Dissection of Mosquito Ovaries, Midgut, and Salivary Glands for Microbiome Analyses at the Organ Level
Summary
Microbial communities in mosquitoes hold great promise for vector biocontrol strategies. Most symbionts are uncultivable, requiring metagenomic analyses. We describe a method to dissect female mosquitoes and separate ovaries, midgut, and salivary glands preventing cross-contamination, facilitating microbiome studies at the organ level, and enhancing understanding of microorganisms’ roles in mosquito biology.
Abstract
The global burden of mosquito-transmitted diseases, including malaria, dengue, West Nile, Zika, Usutu, and yellow fever, continues to increase, posing a significant public health threat. With the rise of insecticide resistance and the absence of effective vaccines, new strategies are emerging that focus on the mosquito’s microbiota. Nevertheless, the majority of symbionts remain resistant to cultivation. Characterizing the diversity and function of bacterial genomes in mosquito specimens, therefore, relies on metagenomics and subsequent assembly and binning strategies. The obtention and analysis of Metagenome-Assembled Genomes (MAGs) from separated organs can notably provide key information about the specific role of mosquito-associated microbes in the ovaries (the reproductive organs), the midgut (key for food digestion and immunity), or the salivary glands (essential for the transmission of vector-borne diseases as pathogens must colonize them to enter the saliva and reach the bloodstream during a blood meal). These newly reconstructed genomes can then pave the way for the development of novel vector biocontrol strategies. To this aim, it is required to isolate mosquito organs while avoiding cross-contamination between them or with microorganisms present in other mosquito organs. Here, we describe an optimized and contamination-free dissection protocol for studying mosquito microbiome at the organ level.
Introduction
Mosquitoes spread a wide range of pathogens causing diseases and are a serious threat to public health. Due to an increased prevalence of insecticide resistance among mosquito populations and in the absence of effective vaccines against these pathogens, new biocontrol methods that focus on the mosquito microbiome are emerging. In particular, the intracellular bacterium Wolbachia, which can interfere with pathogen transmission and manipulate host reproduction, stands out1,2,3. In addition, other mosquito symbionts are central to the survival, development, or immune system of their host, as well as in infection and transmission of pathogens, and show great promise for their exploitation to fight vector-borne diseases4,5,6,7,8.
Microorganisms associated with mosquitoes span all domains of microbial life (including bacteria, eukaryotes, and fungi) that interact intimately with their host but also with each other in the different body compartments9,10. Therefore, a better understanding of the microbiota, its potential seasonal variation11, and the mechanisms by which its members interact directly in distinct mosquito tissues can help develop new targeted biocontrol methods or improve existing ones. However, the majority of symbionts remain resistant to cultivation, rendering their characterization impossible.
The advent of second- and third-generation sequencing methods, together with state-of-the-art assembly and binning approaches, have enabled the reconstruction of microbial genomes and accessing the diversity and functional potential of non-cultivable microbes. Here, we present a method for the dissection of the ovaries, midgut, and salivary glands of female mosquitoes while preventing cross-contamination. This protocol can be followed by genomic DNA extraction and subsequent metabarcoding or shotgun metagenomic sequencing to explore the diversity and function of mosquito microbiota at the organ level. We provide an example of mosquito dissection and microbiome data for Culex spp. specimens although this protocol can be extended to vectors of other genera such as Anopheles or Aedes.
Protocol
NOTE: Figure 1 shows a schematic of the method summarizing the different steps of the protocol.
1. Material preparation
- Before starting the experiment, prepare a bleach solution with 0.5% active chlorine (recommended by the World Health Organization) to disinfect the equipment and the workbench.
- In 900 mL of water, dissolve three soluble tablets, each containing 1.5 g of active chlorine. Wait 15 min for the tablets to fully dissolve, resulting in a 0.5% bleach solution.
- Place the 0.5% bleach solution in a spray bottle. Then, spray the solution onto all dissection equipment, including microscopic slides and workbench.
NOTE: The equipment and workbench should be left to dry for ~10 min before use.
- To rinse the mosquitoes before each dissection to prevent contamination of the samples with external bacteria, prepare four 1.5 mL newly sterilized tubes: two containing 96% ethanol and two others containing 1x PBS buffer (phosphate-buffered saline).
- Prepare the tubes to store the samples after the dissection of each mosquito: four 1.5 mL sterile tubes, each containing 100 µL of preservation buffer.
NOTE: Prepare one tube for the salivary glands, one for the ovaries, one for the midgut, and one for the mosquito carcass.
2. Mosquito collection
- Collect and keep adult female individuals alive (from field or laboratory, 4-7 days) until surface sterilization.
3. Surface sterilization of mosquitoes
NOTE: Clean forceps and needles and use new, sterilized tubes between each organ and individual. If bleach is unavailable, ensure to thoroughly flame-sterilize the dissecting forceps and needles between the dissection of each individual and organ to prevent cross-contamination of biological material. Dissection instruments should be dry, without any bleach/ethanol residue, before the next use.
- To avoid females emptying their gut content when exposed to alcohol, cool-anesthetize them on ice or in a freezer at -20 °C (for 4 min so that the tissues and organs do not freeze).
- Transfer individual mosquitoes into a new sterilized 1.5 mL tube with 1 mL of 96% ethanol. Vortex gently (manually) for 30 s (shake the mosquito well in the tube to detach the scales and potential bacterial contaminants on the surface of the insects). Rinse twice using tubes containing ethanol.
- Transfer the mosquito into a new sterilized 1.5 mL tube with 1 mL of sterile 1x PBS solution and vortex gently (manually) for 30 s (to avoid DNA precipitation with ethanol). Rinse twice using tubes containing sterile 1x PBS.
4. Dissection of mosquitoes
- Place several small drops of sterile 1x PBS onto the slide to facilitate the placement of the mosquito and rinsing of dissected organs.
NOTE: The drops of sterile PBS should be large enough to accommodate the mosquito organs. - Take the cleaned mosquito and place it onto a drop of sterile 1x PBS on the microscope slide.
- Dissect the salivary glands with sterilized forceps and needle as described below.
- Hold the mosquito's thorax with tweezers to prevent it from moving. Then, place a needle at the neck, below the head, and gently pull on the mosquito's head to separate it from the thorax.
NOTE: The salivary glands are located in the upper thorax and are connected to the head by the salivary duct. During dissection, it is crucial not to break this duct as the salivary glands will not come with the head when you pull it from the thorax. This is why it is important to have a fresh mosquito, not a frozen one. - When the head separates from the thorax, observe the two salivary glands emerge, each composed of three lobes. Cut the salivary glands at the level of the salivary duct, retrieve them with a needle, and place them into a new drop of sterile 1x PBS to rinse it and prevent inter-organ contaminations.
- Retrieve the salivary glands using a needle and place them into a new sterilized tube containing 100 µL of preservation buffer.
- Hold the mosquito's thorax with tweezers to prevent it from moving. Then, place a needle at the neck, below the head, and gently pull on the mosquito's head to separate it from the thorax.
- Dissect the ovaries with sterilized forceps and needle as follows.
- Place the mosquito on its back. Using forceps to hold the mosquito by the thorax to prevent movement during dissection, puncture the third abdominal segment (starting from the bottom) with a needle.
NOTE: Optionally, remove the legs and wings of the mosquito to facilitate dissection. - While piercing the third abdominal segment, pull down the abdomen to open it and reveal the internal organs. Remove any remaining pieces of mosquito exoskeleton to facilitate the release of the organs.
NOTE: Organs in the abdomen such as the gut, Malpighian tubules, and ovaries are visible. - Retrieve both ovaries with sterilized forceps and place them in a new drop of sterile 1x PBS on the microscope slide to rinse them. Grab the ovaries and place them in the 1.5 mL tube with the 100 µL of preservation buffer.
- Place the mosquito on its back. Using forceps to hold the mosquito by the thorax to prevent movement during dissection, puncture the third abdominal segment (starting from the bottom) with a needle.
- Now that the abdomen is open and the organs are exposed, retrieve the midgut with new sterilized forceps and/or needle.
- Separate the midgut from the foregut and hindgut; then, remove the Malpighian tubules from the midgut.Grab the midgut using a sterile needle and place it on a new sterile 1x PBS drop to rinse it and prevent inter-organ contaminations.
- Transfer to a new sterile 1x PBS drop. Open the midgut with forceps to free and enrich for bacteria as described in Supplementary File 1. Grab the midgut with forceps and pipette all the PBS containing bacteria to store the entire material (liquid and tissue) in a new sterilized 1.5 mL tube with 100 µL of preservation buffer.
- Add again 5 µL of 1x PBS on the previously used place in the microscope slide and directly pipette back PBS to the same 1.5 mL tube to retrieve bacteria that may have stuck to the microscope slide.
- Use sterile forceps to grasp the carcass (remaining body) and store it in a new sterilized 1.5 mL tube with 100 µL of preservation buffer.
- Storage of material until further processing
- Label tubes with 'Species, Organ, Date, Researcher's name'.
- Store dissected ovaries, midgut, salivary glands, and carcass at -20 °C until processing.
- Keep track in a spreadsheet of all associated metadata for the samples, including Species (or genus level only), Collection date, Country, Province, Latitude, Longitude, Name of the person who did the dissection, Gravid female or not, Blood meal or not.
Representative Results
x`Mosquito dissections
Following the protocol, we collected and isolated the ovaries, midgut, salivary glands, and the carcass from two Culex pipiens molestus specimens (including a gravid female) from a laboratory colony. We confirmed clean dissections following the observation of entire (that is, unbroken) and well-isolated organs with no remaining debris under the binocular. The whole body, head, and thorax, dissected salivary glands, ovaries, and midgut of a Culex pipiens molestus specimen are shown in Figure 2. As expected, the midgut and salivary glands were an order of magnitude smaller than the mosquito ovaries. The eggs of a gravid Culex specimen, together with its midgut and Malpighian tubules, are presented in Figure 3. Of note, dissections are more prone to fail when the material is not fresh and tissue is likely to break (Figure 4B,C). We therefore suggest dissecting material right after collection (within less than 12 h) when possible as tissues are still elastic (Figure 4A). Similarly, dissection of frozen material can be performed but there is a much higher risk of failure and cross-contamination between organs due to fragile tissues (Figure 4).
Microbiome data
In addition, we collected and separated the midguts and ovaries from three individual Culex quinquefasciatus specimens from Noumea, New Caledonia, following the same procedure. We extracted DNA from each organ, prepared samples for whole genome sequencing, and performed microbiome data analyses as detailed in Supplemental File 1. A bacterial taxonomic diversity analysis on unassembled quality-filtered short reads using PhyloFlash12 showed distinct dominant taxa in midguts compared to ovaries (Figure 5A). Notably, ovarian bacterial communities were dominated by Wolbachia, with the additional presence of a Spirochaetaceae phylotype in the ovaries of individual 3, while midgut communities exhibited a wider diversity, including Gammaproteobacteria, Spirochaetaceae, and Firmicutes. From the same sequencing data, we reconstructed four Metagenome-Assembled Genomes (MAGs) with completion > 80% and redundancy < 5%, belonging to classes Spirochaetia, Alpha-, and Gammaproteobacteria (Table 1).
As expected, MAGs reconstructed herein did not cover the full taxonomic diversity predicted by the PhyloFlash results due to the specific shortcomings of genome reconstruction using metagenomic short-reads. The MAG assigned to Wolbachia (Ref. MAG 3 in Table 1) was detected in all ovaries and two of the midguts and had higher coverage in the ovaries (Figure 5B). We also reconstructed two MAGs belonging to the Enterobacteriaceae family, including genus Pantoea (Ref. MAG 1 and 2 in Table 1), in the midguts obtained from Culex individuals 2 and 3, that were not detected in the corresponding ovaries (Figure 5B). Finally, we also reconstructed one Spirochaetaceae bacterial genome, Ref. MAG 4 (Table 1), assigned to genus BR149 that was successfully isolated from Culex pipiens midguts by Graña-Miraglia and colleagues13. Interestingly, this MAG was detected in the midguts of individuals 1 and 3, as well as in the ovaries of individual 3 (Figure 5B).
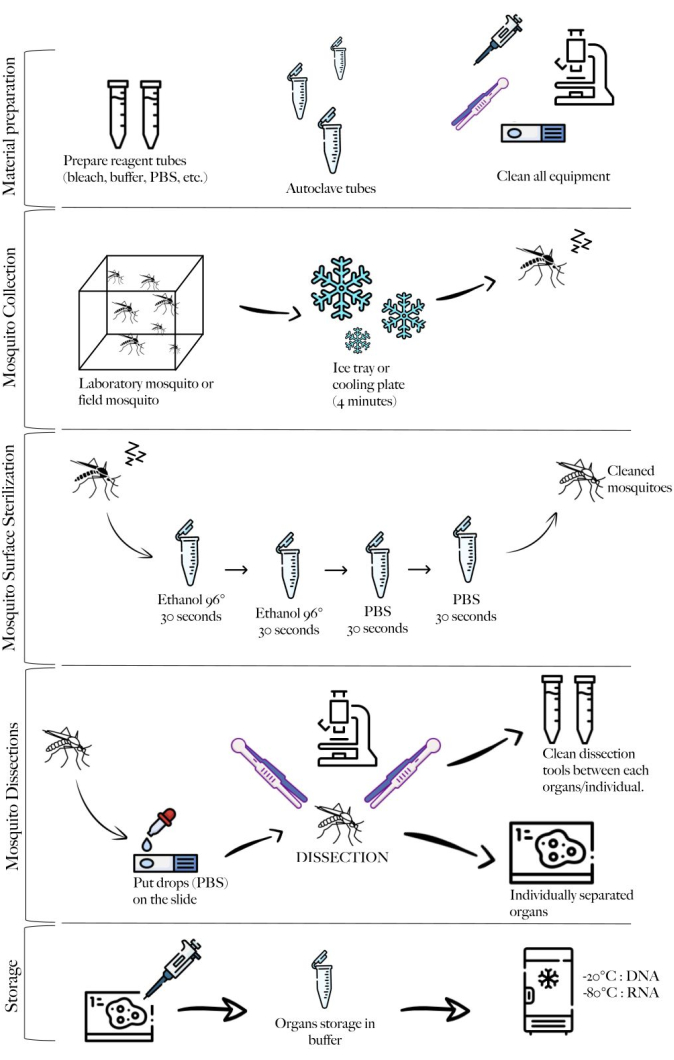
Figure 1: Schematic of the method summarizing the different steps. Material preparation, mosquito collection, mosquito cleaning, mosquito dissections, and storage. Please click here to view a larger version of this figure.
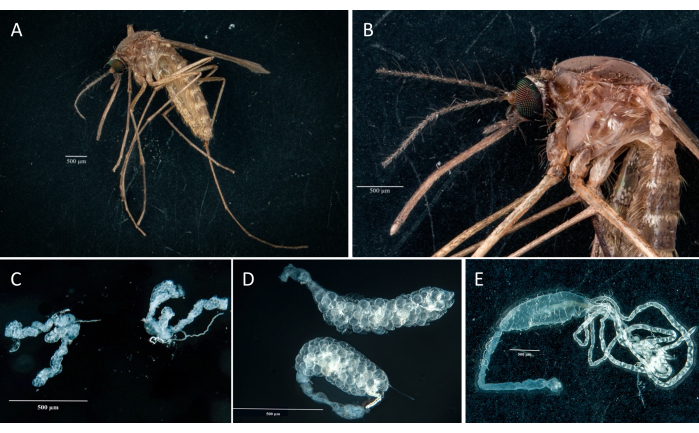
Figure 2: Culex pipiens molestus female. (A) Whole body. (B) Head and thorax. (C) Dissected salivary glands. (D) Dissected ovaries. (E) Dissected gut with Malpighi tubules. Scale bars = 500 µm. Please click here to view a larger version of this figure.
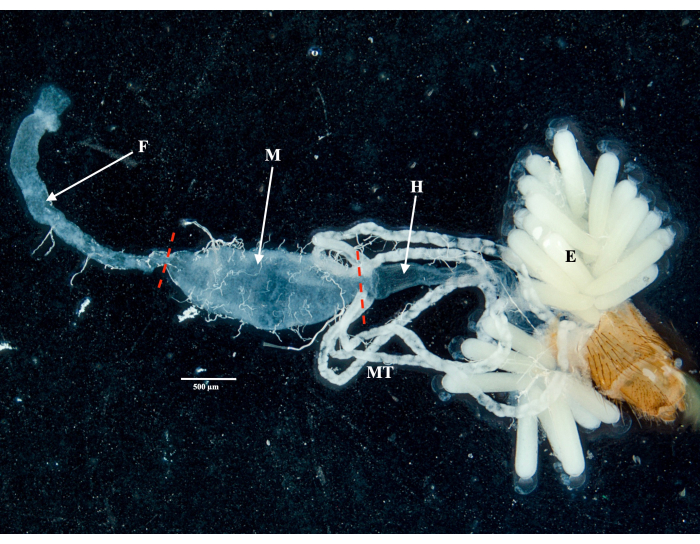
Figure 3: Dissected abdomen of a gravid Culex pipiens molestus female. The foregut, midgut, hindgut, Malpighian tubules, and eggs of the specimen are shown. Red dashes indicate where to cut to separate the midgut from the foregut and hindgut. Scale bar = 500 µm. Abbreviations: F = foregut; M = midgut; H = hindgut; E = eggs; MT = Malpighian tubules. Please click here to view a larger version of this figure.
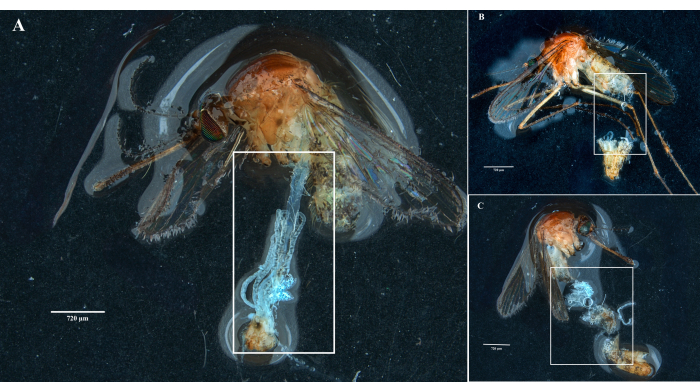
Figure 4: Dissection of fresh and frozen Culex mosquito specimens. White rectangles illustrate (A) mosquito tissues with intact organs from a freshly dissected specimen and (B,C) broken biological material from specimens that have been frozen before dissection. Scale bars = 720 µm. Please click here to view a larger version of this figure.
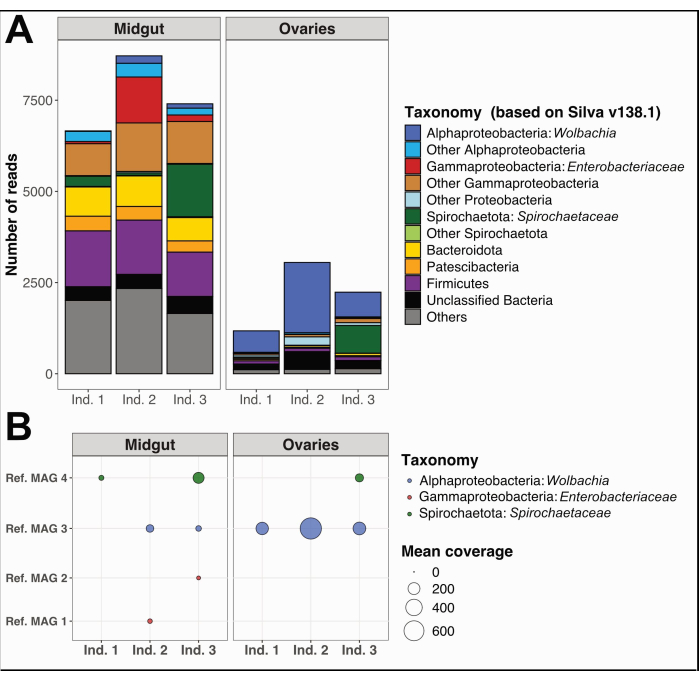
Figure 5: Example microbiome analysis on three Culex quinquefasciatus individuals. (A) Visualization of bacterial diversity estimated through the extraction of SSU rRNA reads with PhyloFlash12 in midguts and ovaries of the three specimens. (B) Mean coverage of the reconstructed MAGs over the samples. Abbreviations: MAG = Metagenome-Assembled Genome; Ind = Individual. Please click here to view a larger version of this figure.
| MAG | Ref. MAG 4 | Ref. MAG 2 | Ref. MAG 1 | Ref. MAG 3 |
| Length (bp) | 1,287,790 | 4,910,866 | 4,751,276 | 1,298,266 |
| Number of contigs | 9 | 162 | 149 | 123 |
| GC % | 34.05 | 55.45 | 54.05 | 34.15 |
| Completion (%) | 84.5 | 97.18 | 98.59 | 91.55 |
| Redundancy (%) | 0 | 2.82 | 4.22 | 0 |
| Domain | Bacteria | Bacteria | Bacteria | Bacteria |
| Phylum | Spirochaetota | Proteobacteria | Proteobacteria | Proteobacteria |
| Class | Spirochaetia | Gammaproteobacteria | Gammaproteobacteria | Alphaproteobacteria |
| Order | WRBN01 | Enterobacterales | Enterobacterales | Rickettsiales |
| Family | WRBN01 | Enterobacteriaceae | Enterobacteriaceae | Anaplasmataceae |
| Genus | BR149 | Pantoea | – | Wolbachia |
Table 1: Reconstructed MAGs from the three Culex quinquefasciatus individuals. Genome size, number of contigs, proportion of GC, estimates of completion, and redundancy based on the single-copy core gene collection available in Anvi'o17 and taxonomy obtained using GTDB18.
Supplemental File 1: Detailed example procedure for microbiome data analysis starting from sample collection, DNA extraction, and whole genome sequencing followed by the bioinformatic workflow for genome reconstruction and estimation of prokaryotic and eukaryotic read proportion Please click here to download this File.
Discussion
We recommend paying particular attention to the sequence of organ dissection, starting with the salivary glands. Indeed, we observed that they were more easily extracted from the thorax of Culex specimens if the mosquito's integrity was preserved. Damage to the abdomen or thorax could reduce pressure in the mosquito's body, impeding the procedure. It is, however, also possible to cut between the thorax and the abdomen and then pull out the salivary glands from the head and thorax (A. B. Failloux, personal communication). Additionally, acquiring proficient dissecting skills can be challenging, so we suggest practicing on an adequate number of specimens prior to the experiment.
Isolating mosquito organs while avoiding cross-contamination in a systematic manner is crucial for a wide range of downstream mosquito microbiota analyses. A genome-resolved metagenomic study following the dissection of single ovaries from Culex pipiens specimens in Southern France allowed the discovery of the first plasmid of Wolbachia de Culex pipiens (pWCP14). Using a similar approach, we investigated the distribution and frequency of pWCP in Culex pipiens and Culex quinquefasciatus specimens from both continental and island areas worldwide, across various environmental and laboratory conditions. Overall, the data revealed a remarkably conserved Wolbachia plasmid element in Culex mosquitoes, suggesting a crucial role for this mobile element in endosymbiont biology15, warranting further analysis.
Here, we provide additional examples of mosquito microbiome analyses on midgut and ovary samples obtained using this systematic procedure. We observed a clear difference in microbiota between tissues (Figure 5), with both shared and organ-specific bacterial taxa. As expected, the presence of Wolbachia was detected in both organs, with higher relative abundance (based on unassembled short reads) and mean coverage of MAGs in the mosquito ovaries compared to the midguts, consistent with the observation that this endosymbiont is transmitted through the ovaries and subsequently spreads to the somatic tissues. Although this study was restricted to samples from New Caledonia, this protocol may facilitate the investigation of Wolbachia's genomic variability on a global scale, as well as its role in various phenotypes, including density regulation of itself and viral protection. Moreover, this work exemplifies how the dissection procedure presented here permits the examination of the taxonomic diversity and potential functional capabilities of mosquito symbionts within midgut samples.
We obtained two enterobacterial draft genomes that were only present in two midgut samples, confirming the lack of contamination between organs for these two specimens. Regarding spirochetes, detected both in the midgut and the ovaries of individual 3, Juma and colleagues, 2020 observed the presence of these bacteria on the surface of egg rafts. The authors suggested that the bacterial communities found in the egg rafts might primarily be maternally inherited from the ovaries, given that the egg rafts were kept in deionized and bacteria-free water. However, they could not rule out the possibility of bacterial colonization occurring right after oviposition and recommended further study on the ovary microbiome16.
While initially designed for specimens of the Culex pipiens species complex, we foresee the applicability of this protocol to a broader community of medical entomologists studying other vectors such as Anopheles or Aedes. By operating at the level of individual organs, this method may enable both intra- and inter-individual genomic comparisons, offering insights into symbiont genomic variability at a fine scale, with the potential of advancing vector control strategies. This method of dissection and isolation of the salivary glands, ovaries, and midgut, preventing microbial cross-contamination, could also be a useful protocol for viral infection dynamics studies within these three organs.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We thank Gilbert Legoff for teaching Jordan Tutagata how to dissect salivary glands from mosquitoes and Giuliano Mucci for helping with photos of the mosquito organs. We thank Anna-Bella Failloux and Nonito Pages for helpful discussion on the protocol. This work was supported by the ERC RosaLind Starting Grant “948135” to JR. We thank the Vectopole platform (IRD, Montpellier) for providing technical support and for the rearing and maintenance of the mosquito populations.
Materials
| Alcohol 96° | Fisher scientific | 10332562 | |
| Binocular magnifier | |||
| Bleach | RAJA | 145517 | 150 tablets of 1.5 g |
| DNA prep kit | Illumina | Provided by MGX sequencing platform | Previously known as Nextera DNA Flex |
| DNA-RNA Shield (50 mL) | Zymo research | ZR1100-50 | Preservation buffer |
| DNeasy Blood and Tissue Kit | Qiagen | 69504 | DNA extraction kit |
| Filter tips 20 µL | Starlab | S1120-3810 | |
| Filter tips 200 µL | Starlab | S1120-8710 | |
| Filter tips 1000 µL | Starlab | S1122-1730-C | |
| Forceps | FST (Fine Science Tools) | 11252-20 | Dumont Forceps #5 |
| Library quantification kit | Roche | Provided by MGX sequencing platform | KAPA Library Quanitification Kits |
| Micropipettes 2-20 µL | Eppendorf | 6.291704 | |
| Micropipettes 20-200 µL | Eppendorf | 6.291703 | |
| Micropipettes 100-1000 µL | Eppendorf | 7.648488 | |
| Microscope slides | Epredia | J1800BMNZ | dimension : 75 mm x 50 mm |
| Needles | Terumo | AN*2719R1 | |
| NGS kit | Agilent | Provided by MGX sequencing platform | Fragment Analyzer Systems HS Genomic DNA 50kb Kit |
| NovaSeq 6000 | Illumina | Provided by MGX sequencing platform | Sequencer |
| PBS Phosphate Buffered Saline (Sterile) | Fisher scientific | 10212990 | |
| Permanent black marker | |||
| Sterile Eppendorf | Dutscher | 33871 | 1.5 mL |
| Support for needles | FST (Fine Science Tools) | 26016-12 | Moria MC1 Pin Holder 12 cm |
Referencias
- Achee, N. L., et al. Alternative strategies for mosquito-borne arbovirus control. PLOS Negl Trop Dis. 13 (1), e0006822 (2019).
- Hoffmann, A. A., et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 476 (7361), 454-457 (2011).
- Porter, J., Sullivan, W. The cellular lives of Wolbachia. Nat Rev Microbiol. 21 (11), 750-766 (2023).
- Coon, K. L., Brown, M. R., Strand, M. R. Mosquitoes host communities of bacteria that are essential for development but vary greatly between local habitats. Mol Ecol. 25 (22), 5806-5826 (2016).
- Didion, E. M., et al. Microbiome reduction prevents lipid accumulation during early diapause in the northern house mosquito, Culex pipiens pipiens. J Insect Physiol. 134, 104295 (2021).
- Gabrieli, P., et al. Mosquito trilogy: Microbiota, immunity and pathogens, and their implications for the control of disease transmission. Front Microbiol. 12, 630438 (2021).
- Garrigós, M., Garrido, M., Panisse, G., Veiga, J., Martínez-de La Puente, J. Interactions between West Nile Virus and the microbiota of Culex pipiens vectors: A literature review. Pathogens. 12 (11), 1287 (2023).
- Zheng, R., et al. Holobiont perspectives on tripartite interactions among microbiota, mosquitoes, and pathogens. ISME J. 17 (8), 1143-1152 (2023).
- Flores, G. A. M., et al. Wolbachia dominance influences the Culex quinquefasciatus microbiota. Sci Rep. 13 (1), 18980 (2023).
- Hegde, S., et al. Interkingdom interactions shape the fungal microbiome of mosquitoes. Anim Microbiome. 6 (1), 11 (2024).
- Suo, P., et al. Seasonal variation of midgut bacterial diversity in Culex quinquefasciatus populations in Haikou city, Hainan province, China. Biología. 11 (8), 1166 (2022).
- Gruber-Vodicka, H. R., Seah, B. K. B., Pruesse, E. phyloFlash: Rapid small-subunit rRNA profiling and targeted assembly from metagenomes. mSystems. 5 (5), e00920-e00920 (2020).
- Graña-Miraglia, L., et al. Spirochetes isolated from arthropods constitute a novel genus Entomospira genus novum within the order Spirochaetales. Sci Rep. 10 (1), 17053 (2020).
- Reveillaud, J., et al. The Wolbachia mobilome in Culex pipiens includes a putative plasmid. Nat Commun. 10 (1), 1051 (2019).
- Ghousein, A., et al. pWCP is a widely distributed and highly conserved Wolbachia plasmid in Culex pipiens and Culex quinquefasciatus mosquitoes worldwide. ISME Commun. 3 (1), 40 (2023).
- Juma, E. O., Kim, C. -. H., Dunlap, C., Allan, B. F., Stone, C. M. Culex pipiens and Culex restuans egg rafts harbor diverse bacterial communities compared to their midgut tissues. Parasit Vectors. 13 (1), 532 (2020).
- Lee, M. D. GToTree: a user-friendly workflow for phylogenomics. Bioinformatics. 35 (20), 4162-4164 (2019).
- Parks, D. H., et al. A complete domain-to-species taxonomy for bacteria and Archaea. Nat Biotechnology. 38 (9), 1079-1086 (2020).

.
