Using Flight Mills to Measure Flight Propensity and Performance of Western Corn Rootworm, Diabrotica virgifera virgifera (LeConte)
Summary
Flight mills are important tools for comparing how age, sex, mating status, temperature, or various other factors may influence an insect’s flight behavior. Here we describe protocols to tether and measure the flight propensity and performance of western corn rootworm under different treatments.
Abstract
The western corn rootworm, Diabrotica virgifera virgifera (LeConte) (Coleoptera: Chrysomelidae), is an economically important pest of corn in the northern United States. Some populations have developed resistance to management strategies including transgenic corn that produces insecticidal toxins derived from the bacterium Bacillus thuringiensis (Bt). Knowledge of western corn rootworm dispersal is of critical importance for models of resistance evolution, spread, and mitigation. Flight behavior of an insect, especially over a long distance, is inherently difficult to observe and characterize. Flight mills provide a means to directly test developmental and physiological impacts and consequences of flight in the laboratory that cannot be obtained in field studies. In this study, flight mills were used to measure the timing of flight activity, total number of flights, and the distance, duration, and speed of flights taken by female rootworms during a 22-h test period. Sixteen flight mills were housed in an environmental chamber with programmable lighting, temperature, and humidity control. The flight mill described is of a typical design, where a flight arm is free to rotate about a central pivot. Rotation is caused by flight of an insect tethered to one end of the flight arm, and each rotation is recorded by a sensor with a time-stamp. Raw data are compiled by software, which are subsequently processed to provide summary statistics for flight parameters of interest. The most difficult task for any flight mill study is attachment of the tether to the insect with an adhesive, and the method used must be tailored to each species. The attachment must be strong enough to hold the insect in a rigid orientation and to prevent detachment during movement, while not interfering with natural wing motion during flight. The attachment process requires dexterity, finesse, and speed, making video footage of the process for rootworms of value.
Introduction
The western corn rootworm, Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae), was identified as a pest of cultivated corn in 19091. Today, it is the most important pest of corn (Zea mays L.) in the U.S. Corn Belt, with larval feeding on corn roots causing most of the yield loss associated with this pest. The annual costs for management and corn production losses due to corn rootworm are estimated to exceed $1 billion2. The western corn rootworm is highly adaptable, and populations have evolved resistance to multiple management strategies including insecticides, crop rotation, and transgenic Bt corn3. Determining spatial dimensions over which tactics must be applied to mitigate local development of resistance, or a resistance hotspot, depends on a better understanding of dispersal4. Mitigation measures will not be successful if they are restricted to too small of a spatial scale around a resistance hotspot, because resistant adults will disperse beyond the mitigation area5. Understanding flight behavior of western corn rootworm is important to create effective resistance management plans for this pest.
Dispersal by flight plays an important role in adult western corn rootworm life history and ecology6, and the flight behavior of this pest can be studied in the laboratory. Several methods may be used to measure flight behavior in the laboratory. An actograph, which restricts flight in a vertical plane, can measure the amount of time an insect is engaged in flight. Actographs have been used to compare flight duration and periodicity patterns of western corn rootworm males and females at different ages, body sizes, temperatures, insecticide susceptibility, and insecticide exposure7,8,9. Flight tunnels, which consist of a tracking chamber and directed air flow, are especially useful for examining insect flight behavior when following an odor plume, such as candidate pheromone components10 or plant volatiles11. Flight mills are perhaps the most common method for laboratory studies of insect flight behavior and can characterize several aspects of flight propensity and performance. Laboratory flight mills have been employed in studies of western corn rootworm to characterize propensity to make short and sustained flights as well as hormonal control of sustained flight12,13.
Flight mills provide a relatively simple way to study insect flight behavior under laboratory conditions by allowing researchers to measure various flight parameters including periodicity, speed, distance, and duration. Many of the flight mills used today are derived from the roundabouts of Kennedy et al.14 and Krogh and Weis-Fogh15. Flight mills can be different in shape and size, but the basic principle remains the same. An insect is tethered and mounted on a radial horizontal arm that is free to rotate, with minimal friction, about a vertical shaft. As the insect flies forward, its path is restricted to circling in a horizontal plane, with the distance traveled per rotation dictated by the length of the arm. A sensor is typically used to detect each rotation of the arm caused by the flight activity of the insect. Raw data include rotations per unit time, and time of day flight occurred. The data are fed into a computer for recording. Data from multiple flight mills are often recorded in parallel, essentially simultaneously, with banks of 16 and 32 flight mills being common. The raw data are further processed by custom software to provide values for such variables as flight speed, total number of separate flights, distance and duration flown, and so forth.
Every insect species is different when it comes to the best method for tethering because of morphological variables such as overall size, size and shape of the target area for attaching the tether, softness, and flexibility of the insect, need and method for anesthetization, potential for fouling the wings and/or head with misplaced or overflow adhesive, and many, many more details. In the cases of visualized tethering of a plataspid bug16 and an ambrosia beetle17, the respective target areas for tether attachment are relatively large and forgiving of imprecise adhesive placement because the head and wings are somewhat well-separated from the attachment site. This is not to downplay the difficulty of tethering these insects, which is demanding for any species. But the western corn rootworm is a particularly challenging insect to tether: the pronotum is narrow and short, making very precise attachment with a minimal amount of adhesive (dental wax in this case) necessary to prevent interference with the opening of the elytra for flight and with the head, where contact with eyes or antennae can affect behavior. At the same time, the tether must be firmly attached to avoid dislodgement by this strong flyer. The demonstration of tethering of rootworm adults is the most important offering in this paper. It should be of help to others who work with this or similar insects where the method visualized here could be a useful option.
This paper describes methods used to effectively tether and characterize the flight activity of western corn rootworm adults that were reared at different larval densities. The flight mills and software used in this study (Figure 1) were derived from designs posted on the internet by Jones et al.18 Tethering techniques were modified from the description in Stebbing et al.9 An array of 16 flight mills was housed in an environmental chamber, designed to control lighting, humidity, and temperature (Figure 2). Using this or similar setup along with the following techniques allows for testing factors that may influence the flight propensity and performance of western corn rootworm, including age, sex, temperature, photoperiod, and many others.
Protocol
1. Rear western corn rootworm for flight tests
NOTE: If the adult’s age must be controlled or known, adults must first be collected in the field followed by rearing their offspring to adulthood for testing. If the age of the beetle or a standardized rearing environment is not of concern, then directly testing field-collected adults may be possible, and the protocol can begin with step 2.
- Collect at least 500 western corn rootworm adults from a cornfield of interest to ensure enough eggs are obtained for rearing adequate numbers of adults. Use a manual aspirator to collect adults from the field.
NOTE: It is recommended to collect adults during peak abundance, around late July in the U.S. Corn Belt, to ensure the collection of both sexes. Most adults will be males if collected earlier, whereas most will be females if collected later. - Place the collected male and female adults into a mesh cage containing chopped corn ear, corn leaf tissue, 1.5% agar solid, and an oviposition substrate. An 18 x 18 x 18-cm cage (mesh size 44 x 32, 650 µm aperture) can hold up to 500 adults at one time.
- Use the corn grown in the field as a source of corn ear, which will be picked at the R3, or milk stage of kernel development19. The R3 kernel is yellow outside, while the inner fluid is milky white due to accumulating starch. Corn ears can be frozen and stored for up to a year until they are needed. To feed the rootworm, remove the husk and chop the corn into horizontal cross-sections about 3 cm thick. Chopped corn is the primary diet for the adults and should be changed out twice a week.
- Obtain leaves from greenhouse-grown corn plants of any age. Amount of leaf tissue will vary with the number of adults in the cage. Avoid using field plants, as they may introduce disease.
- To make the solid agar, mix 15 g of agar powder with 1 L of DI water. Heat the mixture until boiling. Pour the liquid into Petri dishes (100 mm x 15 mm) while it is hot. Place a lid on the Petri dish once cool and place them into cold storage (6° C). Agar provides adults with a source of moisture and should be changed out twice a week.
- To prepare an oviposition substrate, place 40 g of sieved field soil (<180 µm) into a Petri dish. Moisten the soil with deionized water. Ensure that the soil at the bottom of the Petri dish appears wet. Score the top of the moistened soil with a needle tool. Remove the oviposition substrate weekly and place in an incubator at 25° C and 60% RH for at least one month.
- After incubating eggs for one month, wash the contents of the oviposition substrate through a 250-µm sieve until all soil has been removed. Quantify the eggs by placing washed eggs in a 10-mL graduated cylinder. There are approximately 10,000 eggs per 1 mL.
- Place the quantified eggs into a 44-mL container and cover with sieved field soil (<180 µm). Western corn rootworm eggs undergo obligate diapause through the winter20. To break diapause, place eggs into cold storage (6° C) for at least 6 months.
NOTE: Eggs may be kept in cold storage for longer than 5 months, but egg viability decreases with time. After 12 months, there may be little to no hatch. - After a minimum of 5 months, remove eggs from the cold storage and place in an incubator at 25° C and 60% RH. Neonates hatch as early as 16 days after removal from cold storage.
- Once the eggs hatch, place three germinated kernels at the bottom of a 44-mL plastic container with roots exposed (i.e., not covered with soil). Use a soft bristle brush to transfer 12 neonates to the surface of the roots.
- Add 4.5 mL of DI water to 40 mL of sieved soil (<600 µm). Place the moistened soil over the germinated kernels that have been infested with neonates and cover the container with mesh fabric to prevent larvae from escaping.
- On the same day that the 44-mL plastic container is set up with neonates, prepare a 473-mL container with corn kernels that have not germinated. The rootworm larvae will be transferred to this larger container later. The number of kernels determines the desired larval density per plant. Add 120 g of soil mixture consisting of 50% sieved field soil (<600 µm) and 50% potting soil moistened with 20 ml of deionized water.
- After 7 days, transfer all contents of the 44-mL container to the 473-mL container. The larvae will be second instars at the time of transfer.
NOTE: This transfer to a larger container is necessary to supply larvae with enough root mass for feeding through pupation. - Observe the emergence of adults typically around 26 days after egg hatch. Adults are active fliers upon emergence and may escape the 473-mL container when attempting to collect them by hand. Instead, use a vacuum with an aspirator to collect adults.
- Segregate adults by sex and/or date if needed for comparative testing. Sex of western corn rootworm can be determined by observing the morphology of the prothoracic basitarsi21. Males have broad, square-shaped prothoracic basitarsi, whereas those of females are narrow and conical-shaped.
- Place beetle into a 45-mL clear polystyrene plastic vial and cover with a lid with 6 small (~1 mm diameter) holes.
- Anesthetize the beetle. Place the end of a tube attached to a CO2 tank regulator over the holes in the lid and allow a gentle flow of CO2 to enter the tube for approximately 10 to 15 s until the adult loses its grip on the wall of the vial.
NOTE: The anesthetized insect will remain immobilized for about 1 min. - Place the anesthetized beetle, ventral side up, on an inverted plastic petri dish bottom. Carefully place the non-inverted lid of the petri dish over the beetle. Ensure that the tarsi of the beetle press against the lid, allowing easy observation of the prothoracic basitarsi under a dissecting microscope.
- If the experiment requires that beetles be mated prior to flight, then use males at least 5 days old to mate with the newly emerged females.
NOTE: Use of older males ensures that they are sexually mature upon their introduction to virgin females. Females are sexually mature upon adult emergence, whereas males require 5 to 7 days of post-emergence development to reach sexual maturity22,23.
2. Start the flight mill software program prior to flight testing
NOTE: The flight mill program files (.vi file extensions which run in a commercial software platform, see Table of Materials) and details for their use are provided for download via links ("data analysis routine" and "Circular Flight Mill Instructions", respectively) in the "Flight mill wiring and software" section on the Jones et al.18 website. If the programs no longer function in newer or future versions of the software platform, or if the user wants to add new capabilities, the routines provided by Jones et al. 18 can be modified by the user as needed.
- Open the flight mill software program (Figure 3).
- Enter the information under the Initialization tab.
- Set the Start Time and End Time for the desired duration of the flight test.
NOTE: All adults should be tethered and mounted on flight mills by 30 min prior to the Start Time. It may take an experienced person 30 min to 45 min to tether and prepare 16 beetles for flight testing (see Section 3). - Set the Min Threshold (min) to 0. This ensures that any detection of the flight arm passing will be recorded, and is the default recommended by Jones et al. 18.
- Set the Max Threshold (min) to 1. Here, 1 min was used. This value means that 1 min must elapse between sensor detection of the flight arm to "call" the end of a flight.
- Enter a name for the file.
- Set Raw Data Log Interval (min) to 1. This value controls the interval over which the raw data will be compiled for output reporting. Here, it is set to 1 min. Thus, the output of revolutions, for example, will be logged per minute.
NOTE: The actual time interval between electronic scanning of sensor activity is very short, but a 1-min interval allows logging at a fine enough scale for most research purposes, while restricting the number of lines in the spreadsheet output to a reasonable number for examining by eye.
- Set the Start Time and End Time for the desired duration of the flight test.
- Under the Subject Information tab, fill in the columns labeled ID, diet, sex, species, and comments as desired.
- Click on the START button located on the left side of the screen display. The program will begin collecting raw data once the Current Time matches the Start Time.
3. Tether western corn rootworm to flight mill
- Bend a 40-mm length 28-gauge steel wire 90° at the center.
NOTE: The wire may also be of another metal such as copper or brass. - Take a small amount of dental wax, slightly larger than a pinhead, and roll it between the fingertips until a ball is formed. Ensure that fingers are clean to prevent debris, dirt, and oil from incorporating into the wax, because it may prevent the wax from adhering to the insect.
- Push one end of the 40 mm the bent wire into the center of the ball of wax.
- Anesthetize the test adult with CO2 as described above (see 1.11.1 and 1.11.2).
- Place the anesthetized adult on a flat surface and position its dorsal side up. If the beetle does not lie completely flat on the surface, reposition the legs so that it does. It is important that the beetle lie as flat as possible on the surface to ensure the correct positioning of the wire.
- Briefly (< 1 s) heat the dental wax on the wire with a butane lighter. If the wax is heated for too long, the melted wax will drop off of the wire. Do not reuse the wax if it has fallen off from the wire, as it will not effectively adhere to the insect cuticle.
- Carefully place the end of the steel wire with the melted dental wax on the dorsal surface of the pronotum, while pointing the other end of the wire, (i.e., the end without dental wax), along the midline of the abdomen. Alternatively, point the end of the wire without the dental wax toward the head if desired. In that case, a flying beetle will push the flight arm instead of pulling it. Be sure that the melted wax does not get on the elytra or its sutures, as it may prevent or hinder flight.
- Place the free end of the wire into the opening of the hollow metal tube of the flight mill arm. Ensure that the wire fits tight enough to hold in place by friction. The tethered beetle may be positioned to fly either clockwise or counter-clockwise.
- Immediately after mounting a beetle, tear a small piece (~1-cm dia) of tissue paper from a larger tissue. Offer the tissue piece to the tethered beetle hanging from the flight mill for tarsal contact; most beetles will grasp the tissue and hold it against gravity until they release it at the beginning of their first flight activity. This will greatly reduce initial escape or landing flight behavior.
NOTE: Human presence in the flight-testing room should be limited to attaching and removing adults from the flight mills. The test period usually does not begin until at least 30 min have elapsed since attachment (see Note under 2.2.1), and humans should not be present in the flight room during this time or during the test period itself. - Remove all flight-tested adults after completion of a flight mill test. Remove the wax bead connecting the tether to the pronotum by gently peeling the wire away from the pronotum. The wax will separate easily without damaging the cuticle, making the insect available for further experimentation if desired.
4. Save the data collected from the flight mill program.
- The program may be set to either MANUAL or AUTO. If the program is set to manual, then the user must end the program by clicking the STOP button. If the program is set to AUTO, then the program will stop collecting raw data once the Current Time matches the End Time.
- Click EXIT after the flight-testing period has ended.
- Ensure that a TDMS file is saved under the file name entered during program initialization (step 2.2).
- Click on the TDMS file and save the document as a spreadsheet (.xlsx).
5. Retrieve flight parameters from the saved spreadsheet (.xlsx)
NOTE: A spreadsheet can be custom designed to manipulate the raw data output from the flight mill software. Here, the software program was the same as described by Jones et al. 18, but an additional routine was added to recognize and summarize the longest uninterrupted flight by an individual insect during the test period.
- For each individual that engaged in flight activity, the spreadsheet will include the following information: flight number, total revolutions, start time, end time, and flight duration in minutes.
- To calculate the total distance flown during the test period, sum the column labeled ‘Total Revs’ and multiply it by the distance flown per revolution. Distance per revolution depends on the length of the flight arm from the central pivot to the attached insect. For example, if this distance is 15.9 cm, each revolution is equivalent to one meter flown. The total number of revolutions may also be found in the ‘Test Stats’ tab.
- To calculate the total duration flown during the test period, sum the column labeled ‘Flight Duration (min)’.
- To determine the distance and duration of the longest uninterrupted flight, go to the ‘Test Stats’ tab and look under the column labeled ‘Longest Flight #’.
- Flight speed can be calculated by dividing distance flown by flight duration. For insects, speed is commonly expressed in m/s or km/h.
Representative Results
Figure 4 shows representative examples of outputs expected after flight testing. Flight data were obtained from experimental work conducted in the Department of Entomology at Iowa State University. Six-day-old, mated female western corn rootworm adults were tethered to flight mills and placed in a controlled environmental chamber set at 14:10 L:D, 60 % RH, and 25° C. The beetles were left on the flight mills for 22 consecutive hours beginning 30 min before initiation of simulated dawn, and their flight activity was recorded (Figure 4). Dawn and dusk were simulated by a programmed, gradual change in light intensity from full-off to full-on at dawn (or vice-versa at dusk) over a 30-minute period. The first tab in the resulting spreadsheet summarizes the individual adults that were tested, using information entered from step 2.3. The subsequent tabs include flight data for each individual. The last two tabs are labeled ‘RAW DATA’ and ‘Test Stats’. ‘RAW DATA’ includes time of flight activity for all individuals. ‘Test Stats’ indicates the longest uninterrupted flight for each beetle, and summaries of the duration of the longest uninterrupted flight in minutes, the total time spent in flight during the test period in minutes, and the total number of revolutions during the test period. Time stamps for beginning and end of each independent flight allow analyses of flight periodicity.
For the female beetle tethered to flight mill #2 (Figure 4B), the spreadsheet displays the number of flights, total revolutions per flight, start and end time of each flight, and the duration of each flight. This female engaged in several independent flights, most of which were very short. However, in flight #5 the female traveled 1,258 m (which equals the number of revolutions in this case, because the distance per revolution was 1 m) over a 37.8-min period of uninterrupted flight. The female beetle tethered to flight mill #1 (Figure 4C) did not engage in flight during the test period, so a blank spreadsheet is displayed.
As an example, results are presented from a simple comparison of flight characteristics between two groups of female western corn rootworm. Adults were collected in commercial cornfields from two locations in Iowa and allowed to oviposit in the laboratory. Eggs were collected, and offspring reared as described in Step 1 of the protocol at a post-neonate density (step 1.9) of 12 larvae per 36 seedlings. The resulting adult females were tethered and tested as described in Steps 2 and 3. Table 1 shows a summary of the flight parameters from the raw data retrieved from the flight mill software as described in Steps 4 and 5. Total flight parameters refer to the sum of all flights of an individual during the 22-h test period, whereas the longest flight parameters refer to the longest uninterrupted flight during the test.
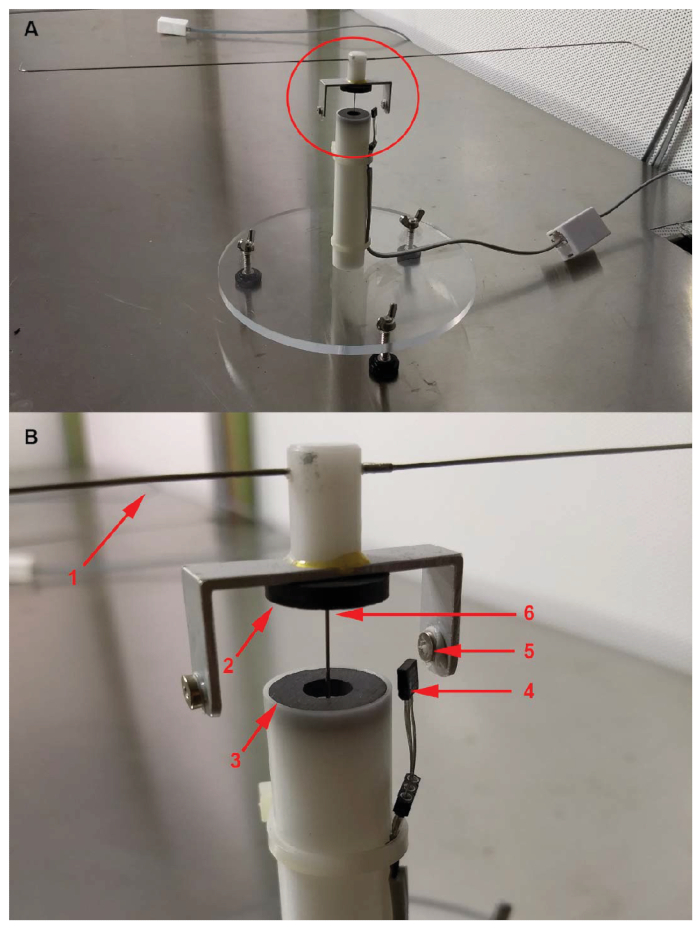
Figure 1. Insect flight mills used for tethered experiments. (A) Entire insect flight mill and (B) working portion of the flight mill. (A) Working portion of the flight mill is circled, (B) (1) 1 m hypodermic tube flight arm, (2, 3) repelling ferrite ring magnets, (4) digital Hall effect sensor, (5) small nickel ring magnet used to trigger the sensor, and (6) hypodermic thin wall tube ("central pin") that separates the repelling magnets (2,3). Flight mills modified slightly from the original design of Jones et al.18 Please click here to view a larger version of this figure.

Figure 2. Components of the flight mill environmental chamber. (A) Exterior chamber features include (1) Intellus controller, (2) control panel, and (3) main power disconnect. (B) Interior chamber features include (1) unit coolers (behind ceiling panel), (2) LED modules, (3) shelving units, and (4) pan-type humidifier. Please click here to view a larger version of this figure.
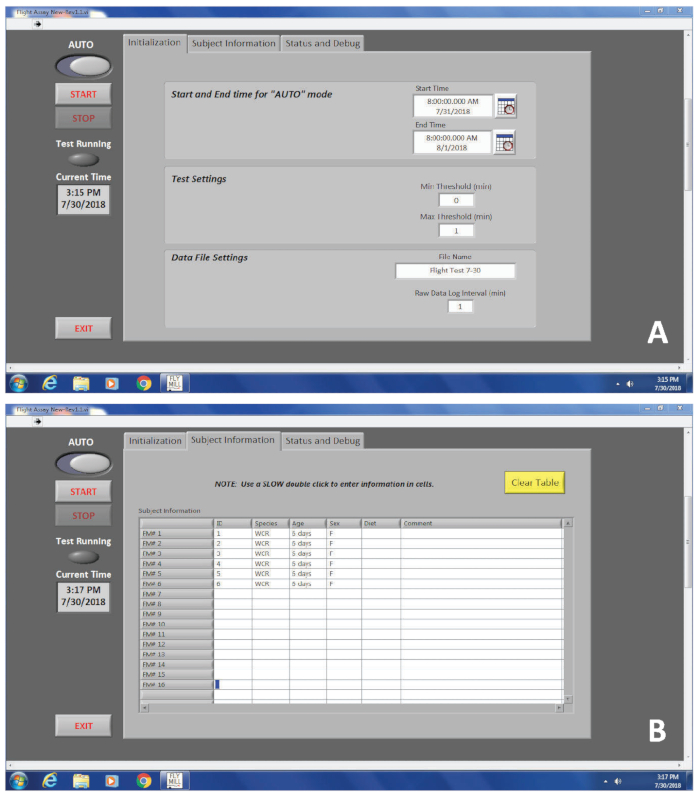
Figure 3. An interface of the flight mill software program. (A) The first tab, labeled ‘Initialization’, requires information including start and end times, and the file name. (B) The second tab, labeled ‘Subject Information’, does not require any information to be entered, but is used to differentiate between multiple individuals evaluated in a single flight test. Please click here to view a larger version of this figure.
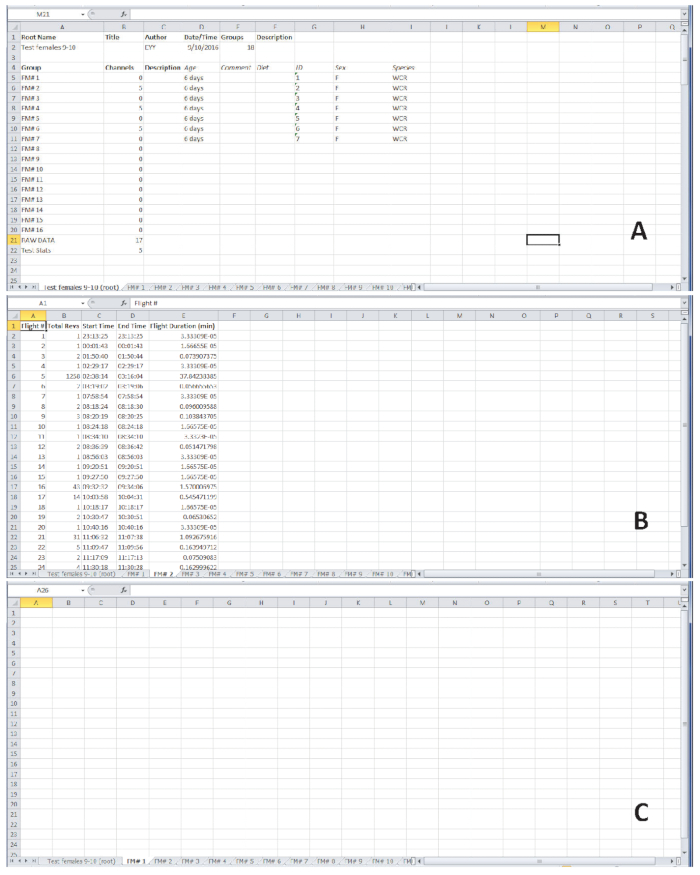
Figure 4. Representative flight data from 6-day-old female western corn rootworm beetles. (A) The first tab of the output summarizes the information on seven individuals flight tested on a particular day. (B) Flight data for the female on flight mill #2 (FM#2), which engaged in multiple independent flights during the 22-hour test period. (C) The female placed on flight mill #1 (FM#1) did not engage in flight during the 22-hour test period, resulting in a blank spreadsheet. Please click here to view a larger version of this figure.
| Location | |||
| Ames | Nashua | ||
| Sample size1 | 23 | 31 | |
| Total flight distance (m) | 387.83 ± 146.21 | 949.10 ± 267.73 | |
| Total flight duration (min) | 14.34 ± 5.06 | 37.01 ± 10.51 | |
| Total flight speed (m/s) | 0.42 ± 0.04 | 0.44 ± 0.06 | |
| Longest flight distance (m) | 184.48 ± 81.82 | 590.13 ± 186.01 | |
| Longest flight duration (min) | 6.27 ± 2.26 | 22.15 ± 7.67 | |
| Longest flight speed (m/s) | 0.46 ± 0.04 | 0.44 ± 0.03 | |
| 1 Flew at least 1 minute | |||
Table 1. Mean (± SE) performance on flight mills of female western corn rootworm from two locations in Iowa. Longest flight refers to the longest uninterrupted (i.e., continuous) flight performed by each individual during the test period.
Discussion
Characterizing western corn rootworm flight behavior is important for devising effective resistance management plans. Flight behavior of this pest has been studied in the laboratory using various methods including actographs, flight tunnels, and flight mills. Flight mills, as described and illustrated in this paper, allow insects to make uninterrupted flights so that researchers can quantify flight parameters such as distance, duration, periodicity, and speed of individual flights, over an entire test period.
The most challenging step in the protocol for flight mill experimentation with western corn rootworm, as it is for most insect species, is properly applying a tether to the adult (Step 3). This can be a difficult task due to the small amount of surface area available on the pronotum for attachment of the wire, as well as the copious amount of natural waxes on the cuticle surface. The task is made more difficult by the limited time available to apply the tether before the insect begins to stir as it emerges from CO2 anesthetization. It is important that the tether is lined up correctly and adheres to the beetle’s pronotum throughout the testing period. If the tether is misaligned, the beetle may have a difficult time engaging in flight while on the flight mill, resulting in artifactually lower distance, duration, and speed. The beetle may escape during the test period if the dental wax does not adhere the wire strongly enough to the pronotum. Therefore, it is important to have clean, steady hands, a good sense for warming the wax to a workable temperature, and confidence while tethering beetles, all of which are attainable with adequate practice.
A decision must be made about what constitutes an independent flight event so that the Max Threshold value can be set (Step 2.2.3). An individual may make no flights, one flight, or dozens of flights during a test period, depending on its stop-and-go activity, but also on the assigned Max Threshold value. The default value reported by Jones et al.18 is 5 s. In this study of western corn rootworm, the Max Threshold was set at 1 min. The most appropriate setting is a judgment call based on the insect species and the goals of the researcher. There are trade-offs. An insect that quits flying but continues to circle for one or more revolutions because of momentum will have those revolutions incorrectly counted as part of the previous flight when the value is set to 1 min. If the value is set at 5 s, most of the extra non-flight revolutions will not be counted and logging of that flight will be correctly terminated. On the other hand, sometimes an insect slows its flight substantially in an effort to control its direction, to land, or for other reasons, then resumes flying at higher speed without ever having stopped active flight. Such behavior on flight mills is common and has been observed in western corn rootworm; it would often be logged as two separate flights when the maximum threshold is set to 5 s, but would be correctly recorded as an uninterrupted flight when the threshold is 1 min. Under the 1-min threshold, however, the flight of an insect that truly stops flying then resumes flight within 1 min would be incorrectly recorded as not having stopped.
A minimum flight threshold (e.g., at least one flight of at least one minute) may be used to exclude from further analyses any adults that may have been damaged during handling or were otherwise in poor health. The trade-off of protecting against such false-zeros (or false very short flights) is the possibility of excluding true-zeros (or true very-short flights), i.e., individuals that were healthy but were not motivated to fly. The researcher must decide how to handle zeros (or very short flights) based on the goals of the experiment, as well as which type of error is most likely and which is least desirable when it comes to interpreting the results. In addition, a common problem occurs when the position of the flight arm supporting an inactive beetle happens to be directly over, or very near, the sensor, where small movements of the arm caused by non-flight movements of the insect or slight air currents in the chamber may be falsely recorded as revolutions. To prevent this methodological artifact from inflating the frequency of shorter flight durations, it is recommended to exclude all flights lasting ≤1 min from analyses. This kind of artifactual reading, if it goes on for a longer time, can also result in a nonsensically high speed (e.g., > 2 m/s) for a recorded "flight"; when detected, those "flight" data should be deleted for that individual.
Although flight mill studies have provided important insights into western corn rootworm flight behavior, as with any species there are complications in relating tethered flight to natural flight in the field24. An insect on a flight mill is suspended, which provides vertical support for its weight. Thus, the energy expended to provide lift during natural flight may not be invested by tethered insects on flight mills25. On the other hand, a tethered insect must provide more thrust than in free-flight to overcome friction at the pivot, the added weight of the flight arm, and aerodynamic drag from the flight arm25,26. Natural flight of western corn rootworm also sometimes occurs at altitudes above its flight boundary layer27, where the distance covered during flight can be strongly influenced by wind speeds that are much greater than the insect's unaided flight speed28. Flight mills impose unidirectional flight, so that distance flown may overestimate total displacement in the field where the flight path may be meandering. Providing tarsal contact with a small piece of tissue after mounting the insect on the flight mill (step 3.9) reduces initial escape flight as well as flight activity associated with an attempt to land. However, once the beetle drops the tissue during an experiment, the same problem of inability to terminate flight by landing is encountered. Alternative actograph systems have been used in laboratory flight experiments with tethered8,9 or untethered7 western corn rootworm. While they alleviate the problem of flight termination by allowing spontaneous tarsal contact, the trade-off is the inability to measure flight distance or speed. Despite these limitations, the flight mill is very useful as a comparative tool for examining how a variety of developmental, biotic, and abiotic factors influence an insect’s propensity to engage in flight, and how flight behavior itself is affected. When combined with other evidence, such as that provided by mark-capture experiments29, trap data30, and estimates of gene flow31, the unique insights obtained from flight mill experiments contribute toward a holistic understanding of western corn rootworm dispersal in the field and its population-level consequences.
Disclosures
The authors have nothing to disclose.
Acknowledgements
E.Y.Y.’s graduate assistantship was supported by the National Science Foundation I/UCRC, the Center for Arthropod Management Technologies, under Grant No. IIP-1338775, and industry partners.
Materials
| Butane multi-purpose lighter | BIC | UXMPFD2DC | To soften wax when tethering |
| Clear polystyrene plastic vial (45-ml) | Freund Container and Supply | AS112 | To hold beetle while anesthetizing |
| Dehydrated culture media, agar powder | Fisher Scientific | S14153 | To make agar for holding moisture for adults |
| Delrin rod (1" diameter, 3.75" long) | Many suppliers: can use cheapest on the internet. | For post of flight mill | |
| Dental wax | DenTek | 47701000335 | Adheres wire tether to prothorax |
| Ferrite ring magnets (OD: 0.69”, ID: 0.29”, Thickness: 0.118”; 7oz pull) | Magnet Shop | 63B06929118 | Opposing – to generate the float. |
| Hall effect sensor | Optikinc | OHN3120U | Look under magnetic sensors on the left side of the Optekinc website then look for the part number. A link is given for current suppliers. |
| Hypodermic tubing (22 gauge; 0.0358” OD x 0.01975” ID x 0.004” wall) | Small Parts, Inc. | HTX-22T-12 | Used for flight mill arms and main axis rod. |
| Incubator (104.1 x 85.4 x 196.1 cm) | Percival Scientific | I-41VL | |
| LabVIEW Full Development System software, system-design platform | National Instruments (See http://www.ni.com/en-us/shop/labview/select-edition.html) | LabVIEW 2018 (Full Edition) | Provides environment needed to run flight mill files (.vi extensions) available for download from Jones et al.18 at http://entomology.tfrec.wsu.edu/VPJ_Lab/Flight-Mill. LabVIEW 2018 Full is compatible with Win/Mac/Linux operating systems. |
| Mesh cage (18 x 18 x 18 cm) | MegaView Science Co. Ltd. | BugDorm-4M1515 | mesh size = 44 x 32, 650 µm aperture |
| Needle tool | BLICK | 34920-1063 | For scoring soil surface for egg laying in laboratory |
| Nickel ring magnets (3/16” OD x 1/16” ID x: 1/16” thick) | K&J Magnetics | R311 | Used to trigger the digital hall effect sensor. |
| Petri dish (100 mm x 15 mm) | Fisher Scientific | S33580A | |
| Plastic container (44-ml) | Dart | 150PC | For initial rearing of young larvae |
| Plastic container (473-ml) | Placon | 22885 | For rearing of older larvae |
| Round brush (size 2) | Simply Simmons | 10472906 | For transferring freshly hatched neonates to surface of roots |
| Sieve (250-µm) | Fisher Scientific | 08-418-05 | To separate eggs from soil |
| Steel wire (28-gauge) | The Hillman Group | 38902350282 | |
| Teflon rod (3/8" diameter, 3/4" length) | United States Plastic Corporation | 47503 | To accept the rotating arm. |
| Vacuum | Gast Manufacturing, Inc. | 1531-107B-G288X | For aspirating adults in laboratory |
| White poly chiffon fabric | Hobby Lobby | 194811 | To prevent escape of larvae from rearing container |
References
- Gillette, C. P. Diabrotica virgifera Lec. as a corn root-worm. Journal of Economic Entomology. 5 (4), 364-366 (1912).
- Rice, M. E. Transgenic rootworm corn: assessing potential agronomic, economic, and environmental benefits. Plant Management Network. , (2004).
- Gray, M. E., Sappington, T. W., Miller, N. J., Moeser, J., Bohn, M. O. Adaptation and invasiveness of western corn rootworm: Intensifying research on a worsening pest. Annual Review of Entomology. 54 (1), 303-321 (2009).
- Martinez, J. C., Caprio, M. A. IPM use with the deployment of a nonhigh dose Bt pyramid and mitigation of resistance for western corn rootworm (Diabrotica virgifera virgifera). Environmental Entomology. 45 (3), 747-761 (2016).
- Miller, N. J., Sappington, T. W. Role of dispersal in resistance evolution and spread. Current Opinion in Insect Science. 21, 68-74 (2017).
- Spencer, J. L., Hibbard, B. E., Moeser, J., Onstad, D. W. Behaviour and ecology of the western corn rootworm (Diabrotica virgifera virgifera LeConte). Agricultural and Forest Entomology. 11, 9-27 (2009).
- VanWoerkom, G. J., Turpin, F. T., Barret, J. R. Influence of constant and changing temperatures on locomotor activity of adult western corn rootworms (Diabrotica virgifera) in the laboratory. Environmental Entomology. 9 (1), 32-34 (1980).
- Naranjo, S. E. Comparative flight behavior of Diabrotica virgifera virgifera and Diabrotica barberi in the laboratory. Entomologia Experimentalis et Applicata. 55 (1), 79-90 (1990).
- Stebbing, J. A., et al. Flight behavior of methyl-parathion-resistant and -susceptible western corn rootworm (Coleoptera: Chrysomelidae) populations from Nebraska. Journal of Economic Entomology. 98 (4), 1294-1304 (2005).
- Dobson, I. D., Teal, P. E. A. Analysis of long-range reproductive behavior of male Diabrotica virgifera virgifera LeConte and D. barberi Smith and Lawrence to stereoisomers of 8-methyl-2decyl propanoate under laboratory conditions. Journal of Chemical Ecology. 13 (6), 1331-1341 (1987).
- Spencer, J. L., Isard, S. A., Levine, E. Free flight of western corn rootworm (Coleoptera: Chrysomelidae) to corn and soybean plants in a walk-in wind tunnel. Journal of Economic Entomology. 92 (1), 146-155 (1999).
- Coats, S. A., Tollefson, J. J., Mutchmor, J. A. Study of migratory flight in the western corn rootworm (Coleoptera: Chrysomelidae). Environmental Entomology. 15 (3), 620-625 (1986).
- Coats, S. A., Mutchmor, J. A., Tollefson, J. J. Regulation of migratory flight by juvenile hormone mimic and inhibitor in the western corn rootworm (Coleoptera: Chrysomelidae). Annals of the Entomological Society of America. 80 (5), 697-708 (1987).
- Kennedy, J. S., Ainsworth, M., Toms, B. A. Laboratory studies on the spraying of locusts at rest and in flight. Anti-Locust Bull. L. 2, 64 (1948).
- Krogh, A., Weis-Fogh, T. A Roundabout for studying sustained flight of Locusts. Journal of Experimental Biology. 29, 211-219 (1952).
- Attisano, A., Murphy, J. T., Vickers, A., Moore, P. J. A simple flight mill for the study of tethered flight in insects. Journal of Visualized Experiments. (106), e53377 (2015).
- Okada, R., Pham, D. L., Ito, Y., Yamasaki, M., Ikeno, H. Measuring the flight ability of the ambrosia beetle, Platypus quercivorus (Murayama), using a low-cost, small, and easily constructed flight mill. Journal of Visualized Experiments. (138), e57468 (2018).
- Jones, V. P., Naranjo, S. E., Smith, T. J. . Insect ecology and behavior: laboratory flight mill studies. , (2010).
- Abendroth, L. J., Elmore, R. W., Boyer, M. J., Marlay, S. K. . Corn Growth and Development. , (2011).
- Meinke, L. J., Sappington, T. W., Onstad, D. W., Guillemaud, T., Miller, N. J., Komáromi, J., Levay, N., Furlan, L., Kiss, J., Toth, F. Western corn rootworm (Diabrotica virgifera virgifera LeConte) population dynamics. Agricultural and Forest Entomology. 11, 29-46 (2009).
- Hammack, L., French, B. W. Sexual dimorphism of basitarsi in pest species of Diabrotica and Cerotoma (Coleoptera: Chrysomelidae). Annals of the Entomological Society of America. 100 (1), 59-63 (2007).
- Guss, P. L. The sex pheromone of the western corn rootworm (Diabrotica virgifera). Environmental Entomology. 5 (2), 219-223 (1976).
- Hammack, L. Calling behavior in female western corn rootworm beetles (Coleoptera: Chrysomelidae). Annals of the Entomological Society of America. 88 (4), 562-569 (1995).
- Minter, M., Pearson, A., Lim, K. S., Wilson, K., Chapman, J. W., Jones, C. M. The tethered flight technique as a tool for studying life-history strategies associated with migration in insects. Ecological Entomology. 43, 397-411 (2018).
- Ribak, G., Barkan, S., Soroker, V. The aerodynamics of flight in an insect flight-mill. PLoS One. 12 (11), e0186441 (2017).
- Riley, J. R., Downham, M. C. A., Cooter, R. J. Comparison of the performance of leafhoppers on flight mills with that to be expected in free flight. Entomologia Experimentalis et Applicata. 83, 317-322 (1997).
- Isard, S. A., Spencer, J. L., Mabry, T. R., Levine, E. Influence of atmospheric conditions on high-elevation flight of western corn rootworm (Coleoptera: Chrysomelidae). Environmental Entomology. 33 (3), 650-656 (2004).
- Chapman, J. W., Reynolds, D. R., Wilson, K. Long-range seasonal migration in insects: mechanisms, evolutionary drivers and ecological consequences. Ecology Letters. 18, 287-302 (2015).
- Spencer, J. L., Mabry, T. R., Vaughn, T. T. Use of transgenic plants to measure insect herbivore movement. Journal of Economic Entomology. 96 (6), 1738-1749 (2003).
- Isard, S. A., Spencer, J. L., Nasser, M. A., Levine, E. Aerial movement of western corn rootworm, Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae): diel periodicity of flight activity in soybean fields. Environmental Entomology. 29 (2), 226-234 (2000).
- Kim, K. S., Sappington, T. W. Genetic structuring of western corn rootworm (Coleoptera: Chrysomelidae) populations in the U.S. based on microsatellite loci analysis. Environmental Entomology. 34 (2), 494-503 (2005).

