Monitoring Dopamine Release in a Rat Model with VTA Dopaminergic Neuron Receptor Manipulation
Abstract
Source: Wickham, R. J., et al. Combined Infusion and Stimulation with Fast-Scan Cyclic Voltammetry (CIS-FSCV) to Assess Ventral Tegmental Area Receptor Regulation of Phasic Dopamine. J. Vis. Exp. (2020).
This video demonstrates the implantation of electrodes and cannulas in an anesthetized rat to measure dopamine release in the nucleus accumbens using a potentiostat and the infusion of drugs to modulate dopaminergic activity in the ventral tegmental area.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Presurgical preparations
- Electrode solution preparation
- To make the electrode backfill solution, prepare a solution of 4 M potassium acetate with 140 mM potassium chloride.
- Electrode preparation
- Using vacuum suction, insert a T-650 carbon fiber (7 µm in diameter) into a borosilicate glass capillary (length = 100 mm, diameter = 1.0 mm, inside diameter = 0.5 mm).
- Once the carbon fiber has been placed inside the glass capillary, place the glass capillary into a vertical electrode puller, with the heating element roughly in the middle of the capillary. Set the heater to 55 with the magnet turned off.
- After the capillary is pulled, carefully raise the upper capillary holder so that the tip of the electrode is not surrounded by the heating element.
- Using sharp scissors, cut the carbon fiber that is still connecting the two pieces of the capillary. This will result in two separate carbon fiber microelectrodes.
- Under a light microscope, carefully cut the exposed carbon fiber with a sharp scalpel so that it extends approximately 75-100 µm beyond the end of the glass.
- Using a light microscope, ensure that the electrode is free of cracks along the capillary and that the seal where the carbon fiber exits the capillary is difficult to notice and free from cracks.
NOTE: A good seal will help reduce noise during recordings.
- Reference electrode fabrication
- Solder a gold pin to a 5 cm silver wire.
- Attach the anode to a metal paper clip or other conductor, the cathode to a pin, and apply a voltage (~2 V) while the paper clip and silver wire is submerged in 0.1 M HCl.
- Cease the voltage once a white coating (AgCl) appears on the silver wire.
- Preparing electrodes for implantation
- Solder a gold pin to a thin insulated wire (~10 cm in length, <0.50 mm diameter).
- Remove ~5 cm of insulation from the wire opposite to the gold pin.
- Fill the electrode approximately halfway with the electrode solution.
- Insert insulated wire into the electrode.
NOTE: The wire should make contact with the carbon fiber inside the electrode.
2. Electrode implantations
- Give adult, male, Sprague Dawley rats (250−450 g) an intraperitoneal injection (1.5 g/kg or 1 mL/kg volume) of 0.5 g/mL urethane dissolved in sterile saline. Start with an initial urethane dose of 1.0−1.2 g/kg. If the animal is still responsive to the noxious stimulus test (tail pinch) 20 min after urethane administration, administer an additional 0.3−0.5 g/kg urethane for a 1.5 g/kg total dose.
NOTE: To prepare the 0.5 g/mL urethane solution, add 10 g of urethane to 10 g (~10 mL) of saline. Urethane is a carcinogen and must be handled with care. However, it is an important anesthetic, as it does not alter levels of dopamine, as do other anesthetics such as ketamine/ xylazine and chloral hydrate. - Once the animal is deeply anesthetized and is not responsive to noxious stimuli (e.g., toe pinch), place it in the stereotaxic frame. Apply ophthalmic lubricant to each eye of the rat.
NOTE: This is a non-survival surgery, but a good aseptic technique is encouraged. - Clean the rat's scalp using a two-stage scrub (i.e., an iodopovidone scrub followed by a 70% ethanol scrub; perform with a 3-cycle repetition).
- Cut away the scalp tissue using sterilized needle nose tweezers and surgical scissors. Remove a significant amount of tissue to make room for the various implantations outlined below.
- Gently clean the skull surface using sterilized cotton tip applicators. Then apply 2−3 drops of 3% hydrogen peroxide to help identify the lambda and bregma.
- Using a stereotaxic or hand drill (1.0 mm, ~20,000 rpm), drill a 1.5 mm diameter hole 2.5 mm anterior to the bregma and 3.5 mm lateral to the bregma. Partially (about halfway, until it is firmly in place) implant a screw (1.59 mm O.D., 3.2 mm long) in this hole. It is recommended to use sterile saline to irrigate while drilling to prevent thermal injury.
- For the reference electrode, drill a 1.0 mm diameter hole 1.5 mm anterior and 3.5 mm lateral to the bregma, in the left hemisphere.
- By hand, insert ~2 mm of reference wire into this hole while wrapping the reference wire around and under the head of the implanted screw.
- Fully implant the screw, pinning down the reference electrode in place.
- In the right hemisphere, drill a 1.5 mm diameter hole 1.2 mm anterior and 1.4 mm lateral to the bregma.
- Gently remove the dura using tweezers.
- For the stimulating electrode, drill a square hole (2 mm anterior-posterior, 5 mm medial-lateral) centered at 5.2 mm posterior and 1.0 mm lateral to the bregma.
- Using the stereotactic arm bars, lower the bipolar stimulating electrode/guide cannula 5 mm below the dura. In case of bleeding during the implantation of the electrode, use sterile cotton swabs and gauze to minimize bleeding.
NOTE: The bipolar stimulating electrode used in this method is prefitted with a guide cannula (Table of Materials). The internal cannula used with this item should be flushed with the prongs on the bipolar stimulating electrode when fully inserted into the guide cannula. This will allow the internal cannula to sit directly in between the two prongs of the stimulator, which sit about 1 mm apart. A similar protocol is described elsewhere. - Using the stereotactic arm bars, lower the carbon fiber microelectrode 4 mm below the dura. This location is at the most dorsal portion of the striatum.
- Connect the reference wire and carbon fiber to a potentiostat.
- Apply a triangular waveform (-0.4−1.3 V, 400 V/s) for 15 min at 60 Hz, and again for 10 min at 10 Hz.
NOTE: Typically, when applying waveforms to carbon fiber microelectrodes in the brain, oxide groups are added to the surface of the carbon fiber. Equilibrium of this reaction must be reached prior to recording; otherwise, significant drift will occur19. Cycling the electrode at higher frequencies (60 Hz) allows the carbon fiber to achieve equilibrium faster.
3. Optimizing carbon fiber and stimulating electrode/guide cannula locations
- Set the stimulator to produce a bipolar electrical waveform, with a frequency of 60 Hz, 24 pulses, 300 µA current, and a pulse width of 2 ms/phase.
- Gently lower the stimulator in increments of 0.2 mm from 5 mm to 7.8 mm below the dura. At each increment, stimulate the VTA.
NOTE: At more dorsal depths (5−6 mm), stimulation of the brain will typically (~80% of the time) cause the whiskers of the rat to twitch. At further depths, the whiskers will cease twitching, which occurs between 7.5−8.2 mm below the dura. When the whiskers cease twitching, the stimulating electrode will be near or at the VTA. This will not occur in every rat, and lack of whisker twitching should not be taken as a sign that the bipolar stimulating electrode/infusion cannula is misplaced. Whisker twitching may not occur for all anesthetics (e.g., isoflurane). - Continue to lower the bipolar stimulating electrode/guide cannula until a stimulation produces phasic DA release at the carbon fiber microelectrode (currently in the dorsal striatum).
NOTE: DA release in the dorsal striatum will not always occur if the bipolar electrode is implanted in the VTA, but observation of DA release in the dorsal striatum upon VTA stimulation is usually a good sign that a good signal will be observed in the NAc core. - Lower the carbon fiber microelectrode until it is at least 6.0 mm below the dura. This is the most dorsal part of the NAc core.
- Stimulate the VTA and record the peak amplitude of the DA peak.
- Lower or raise the carbon fiber microelectrode at the site that produces the greatest DA release.
- Ensure that the DA response peak is a clear oxidation peak at 0.6 V and a reduction peak at -0.2 V. These peaks indicate DA.
4. Combination infusion and stimulation FSCV recording
NOTE: Figure 1 shows the timeline for recording before and after VTA microinfusion.
- Once the carbon fiber and stimulating electrode/guide cannula location has been optimized, stimulate for ~20−30 min.
NOTE: Under the current stimulation parameters, do not stimulate any more than once every 3 min, to allow for vesicular reloading22. - After achieving a stable baseline (<20% variation over five stimulations), gently lower the internal cannula by hand into the guide cannula that is prefitted into the bipolar stimulator.
- Take an additional 2−3 baseline recordings to ensure that the cannula insertion itself did not change the evoked signal. In some cases, the insertion and removal of the internal cannula can damage the VTA. If the signal drastically changes over this baseline period (>20%), then take an additional 3−4 recordings until the baseline restabilizes.
- Using a syringe pump and micro syringe, infuse 0.5 µL of solution (e.g., 0.9% saline, N-methyl-D-aspartate [NMDA], (2R)-amino-5-phosphonovaleric acid [AP5]) into the VTA over a 2 min period.
- Post infusion, leave the internal cannula for at least 1 min prior to removal.
NOTE: Some drugs may require leaving the internal cannula for a longer time based on the drug kinetics, and removal of the internal cannula may cause the drug to travel back up through the internal cannula. If there is concern, one could leave the internal cannula in the guide cannula during the entirety of the recording. Otherwise, recording can begin after this 1 min interval. - Continue recording every 3 min to measure post-infusion effects.
NOTE: If infusing a control solution, and no effect is observed, it is possible to infuse a second time10. If there is altered DA release caused by inserting the internal cannula or saline infusion, the signal typically recovers to baseline within 30 min.
Representative Results
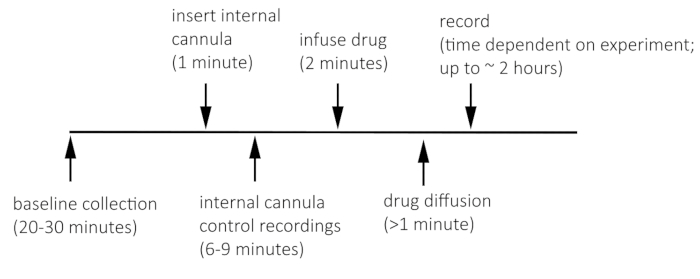
Figure 1: Timeline for recording before and after VTA microinfusion.
Disclosures
The authors have nothing to disclose.
Materials
| Electrode Filling Solution/Supplies | |||
| Micropipette | World Precision Instruments | MF286-5 (28 gauge) | |
| Potassium Acetate | Sigma | 236497-100G | |
| Potassium Chloride | Sigma | P3911-25G | |
| Electrode Supplies | |||
| Carbon fiber | Thornel | T650 | |
| Electrode puller | Narishige International | PE-22 | Note: horizontal pullers can be used as well |
| Glass capillary | A-M systems | 626000 | |
| Insulated wires for electrodes | Weico Wire and Cable Incorporated | UL 1423 | Length; 10 cm; diameter,0.4mm; must get custom made; insulated material should cover 5 cm of the wire |
| Light Microscope (for viewing and cutting electrode) | Fischer Scientific | M3700 | |
| Pin | Phoenix Enterprises | HWS1646 | To be soldered onto the insuled electrode wire and reference electrode; connects to headstage |
| Putty | Alcolin | 23922-1003 | Used to place electrode on while cutting the carbon fiber |
| Scalpal Blade | World Precision Instruments | 500239 | For cutting carbon fiber to the apprpriate length |
| Silver Wire | Sigma | 327026-4G | |
| FSCV Hardware/Software | |||
| Faraday Cage | U-Line | H-3618 (36" x 24" x 42") | |
| Potentiostat | Univ. of N. Carolina, Electronics Facility | ||
| Stimulating electrode | PlasticsOne | MS303/2-A/SPC | when ordering, request a 22 mm cut below pedestal |
| TarHeel HDCV Software | University of North Carolina-Chapel Hill | – | https://chem.unc.edu/critcl-main/criticl-electronics/criticl-electronics-hardware/ for ordering information |
| UEI breakout box | Univ. of N. Carolina, Electronics Facility | https://chem.unc.edu/critcl-main/criticl-electronics/criticl-electronics-hardware/ for ordering information | |
| UEI power supply | Univ. of N. Carolina, Electronics Facility | https://chem.unc.edu/critcl-main/criticl-electronics/criticl-electronics-hardware/ for ordering information | |
| Stimulator Hardware | |||
| Neurolog stimulus isolator | Digitimer Ltd. | DS4 | Neurolog 800A |
| Infusion/Stimulation Supplies | |||
| Infusion Pump | New Era Syringe Pump | NE-300 | |
| Internal Cannula | PlasticsOne | C315I/SPC INTERNAL 33GA | |
| Microliter Syringe | Hamilton | 80308 | |
| Tubing | PlasticsOne | C313CT/ PKG TUBING 023 X 050 PE50 | |
| Surgical Supplies | |||
| Cannula Holder | Kopf Instruments | 1776 P-1 | |
| Cotton Tip Applicators | Vitality Medical | 806 | |
| Electrode Holder | Kopf Instruments | 1770 | |
| Heating Pad | Kent Scientific | RT-0501 | |
| Povidone Iodine | Vitality Medical | 29906-004 | |
| Screws | Stoelting | Bone Anchor Screws/Pkg.of 100 | 1.59 mm O.D., 3.2 mm long |
| Silver wire reference with AgCl | InVivo Metric | E255A | |
| Square Gauze | Vitality Medical | 441408 | |
| Stereotax | Kopf Instruments | Model 902 (Dual Arm Bar) | |
| Drugs for infusions | |||
| ((2R)-amino-5-phosphonovaleric acid | Sigma Aldrich | A5282 | |
| N-methyl-D-aspartate | Sigma Aldrich | M3262 | |
| Mecamylamine hydrochloride (M9020-5mg) | Sigma Aldrich | M9020 | |
| Scopolamine hydrobromide (S0929-1g) | Sigma Aldrich | S0929 |

