and disperse repeatedly to mix.
- Spin the solution containing lipids in CHCl3 and 9-AA at 16,000 x g for 30 s, and then deposit the supernatant as droplets of 0.35 µL (three replicates for each sample) on the MALDI target (sample plate) to be analyzed ('dried droplet' deposition method).
- Let the droplets air dry at least for 10-15 min.
3. Lipid analysis by MALDI-TOF/MS
- Acquire mass spectra of samples in triplicates on a MALDI-TOF mass spectrometer.
- After calibration with lipid standards (see Table of Materials), set the analyses in the negative ion mode and optimize the detection m/z range from 200 Th to 2,000 Th for small molecule analysis.
- Keep the laser fluence 5% above the threshold (of CL and MLCL) to have a S/N (at least 2).
- Acquire spectra in reflector mode using delayed pulsed extraction. For each mass spectrum, average 2,000 single laser shots (sum of 4 x 500). Apply gated matrix suppression to 400 Th to prevent detector saturation.
4. How to calculate the (MLCL + CLi)/CLm ratio
- Run the MALDI-TOF/MS instrument software (see Table of Materials) to analyze the acquired spectra.
- Using the Open command, open the Spectrum Browser dialog that allows selecting and loading the spectrum of interest.
- On the menu bar, click on the icons Smooth Mass Spectrum and Subtract Mass Spectrum Baseline.
- Click on File > Export > Mass Spectrum and choose the ASCII format for exporting the spectrum as a two-column table with pairs of data points: m/z (x) and intensity (y). Copy and paste the coordinates in a spreadsheet program (*.xls).
- Repeat steps 4.2 to 4.4 for the triplicates of each analyzed sample, pasting the coordinates in the same spreadsheet program file as shown in Figure 1 (Step 1).
- Following the x-ranges shown in Figure 1 (Step 2) for each species listed, calculate the sum of y1, y2, and y3 values (triplicates) by the SUM function to get the peak area (Figure 1; Step 3).
- Perform the average of the triplicates area values by AVERAGE function (Figure 1; Step 3).
- Place the average area values for each species in the column as shown in Figure 1 (Step 2).
- In order to consider only the first isotopologue of the CLm 72:7 species as in28, calculate the isotopic correction for the overlapping between the M + 2 isotopologue of the CLm 72:8 and the monoisotopic peak of CLm 72:7 as shown in Figure 1 (Step 4).
- Finally, calculate the (MLCL + CLi)/CLm ratio as shown in Figure 1 (Step 5).
Representative Results
In this study, a simple and rapid method for isolating leukocytes from 1 mL of whole blood and obtaining CL fingerprinting by MALDI-TOF/MS has been described (see Figure 2). Figure 3 shows the comparison of representative CL fingerprinting of leukocytes, obtained from control subjects and BTHS young boys, in the CL and MLCL mass (m/z) range. Table 1 lists CL and MLCL species detected in these mass spectra.
Defects in the TAFAZZIN gene typically determine a lipid profile specific for the BTHS, i.e., the appearance of MLCL and CLi forms together with the reduction of CLm forms in the CL fingerprint. The lipid profile of leukocytes of control subjects typically exhibits only two molecular species of CLm (upper panel of Figure 3). The only peaks of interest present in this mass spectrum are CLm species: tetralinoleoyl CL (18:2)4 at m/z 1,448.0 and CL (18:2)3 (18:1)1 at m/z 1,450.0. This method allows their visualization in the MALDI lipid profile so that the two CL species can be easily identified and measured in the healthy phenotype.
The representative CL fingerprinting of a boy affected by BTHS shows a different pattern of MALDI signals (lower panel of Figure 3). The signals attributable to CLm species are lower, while peaks assigned to two CLi species (at m/z 1,404.0 and 1,430.0) and two MLCL species (at m/z 1,165.8 and 1,191.8) are also found (see Table 1). In summary, data in Figure 3 clearly show that the mitochondrial CL and MLCL species can be readily detected by MALDI-TOF/MS analyses of leukocytes.
A previous study has demonstrated that the (MLCL + CLi)/CLm ratio calculated in lipid profiles of leukocytes obtained by MALDI-TOF/MS can be used as a diagnostic parameter for BTHS28. In that study, CL fingerprinting of leukocytes isolated from healthy subjects and BTHS boys was compared28. Here, results obtained from the MALDI lipid profile of leukocytes isolated from 11 pediatric heart failure patients (one BTHS-affected and 10 non-BTHS-affected) were added.
Figure 4 shows calculated (MLCL + CLi)/CLm ratios in MALDI mass spectra of lipid mini-extracts of leukocytes from nine BTHS patients, 10 non-BTHS-affected (i.e., heart failure patients), and 22 healthy subjects; the ratio has been calculated by considering the area of CL and MLCL peaks detected in the mass spectra, as described, but intensities can also be used with similar results (data not shown).
The (MLCL + CLi)/CLm ratio for BTHS-patients was 7.2 ± 2.9, while those calculated for healthy subjects and heart failure patients were 0.19 ± 0.10 and 0.13 ± 0.07, respectively (mean ± SD). It can be seen that control groups (both healthy subjects and heart failure patients) and BTHS-patients are separated by more than one order of magnitude, showing that this method has strong diagnostic power.
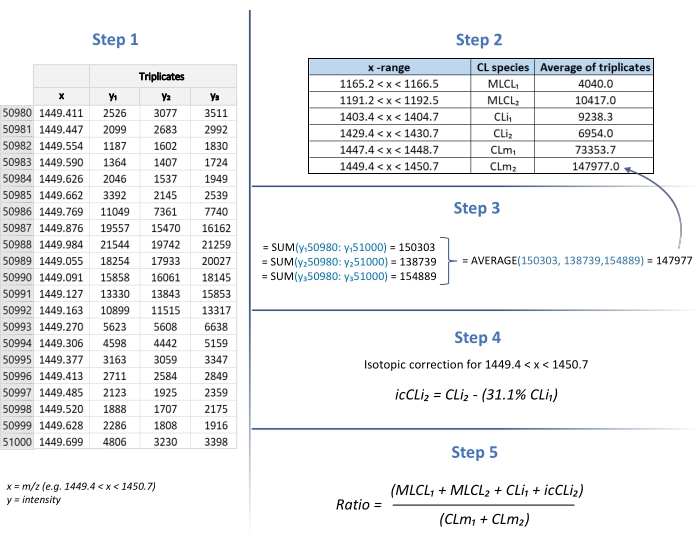
Figure 1: (MLCL+CLi)/CLm ratio determination. The calculation of the (MLCL + CLi)/CLm ratio is performed using the Excel program. Step 1: Table of m/z (x) / intensity (y) for species of interest (in triplicates). Step 2: Table of x-ranges to calculate each peak area. Step 3: Calculation of SUM and AVERAGE of y1, y2, and y3 values (triplicates). Step 4: Calculation of isotopic correction. Step 5: Calculation of the (MLCL+CLi)/CLm ratio. Please click here to view a larger version of this figure.
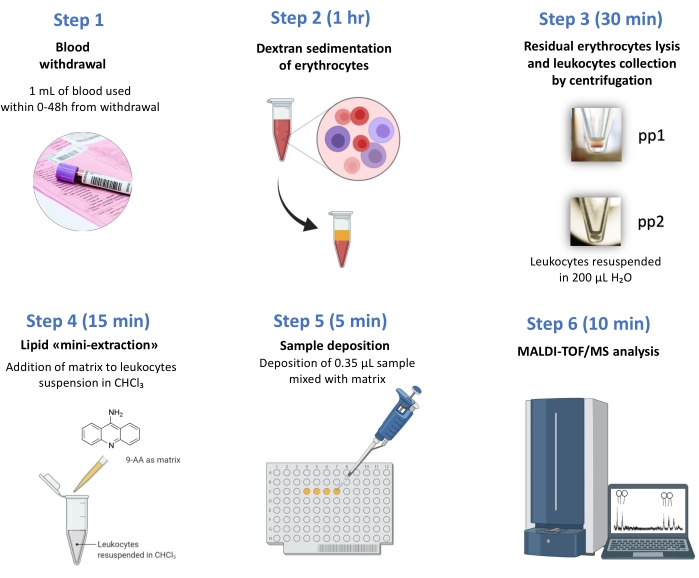
Figure 2: Work-flow chart. From the blood withdrawal to the test results, only 2 h are necessary. MALDI-TOF/MS-based BTHS diagnosis in few steps: (1) Blood withdrawal; (2) Dextran sedimentation of erythrocytes, (3) residual erythrocytes lysis (pp1) and leukocytes collection (pp2) by centrifugation, (4) lipid "mini-extraction", (5) sample deposition, (6) MALDI-TOF/MS analysis. Please click here to view a larger version of this figure.
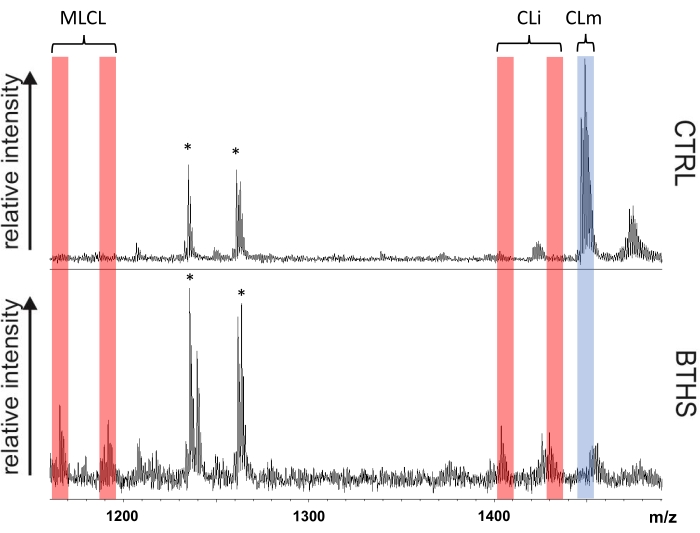
Figure 3: BTHS diagnostic leukocyte CL fingerprints. Comparison of cardiolipin fingerprints of leukocytes isolated from a control subject (CTRL) and a representative Barth syndrome patient (BTHS) is shown. Red bands highlight peaks of the immature form of cardiolipin (CLi) and monolysocardiolipin (MLCL) species, while the blue band marks the peaks of the mature forms of CL (CLm). Signals labeled with an asterisk refer to gangliosides and are not of interest to this study. Please click here to view a larger version of this figure.
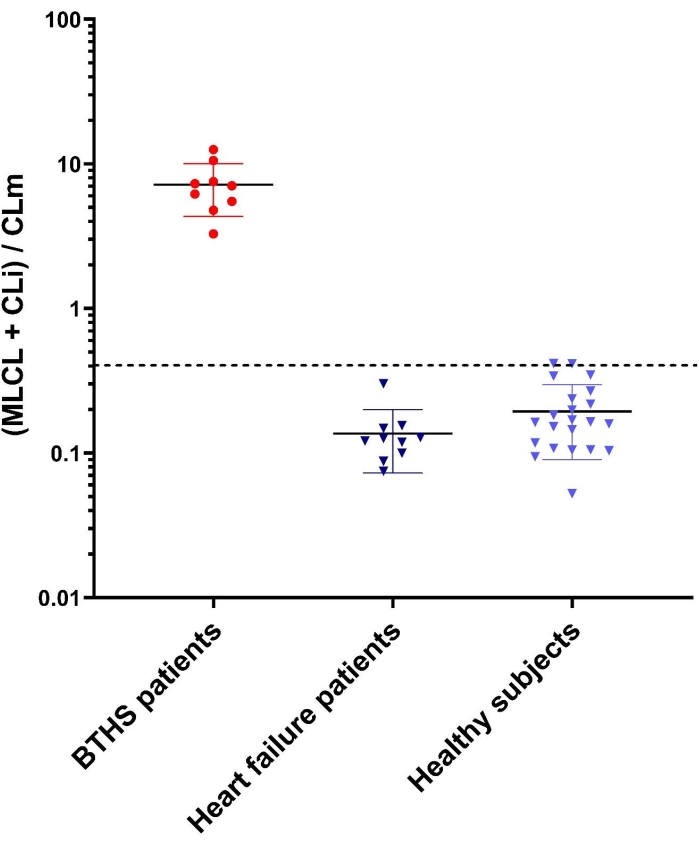
Figure 4: (MLCL + CLi)/CLm ratios in controls and BTHS patients. Error bars indicate standard deviations obtained from the mean of measurements of all subjects for each of the three groups: nine BTHS patients, 10 non-BTHS-affected heart failure patients, and 22 healthy subjects. Each sample was analyzed by MALDI-TOF/MS (in triplicate). The y-axis shows a logarithmic scale. The upper cut-off value of controls, calculated as in references22,28 is 0.4. Please click here to view a larger version of this figure.
| m/z [M-H]– | Assignment |
| 1165.8 | MLCL 52:2 |
| 1191.8 | MLCL 54:1 |
| 1404.0 | CLi 68:2 |
| 1430.0 | CLi 70:3 |
| 1448.0 | CLm 72:8 |
| 1450.0 | CLm 72:7 |
Table 1: List of detected peaks assigned to CL and MLCL species in the MALDI-TOF mass spectra.
Discussion
Barth syndrome is an inborn error of metabolism and a life-changing condition that is likely to be under-diagnosed2,6. As mentioned before, a contributing factor may be the lack of a straightforward diagnostic test. Here, a simple and fast method to measure MLCL/CL ratio by MALDI-TOF/MS in leukocytes for BTHS screening was described. Moreover, MALDI-TOF mass spectrometers are widely distributed among clinical laboratories worldwide and do not require high analytical expertise31,32.
The current method has a major advantage over existing approaches in that it can simultaneously detect in a single run of MS analysis the CL and MLCL species in the total lipid profile of isolated leukocytes, avoiding time-consuming steps of standard lipid extraction and separation via HPLC. In addition to the time factor, having more steps in the workflow can potentially introduce errors. The present method is easy even for operators without relevant experience in lipidomics.
The method for BTHS screening described here not only relies on the decrease of CLm levels but also considers the increase of CLi species, which along with the presence of MLCL species specifically suggest the CL metabolism defect, i.e., mutations in the TAFAZZIN gene. This approach is successful owing to the use of 9-AA as the matrix for MALDI analysis, which is particularly suitable for the detection of the CL species23,25, even when these species are minor components in complex lipid profiles.
Comparing these results to those obtained in LC/MS studies on leukocytes or bloodspots20,21,22, the mean MLCL/CL ratio in BTHS patients is around 10 in all reports. On the contrary, the mean ratio in controls varies and it is found to be ~0.001 in22, 0.01 in21, and finally 0.1 in28. The reason for the last value being slightly higher is the conservative approach undertaken in the calculations28. Indeed, the method previously reported in28 and the one described here include measurements for MLCL and CLi also in controls' mass spectra, where these are nearly absent. This in fact means accounting for noise levels, which artificially increase the ratio in healthy controls. The reason for this cautionary approach is that low-performance MALDI-MS instruments may not be sensitive enough to detect low levels of the lipids of interest. By considering noise levels, this method assumes a basal level of undetectable MLCL and CLi in controls, and of CLm in patients. Notably, even with such a precautional approach, the application of the MLCL/CL ratio yields a >10-fold difference in the MLCL/CL ratio between BTHS patients and control groups (see Figure 4). Therefore, the assay described here maintains 100% sensitivity and specificity21,22,28.
One limitation of this method is in that it employs 1 mL of blood, although it can be scaled down to 500 µL in practice. This is certainly a drawback when compared to LC/MS methods employing bloodspots, where the latter can be shipped with regular mail and are usually made of ~50 µL of blood. However, this MALDI method has the major advantage of being performable by operators without previous experience in MS analyses, and within only 2 h from withdrawal.
In summary, the proposed assay is simple and can be easily integrated into the routine work of a clinical biochemistry laboratory. This could potentially increase the number of samples analyzable, hence contributing to the identification of new hidden cases.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We are grateful to the individuals with BTHS and their families for participating in our research. We thank the Barth Syndrome Foundation US and the Barth Syndrome UK Trust for their support and for helping with the collection of the blood samples at the annual meeting in Bristol. This study was funded by Barth Syndrome Foundation US, Barth Italia Onlus, and Apulia Region.
Materials
| 1,1′,2,2′-tetratetradecanoyl cardiolipin | Avanti Polar Lipids | 750332 | Lipid standard for MALDI-TOF calibration |
| 1,1′2,2′-tetra- (9Z-octadecenoyl) cardiolipin | Avanti Polar Lipids | 710335 | Lipid standard for MALDI-TOF calibration |
| 1,2-di- (9Z-hexadecenoyl)-sn-glycero-3-phosphoethanolamine | Avanti Polar Lipids | 878130 | Lipid standard for MALDI-TOF calibration |
| 1,2-ditetradecanoyl-sn-glycero-3-phosphate | Avanti Polar Lipids | 830845 | Lipid standard for MALDI-TOF calibration |
| 1,2-ditetradecanoyl-snglycero-3-phospho-(1′-rac-glycerol) | Avanti Polar Lipids | 840445 | Lipid standard for MALDI-TOF calibration |
| 1,2-ditetradecanoyl-sn-glycero-3-phospho-L-serine | Avanti Polar Lipids | 840033 | Lipid standard for MALDI-TOF calibration |
| 2-Propanol, ACS reagent, ≥99.5% | Merck Life Science S.r.l. | 190764 | |
| 9-Aminoacridine hemihydrate, 98% | Acros Organics | 134410010 | |
| Acetonitrile, ACS reagent, ≥99.5% | Merck Life Science S.r.l. | 360457 | |
| Chloroform, ACS reagent, ≥99.8% | Merck Life Science S.r.l. | 319988 | |
| Dextran from Leuconostoc spp. Mr 450,000-650,000 | Merck Life Science S.r.l. | 31392 | |
| Flex Analysis 3.3 | Bruker Daltonics | Software | |
| MALDI-TOF mass spectrometer Microflex LRF | Bruker Daltonics | ||
| Microsoft Excel | Microsoft Office | Software | |
| OmniPur 10X PBS Liquid Concentrate | Merck Life Science S.r.l. | 6505-OP | |
| Potassium chloride, ACS reagent, 99.0-100.5% | Merck Life Science S.r.l. | P3911 | |
| Sodium chloride, ACS reagent, ≥99.0% | Merck Life Science S.r.l. | S9888 |
References
- Barth, P. G., et al. X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): respiratory-chain abnormalities in cultured fibroblasts. Journal of Inherited Metabolic Disease. 19 (2), 157-160 (1996).
- Steward, C. G., et al. syndrome (X linked cardiac and skeletal myopathy, neutropenia, and organic aciduria): rarely recognised, frequently fatal [abstract]. Archives of Disease in Childhood. 89, 48 (2004).
- Clarke, S. L. N., et al. Barth syndrome. Orphanet Journal of Rare Diseases. 8, 23 (2013).
- Zegallai, H. M., Hatch, G. M. Barth syndrome: cardiolipin, cellular pathophysiology, management, and novel therapeutic targets. Molecular and Cellular Biochemistry. 476 (3), 1605-1629 (2021).
- Taylor, C., et al. Clinical presentation and natural history of Barth Syndrome: An overview. Journal of Inherited Metabolic Disease. 45 (1), 7-16 (2022).
- Miller, P. C., Ren, M., Schlame, M., Toth, M. J., Phoon, C. A. Bayesian analysis to determine the prevalence of Barth syndrome in the pediatric population. The Journal of Pediatrics. 217, 139-144 (2020).
- Bione, S., et al. A novel X-linked gene, G4.5. is responsible for Barth syndrome. Nature Genetics. 12 (4), 385-389 (1996).
- Whited, K., Baile, M. G., Currier, P., Claypool, S. M. Seven functional classes of Barth Syndrome mutation. Human Molecular Genetics. 22 (3), 483-492 (2013).
- Schlame, M., Ren, M., Xu, Y., Greenberg, M. L., Haller, I. Molecular symmetry in mitochondrial cardiolipins. Chemistry and Physics of Lipids. 138 (1-2), 38-49 (2005).
- Schlame, M., Xu, Y. The function of Tafazzin, a mitochondrial phospholipid-lysophospholipid acyltransferase. Journal of Molecular Biology. 432 (18), 5043-5051 (2020).
- Schlame, M., Rua, D., Greenberg, M. L. The biosynthesis and functional role of cardiolipin. Progress in Lipid Research. 39 (3), 257-288 (2000).
- Mileykovskaya, E., Dowhan, W. Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochimica et Biophysica Acta. 1788 (10), 2084-2091 (2009).
- Claypool, S. M., Koehler, C. M. The complexity of cardiolipin in health and disease. Trends in Biochemical Sciences. 37 (1), 32-41 (2011).
- Ren, M., Phoon, C. K., Schlame, M. Metabolism and function of mitochondrial cardiolipin. Progress in Lipid Research. 55, 1-16 (2014).
- Paradies, G., Paradies, V., Ruggiero, F. M., Petrosillo, G. Role of cardiolipin in mitochondrial function and dynamics in health and disease: Molecular and pharmacological aspects. Cells. 8 (7), 728 (2019).
- Acoba, M. G., Senoo, N., Claypool, S. M. Phospholipid ebb and flow makes mitochondria go. The Journal of Cell Biology. 219 (8), 03131 (2020).
- Schlame, M., et al. Phospholipid abnormalities in children with Barth syndrome. Journal of the American College of Cardiology. 42 (11), 1994-1999 (2003).
- Wortmann, S. B., et al. Inborn errors of metabolism with 3-methylglutaconic aciduria as discriminative feature: proper classification and nomenclature. Journal of Inherited Metabolic Disease. 36 (6), 923-928 (2013).
- Ikon, N., Ryan, R. O. On the origin of 3-methylglutaconic acid in disorders of mitochondrial energy metabolism. Journal of Inherited Metabolic Disease. 39 (5), 749-756 (2016).
- Kulik, W., et al. Bloodspot assay using HPLC-tandem mass spectrometry for detection of Barth syndrome. Clinical Chemistry. 54 (2), 371-378 (2008).
- Vaz, F. M., et al. An improved functional assay in blood spot to diagnose Barth syndrome using the monolysocardiolipin/cardiolipin ratio. Journal of Inherited Metabolic Disease. 45 (1), 29-37 (2022).
- Bowron, A., et al. Diagnosis of Barth syndrome using a novel LC-MS/MS method for leukocyte cardiolipin analysis. Journal of Inherited Metabolic Disease. 36 (5), 741-746 (2013).
- Sun, G., et al. Matrix assisted laser desorption/ionization time-of-flight mass spectrometric analysis of cellular glycerophospholipids enabled by multiplexed solvent dependent analyte-matrix interactions. Analytical Chemistry. 80 (19), 7576-7585 (2008).
- Leopold, J., Popkova, Y., Engel, K. M., Schiller, J. Recent developments of useful MALDI matrices for the mass spectrometric characterization of lipids. Biomolecules. 8 (4), 173 (2018).
- Angelini, R., Babudri, F., Lobasso, S., Corcelli, A. MALDI-TOF/MS analysis of archaebacterial lipids in lyophilized membranes dry-mixed with 9-aminoacridine. The Journal of Lipid Research. 51 (9), 2818-2825 (2010).
- Angelini, R., et al. Lipidomics of intact mitochondria by MALDI-TOF MS. The Journal of Lipid Research. 53 (7), 1417-1425 (2012).
- Angelini, R., Vormieter, G., Corcelli, A., Fuchs, B. A fast method for the determination of PC/LPC ratio in intact horse serum by MALDI-TOF-MS: an easy-to-follow lipid biomarker of inflammation. Chemistry and Physics of Lipids. 183, 169-175 (2014).
- Angelini, R., et al. Cardiolipin fingerprinting of leukocytes by MALDI-TOF/MS as a screening tool for Barth syndrome. The Journal of Lipid Research. 56 (9), 1787-1794 (2015).
- Lobasso, S., et al. A lipidomic approach to identify potential biomarkers in exosomes from melanoma cells with different metastatic potential. Frontiers in Physiology. 12, 748895 (2021).
- Angelini, R., et al. Visualizing cholesterol in the brain by on-tissue derivatization and quantitative mass spectrometry imaging. Analytical Chemistry. 93 (11), 4932-4949 (2021).
- Greco, V., et al. Applications of MALDI-TOF mass spectrometry in clinical proteomics. Expert Review of Proteomics. 15 (8), 683-696 (2018).
- Duncan, M., DeMarco, M. L. MALDI-MS: Emerging roles in pathology and laboratory medicine. Clinical Mass Spectrometry (Del Mar, Calif). 13, 1-4 (2019).

