Preparation of N-(2-alkoxyvinyl)sulfonamides from N-tosyl-1,2,3-triazoles and Subsequent Conversion to Substituted Phthalans and Phenethylamines
Summary
Representative experimental procedures for the synthesis of N-(2-alkoxyvinyl)sulfonamides and subsequent conversion to phthalan and phenethylamine derivatives are presented in detail.
Abstract
Decomposition of N-tosyl-1,2,3-triazoles with rhodium(II) acetate dimer in the presence of alcohols forms synthetically versatile N-(2-alkoxyvinyl)sulfonamides, which react under a variety of conditions to afford useful N– and O-containing compounds. Acid-catalyzed addition of alcohols or thiols to N-(2-alkoxyvinyl)sulfonamide-containing phthalans provides access to ketals and thioketals, respectively. Selective reduction of the vinyl group in N-(2-alkoxyvinyl)sulfonamide-containing phthalans via hydrogenation yields the corresponding phthalan in good yield, whereas reduction with sodium bis(2-methoxyethoxy)aluminumhydride generates a ring-opened phenethylamine analogue. Because the N-(2-alkoxyvinyl)sulfonamide functional group is synthetically versatile, but often hydrolytically unstable, this protocol emphasizes key techniques in preparing, handling, and reacting these pivotal substrates in several useful transformations.
Introduction
Rhodium(II)-azavinyl carbenoids have recently emerged as an exceptionally versatile reactive intermediate en route to numerous valuable products.1,2,3,4,5,6,7,8,9,10 In particular, many novel uses of these intermediates for production of heterocycles10 have provided chemists with new and efficient synthetic strategies. Toward this end, our group initiated development of a new protocol for the synthesis of phthalans11 that would capitalize on recent advancements in the inter- and intramolecular additions of oxygen-based nucleophiles to Rh(II)-azavinyl carbenoids derived from N-sulfonyl-1,2,3-triazoles.12,13,14,15,16,17 Our approach features a straightforward two-step protocol for converting terminal alkynes such as 1 into N-sulfonyl-1,2,3-triazoles 2 bearing a pendent alcohol (Figure 1). Subsequently, a Rh(II)-catalyzed denitrogenation / 1,3-OH insertion cascade from 2 provides phthalans 3 having a reactive N-(2-alkoxyvinyl)sulfonamide functional group.
Since the N-(2-alkoxyvinyl)sulfonamide moiety is a potentially versatile, but relatively underexplored N– and O-containing synthon,16,17,18,19,20,21,22,23,24,25,26,27 we became interested in studying the reactivity of its fused enol-ether/ene-sulfonamide system under a variety of conditions (Figure 2). After screening various reducing protocols, two methods were identified which led to stable phthalan and/or phenethylamine-containing products (Figure 2, 3 → 4/5). First, it was discovered that a standard hydrogenation of N-(2-alkoxyvinyl)sulfonamide 3a with catalytic palladium on carbon (Pd/C) selectively reduces the C=C bond to yield phthalan 4. Alternatively, treatment of 3a with sodium bis(2-methoxyethoxy)aluminum hydride in diethyl ether/toluene provides the uniquely substituted phenethylamine derivative 5. We believe that both of these transformations are valuable, as they lead to product classes with potential biological activity including neuroactive properties arising from the embedded phenethylamine, and in the case of 4, metal-chelation via the cis-oriented N– and O-atoms.
While investigating acid-promoted additions to exploit the electron-rich C=C bond of 3a, it was found that treatment of this compound with catalytic trimethylsilyl chloride in the presence of alcohols or a thiol yielded ketals 6a-c and thioketal 6e, respectively, while keeping the bicyclic phthalan framework intact. Alternatively, stirring 3a in a 1:1 acetic acid/water solution yields stable hemiketal 6d.
Protocol
1. Synthesis of N-Tosyl Triazole 2a: (2-(1-tosyl-1H-1,2,3-triazol-4-yl)phenyl)methanol
- Add a 3 x 10 mm PTFE magnetic stir bar, 139 mg of 2-ethynylbenzyl alcohol, and 20 mg of copper(I) thiophenecarboxylate (CuTC) to an oven-dried 2 – 5 mL microwave vial and seal the vial securely with a septum cap and crimper. Due to the rapid heating of the microwave, always use a new vial and cap that are free of any defects and make sure the cap is secure and properly fitted.
- Remove air from the vial under vacuum and refill with argon gas three times.
- Add 4 mL of anhydrous chloroform via syringe and commence magnetic stirring.
- Add 0.15 mL of p-toluenesulfonyl azide (TsN3) dropwise via syringe. CAUTION! p-Toluenesulfonyl azide is potentially explosive28 and must be handled using appropriate personal protective equipment.
- Heat the sealed microwave vial at 100 °C in a microwave reactor for 15 min. CAUTION! Do not use a standard microwave oven or a unit unauthorized for chemical synthesis.
NOTE: A commercial microwave reactor was used in this protocol. The absorption level was set to "normal" and the stirring rate was kept at 600 rotations per minute (RPM). It is likely that other microwave reactors designed for chemical synthesis will also work for this protocol, although the ideal time, temperature, and other parameters may vary. - Cool the reaction vessel to room temperature quickly (~2 – 3 min) using a stream of compressed air and transfer the reaction mixture to a 100 mL round-bottomed flask. Wash the reaction vial with an additional 2 x 10 mL of dichloromethane to transfer any residual crude product into the 100 mL round-bottomed flask.
- Add ~1.5 g of silica gel to the same round-bottomed flask and remove the solvents using a rotary evaporator.
- Tightly pack the silica gel adsorbed with the crude product into a solid load cartridge and attach to a 12 g pre-packed silica gel column for automated flash chromatography.
NOTE: An automated purification system, solid load cartridge, and 12 g silica gel column was used in this protocol. Solvent flow rates were maintained at approximately 30 mL/min. Automated flash chromatography is not required for purification; conventional flash chromatography can also be used. However, we favor automation since it typically enables one to isolate compound 2a as quickly as possible before significant decomposition occurs. - Run the column using a continuous gradient of 0 – 100% ethyl acetate in hexanes over 15 min by beginning with pure hexanes and ending with pure ethyl acetate. Collect the major peak as indicated by UV absorbance at 254 nm and concentrate the combined, corresponding fractions on a rotary evaporator to obtain the purified product 2a as an off-white solid.
NOTE: Triazole 2a was typically found to be stable when stored as a solid under argon at 2 – 5 °C for 1 – 2 weeks. However, certain batches of product degraded faster than others, possibly due to DCl contamination from CDCl3. Therefore, we recommend analyzing the purity of product by NMR using CDCl3 neutralized with K2CO3 and using it immediately in subsequent reactions for the best results.
2. Synthesis of N-(2-alkoxyvinyl)sulfonamide phthalan 3a: (Z)-N-(isobenzofuran-1(3H)-ylidenemethyl)-4-methylbenzenesulfonamide
- Add a 3 x 10 mm PTFE magnetic stir bar and 4.6 mg of rhodium(II) acetate dimer to an oven-dried 0.5 – 2 mL microwave vial and seal the vial securely with a septum cap and crimper. Due to the rapid heating of the microwave, always use a new vial and cap that are free of any defects and make sure the cap is secure and properly fitted.
- Remove air from the vial under vacuum and refill with argon gas three times.
- Under an argon atmosphere, dissolve 152 mg of triazole 2a in 1 mL of anhydrous chloroform and transfer the resulting solution to the microwave vessel via syringe. Rinse the flask containing residual triazole two times with an additional 2 mL of chloroform and transfer to the same microwave vessel to ensure that all starting material is transferred.
- Heat the sealed microwave vial at 100 °C in a microwave reactor for 1 h. CAUTION! Do not use a standard microwave oven or a unit unauthorized for chemical synthesis.
NOTE: A commercial microwave reactor was used in this protocol. The absorption level was set to "normal" and the stirring rate was kept at 600 rotations per minute (RPM). It is likely that other microwave reactors designed for chemical synthesis will also work for this protocol, although the ideal time, temperature, and other parameters may vary. - Cool the reaction vessel to room temperature quickly (~2 – 3 min) using a stream of compressed air and filter through a short plug of silica gel, eluting with ethyl acetate.
- Concentrate the filtrate in vacuo using a rotary evaporator with a warm (~30 °C) water bath to obtain the product in sufficient purity to be used immediately for subsequent reactions.
Note that the product decomposes quickly (within 1h) under mildly acidic conditions such as in CDCl3 containing residual DCl, and gradually (within 1 – 3d) when stored neat under argon at 3 – 5 °C. Therefore, we recommend analyzing the purity of product by NMR using CDCl3 neutralized with K2CO3 and using it immediately in subsequent reactions for the best results. - If necessary, purify the product via column chromatography on silica gel using a 0 – 75% gradient of ethyl acetate in hexanes over 15 min by beginning with pure hexanes and ending with 75% ethyl acetate in hexanes.
NOTE: An automated purification system, solid load cartridge, and 12 g silica gel column was used in this protocol. Solvent flow rates were maintained at approximately 30 mL/min. Automated flash chromatography is not required for purification; conventional flash chromatography can also be used. However, we favor automation since it typically enables one to isolate compound 3a as quickly as possible before significant decomposition occurs.
3. Synthesis of Phthalan 4: N-((1,3-dihydroisobenzofuran-1-yl)methyl)-4-methylbenzenesulfonamide
- In a 25 mL round-bottomed flask with a magnetic stir bar, dissolve 211 mg of freshly prepared phthalan 3a in 15 mL of absolute ethanol under an argon atmosphere.
- Add 149 mg of 10 wt% palladium on carbon to the flask, taking care to minimize exposure to air. CAUTION! It is very important to ensure that the reaction mixture is under an argon or nitrogen atmosphere. Palladium on carbon can ignite in the presence of air, hydrogen gas, and/or a flammable solvent. Wear all appropriate personal protective equipment and proactively keep a flame extinguisher and/or bucket of sand nearby to extinguish any flames.
- Fill a standard latex balloon securely attached to a syringe with hydrogen gas. Do not exceed the recommended capacity of the balloon.
- Attach the balloon and syringe to the reaction vessel using a needle to penetrate the septum. Check to ensure that there are no leaks in the balloon and/or septum.
- To replace the argon atmosphere with hydrogen, apply a weak vacuum to the reaction vessel while pinching off the balloon, then after stopping the vacuum, refill the vessel with hydrogen gas. Repeat two additional times.
- Stir the reaction for 24 h and then remove the balloon.
- Purge the flask with argon gas and then filter the solution through a silica gel plug eluting with ethyl acetate. Carefully discard the silica gel containing palladium by dampening the mixture with water and placing in a sealed solid waste container.
- Remove the solvent in vacuo to provide the product.
4. Synthesis of phenethylamine 5: N-(2-(hydroxymethyl)phenethyl)-4-methylbenzenesulfonamide
- In a 10 mL round-bottomed flask, dissolve 169 mg of freshly prepared phthalan 3a with 5 mL of diethyl ether under an argon atmosphere.
- Cool the reaction mixture to 0 °C using an ice-bath and then slowly add 0.52 mL of a ~60 wt% solution of sodium bis(2-methoxyethoxy)aluminum hydride in toluene. CAUTION! Sodium bis(2-methoxyethoxy)aluminum hydride reacts violently with water. Only use this reagent in a moisture-free, inert atmosphere.
- Stir the reaction mixture for 18 h at room temperature.
- Cool the reaction mixture to 0 °C and then carefully add 0.5 mL of methanol dropwise over 2 min. Stir for an additional 2 min at 0 °C. CAUTION! Addition of methanol to sodium bis(2-methoxyethoxy)aluminum hydride is exothermic. Ensure that the solution is sufficiently cold and take care to avoid adding the methanol all at once.
- At 0 °C, add 0.6 mL of saturated aqueous ammonium chloride, remove the ice bath, and stir for 5 min at room temperature.
- Pour the resulting solution into a separatory funnel containing 90 mL of 1M hydrochloric acid and extract the aqueous layer with 60 mL of ethyl acetate three times.
- Wash the combined organic layers with 30 mL of water and then 30 mL of brine before drying over sodium sulfate.
- Filter off the sodium sulfate using a Buchner funnel and concentrate the filtrate in vacuo to obtain the crude phenethylamine product.
NOTE: Typically, the product is sufficiently pure after this step, but occasionally contaminants from decomposition of N-(2-alkoxyvinyl)sulfonamide phthalan 3a may be present. - If necessary, purify the product via column chromatography on silica gel using a 0 – 100% gradient of ethyl acetate in hexanes over 15 min by beginning with pure hexanes and ending with pure ethyl acetate.
NOTE: A purification system, solid load cartridge, and 12 g silica gel column was used in this protocol. Solvent flow rates were maintained at approximately 30 mL/min. Automated flash chromatography is not required for purification; conventional flash chromatography can also be used.
5. Synthesis of ketal 6c: N-((1-(2-hydroxyethoxy)-1,3-dihydroisobenzofuran-1-yl)methyl)-4-methylbenzenesulfonamide
- In a 10 mL round-bottomed flask with a stir bar, dissolve 211 mg of freshly synthesized phthalan 3a in 2 mL of ethylene glycol under an air atmosphere and commence stirring.
- Using a 1 mL syringe equipped with an 18 gauge needle, add 1 drop of trimethylsilyl chloride to the stirring solution.
- Place a rubber septum on the flask with a venting needle open to air and stir the reaction mixture for 18 h at room temperature.
- Transfer the reaction mixture to a 125 mL separatory funnel, rinsing with 50 mL of dichloromethane and then add 10 mL of saturated aqueous sodium bicarbonate and 40 mL of deionized water.
- Mix vigorously, venting often, and separate the organic layer into a clean flask. Extract the aqueous layer an additional three times with 30 mL of dichloromethane each time.
- Combine the organic layers and dry over sodium sulfate.
- Filter off the sodium sulfate using a Buchner funnel and concentrate the filtrate on a rotary evaporator.
- Dissolve the crude product in 10 mL of dichloromethane, add ~750 mg of silica gel to the mixture, and remove the solvent using a rotary evaporator.
- Tightly pack the silica gel adsorbed with the crude product into a solid load cartridge and attach to a 12 g pre-packed silica gel column for automated flash chromatography.
NOTE: A purification system, solid load cartridge, and 12 g silica gel column was used in this protocol. Solvent flow rates were maintained at approximately 30 mL/min. Automated flash chromatography is not required for purification; conventional flash chromatography can also be used. - Run the column using a continuous gradient of 0 – 70% ethyl acetate in hexanes over 15 min by beginning with pure hexanes and ending with pure ethyl acetate. Collect the major peak as indicated by UV absorbance at 254 nm and concentrate the combined, corresponding fractions on a rotary evaporator to obtain the purified product 6c as an off-white solid.
Representative Results
All compounds in this study were characterized by 1H and 13C NMR spectroscopy and electrospray ionization mass spectrometry (ESI-MS) to confirm the product structure and assess purity. Key data for representative compounds are described in this section.
Spectral data are in good agreement with the triazole structure of 2a (Figure 3). In the 1H NMR spectrum of 2a the characteristic C5 proton of the triazole appears at 8.45 ppm as a singlet integrating for 1H. The mass spectrum obtained via ESI-MS generally shows both the MH+ peak and a M-N2 peak corresponding to the loss of dinitrogen.
Synthesis of N-(2-alkoxyvinyl)sulfonamide phthalan 3a via our protocol reliably delivers the product in >90% yield, however, substantial deviation in key parameters such as time, temperature, and heating method significantly impact the efficiency of the reaction (vide infra) and, consequently, the quality of spectral data. Figure 4a depicts the 1H NMR spectrum of pure 3a following a successful experiment. Notable is the absence of the triazole C5 proton peak around 8.5 ppm (cf. Figure 3) and appearance of two doublets at 6.07 and 6.25 corresponding to the vinyl and NH protons, respectively. In the 13C NMR spectrum of 3a, a key resonance is observed at 94.9 ppm that corresponds to the exocyclic vinyl carbon. For comparison, Figure 5 illustrates the 1H NMR spectrum of 3a decomposition products resulting from rapid degradation in CDCl3.
Figure 6 and Figure 7 show 1H/13C NMR and mass spectra that are in good agreement with the structures of reduction products 4 and 5, respectively. The 1H NMR spectrum of 4, which maintains the bicyclic phthalan substructure, shows key signals corresponding to diastereotopic methylene protons 3.49 and 3.14 ppm. Contrastingly, the 1H NMR spectrum phenethylamine 5 displays the same methylene as a simple quartet at 3.27 ppm due to free rotation in the ring-opened product resulting in first-order splitting patterns.
For compounds 6a-e, a characteristic 13C NMR signal corresponding to the ketal, hemiketal or thioketal carbon is found between 95 – 110 ppm, such as the peak at 110.0 ppm observed in the 13C NMR spectrum of 6c (Figure 8). Additionally, mass spectra obtained by ESI-MS typically show a relatively small MH+ peak along with a larger M-RX elimination fragment peak (RX = the corresponding alkoxy or thioalkyl group of 6).
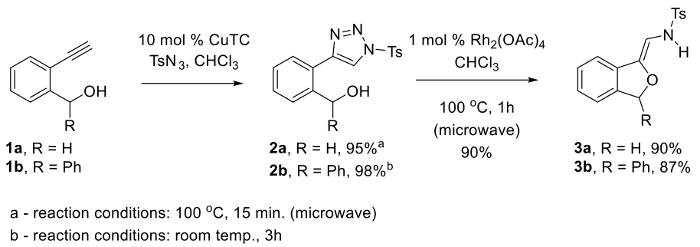
Figure 1. Synthesis of N-tosyl-1,2,3-triazoles 2 via Cu(I)-catalyzed azide-alkyne [3+2] cycloaddition and subsequent conversion to N-(2-alkoxyvinyl)sulfonamide phthalans 3 via Rh(II)-catalyzed alcohol cyclization. Please click here to view a larger version of this figure.
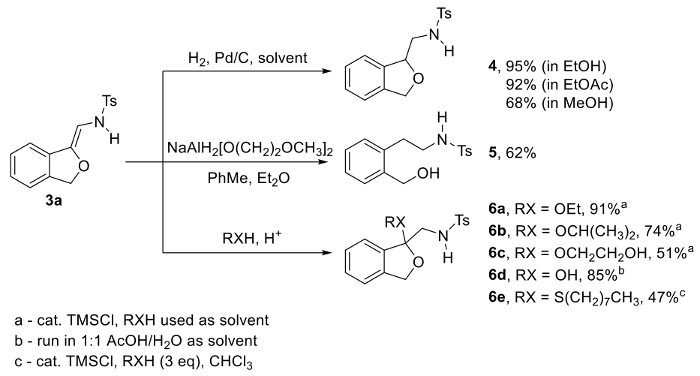
Figure 2. Differential reactivity of N-(2-alkoxyvinyl)sulfonamide phthalan 3a: conversion to reduced phthalan 4 via Pd-catalyzed hydrogenation, conversion to phenethylamine 5 via aluminum hydride reduction, and conversion to ketals 6a-c, hemiketal 6d, and thioketal 6e via acid-promoted addition of alcohols, water, and a thiol, respectively. Please click here to view a larger version of this figure.
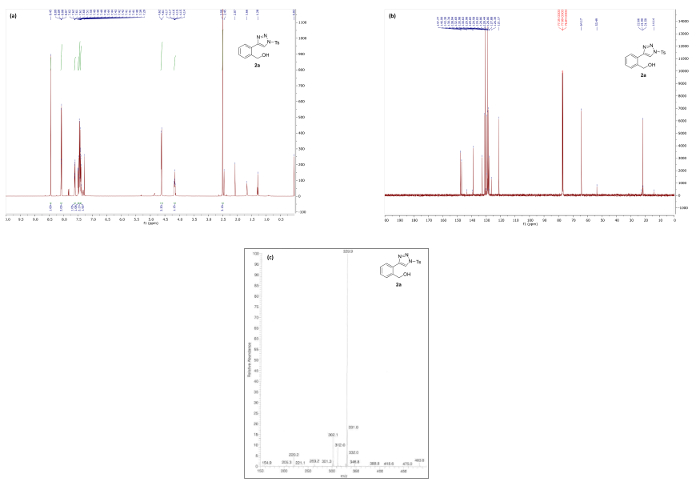
Figure 3. Spectral data for triazole 2a: (a) 1H NMR spectrum; (b) 13C NMR spectrum; and (c) mass spectrum. Please click here to view a larger version of this figure.
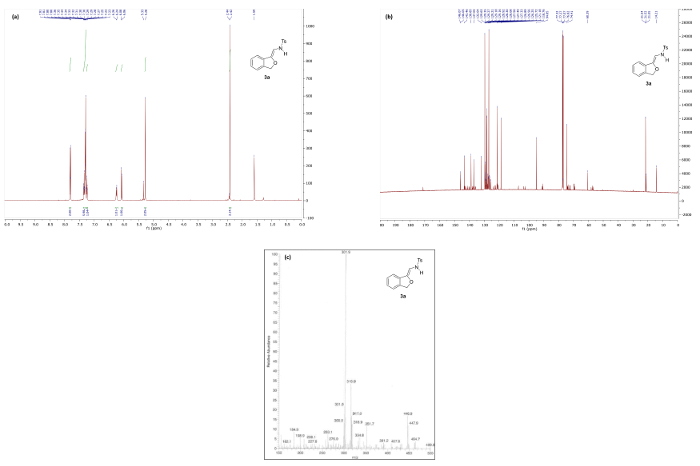
Figure 4. Spectral data for N-(2-alkoxyvinyl)sulfonamide phthalan 3a: (a) 1H NMR spectrum; (b) 13C NMR spectrum (minor peaks are decomposition products resulting from rapid degradation in CDCl3); and (c) mass spectrum. Please click here to view a larger version of this figure.
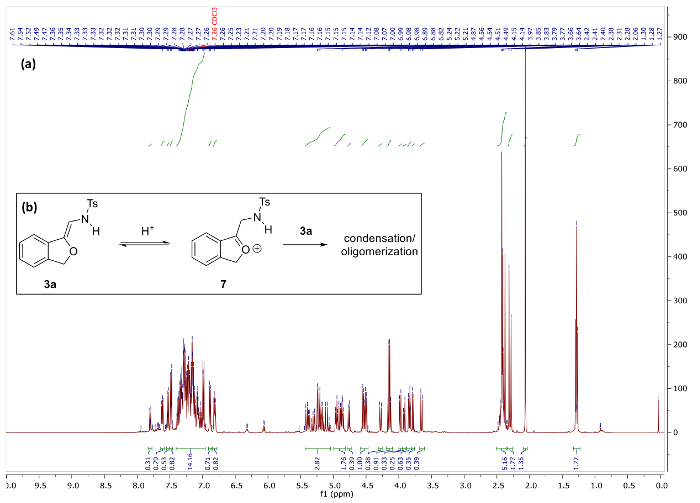
Figure 5. (a) 1H NMR spectra of 3a and decomposition products after storage in CDCl3 for 1h. (b) Hypothesized decomposition mechanism of 3a. Please click here to view a larger version of this figure.
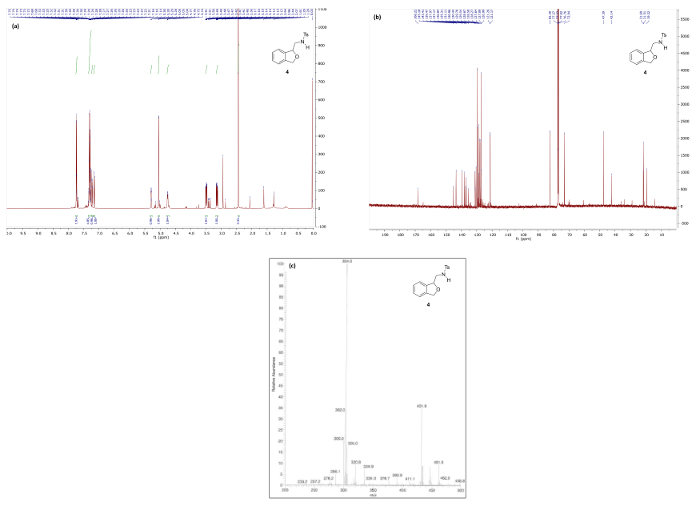
Figure 6. Spectral data for reduced phthalan 4: (a) 1H NMR spectrum; (b) 13C NMR spectrum; and (c) mass spectrum. Please click here to view a larger version of this figure.
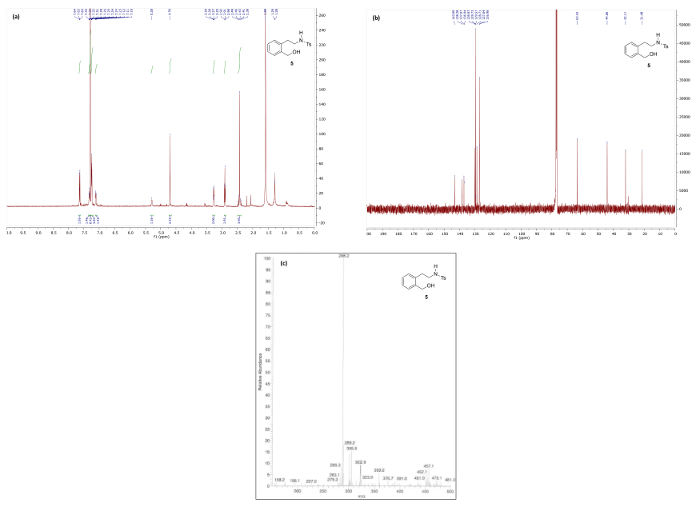
Figure 7. Spectral data for phenethylamine 5: (a) 1H NMR spectrum; (b) 13C NMR spectrum; and (c) mass spectrum. Please click here to view a larger version of this figure.
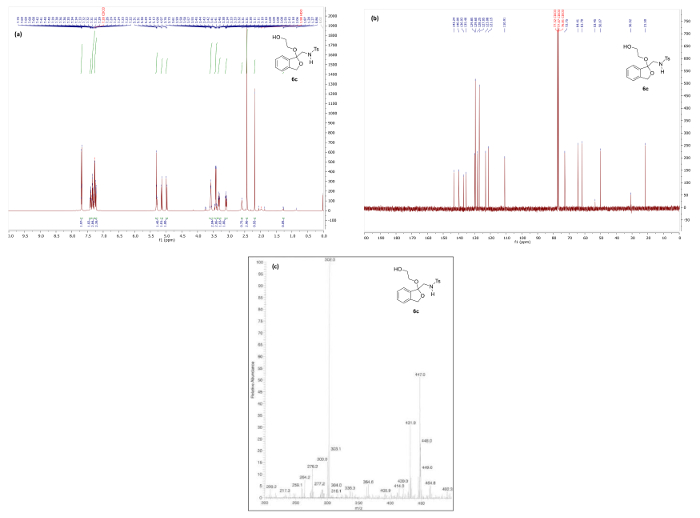
Figure 8. Spectral data for ketal 6c: (a) 1H NMR spectrum; (b) 13C NMR spectrum; and (c) mass spectrum. Please click here to view a larger version of this figure.
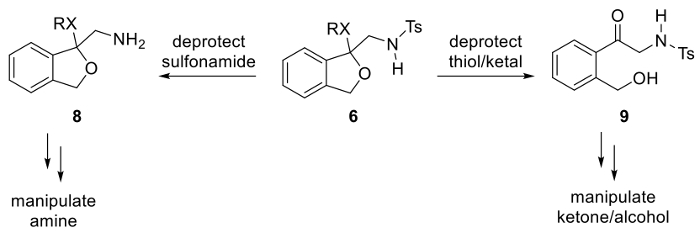
Figure 9. Strategic considerations for manipulation of compound 6, a differentially protected α-aminoketone. Please click here to view a larger version of this figure.
Discussion
Triazoles 2a-b can be cleanly obtained via a Cu(I)-catalyzed azide-alkyne [3+2] cycloaddition (CuAAC) using CuTC as catalyst. Notably, triazole 2a is most efficiently generated at high temperature via a standard reflux in chloroform for 3h or heating to 100 °C for 15 min in a microwave reactor (note that time may vary depending on microwave efficiency); however, triazole 2b is most efficiently prepared via a CuAAC at room temperature. Therefore, effort must be taken to identify the optimal conditions in this substrate-dependent reaction when executing this protocol on a new substrate. In the microwave-promoted synthesis of 2a, care must be taken not to heat the reaction over 100 °C in the microwave, or beyond 15 min, as this typically leads to substantial decomposition.
Microwave heating of triazole 2a with 1 mol% of rhodium(II) acetate dimer at 100 °C in chloroform generates N-(2-alkoxyvinyl)sulfonamide phthalan 3a in high yield and purity. Several attempts were made to modify this procedure, however, only the microwave heating protocol gave good results. For example, performing the reaction by submersing a sealed microwave vial in a conventional oil bath at 100 °C led only to a complex mixture of products. Since both procedures for the synthesis of triazole 2a and phthalan 3a utilize chloroform as solvent and take place at 100 °C, we also made numerous attempts to execute a one-pot protocol for the synthesis of 3a directly from alkyne 1a in the presence of tosyl azide, CuTC, and Rh2(OAc)4 under a variety of conditions, but without success.11
When handling 3a, it is critical to avoid acidic conditions as this will cause rapid decomposition. For example, when 3a is purified via a brief silica gel plug, pure product can be obtained as judged by 1H NMR spectral data (Figure 4). However, storage of 3a on silica or in non-neutralized CDCl3 (which contains trace HCl/DCl) for more than several minutes leads to a complex mixture of products (Figure 5). Presumably, this process occurs via condensation between the nucleophilic furan 3a and its corresponding electrophilic tautomer 7. Notably, CDCl3 that has been neutralized by K2CO3 prior to use slows down the decomposition process of 3a significantly, but not completely. Interestingly, N-(2-alkoxyvinyl)sulfonamide phthalan 3b was found to be stable in mildly acidic, non-neutralized CDCl3 for at least 3 d and in storage for 4 weeks, which suggests that steric hindrance and/or electronic factors can be used to attenuate the reactivity of this functional group.
N-(2-Alkoxyvinyl)sulfonamides such as 3a serve as a pivotal intermediates, and when freshly prepared can be used as unique precursors toward phthalan and phenethylamine derivatives. Catalytic hydrogenation of 3a with 10 mol% Pd/C in EtOH or EtOAc delivers the phthalan 4 in high yield whereas treatment with the sodium bis(2-methoxyethoxy)aluminum hydride provides the ring-opened phenethylamine 5 (Figure 2). In each of these reductions, the solvent has a significant impact on the efficiency of the reaction. Hydrogenation in MeOH generates 4 in similar purity, but significantly lower yield. The aluminum hydride reduction was only found to work when diethyl ether was used as the primary solvent; little to no product is observed when this reaction is attempted in THF, MTBE, 1,4-dioxane, PhMe, or CHCl3.
Dissolving freshly prepared 3a in alcoholic solvent containing catalytic TMSCl provides ketals 6a-c in moderate to high yields. Alternatively, thioketal 6e can be prepared by treating 3a with 3 eq of octane thiol, whereas hemiketal 6d is produced by stirring 3a in a 1:1 mixture of acetic acid and water.
A notable advantage of this ketalization approach is that the resulting compound is a differentially protected α-amino ketone, an often unstable class of compound whenever a basic amine and enolizable ketone are present at the same time.29,30,31,32 Furthermore, as illustrated in Figure 9, differential protection may offer the strategic advantage of manipulating the protected amine or ketone in separate, orthogonal operations.
In the future, we expect that these protocols can be employed for the synthesis of novel, bioactive compounds bearing the privileged phenethylamine substructure and/or phthalan scaffold. In addition, we have demonstrated the utility of N-(2-alkoxyvinyl)sulfonamides as versatile functional groups. Therefore, further investigation of this under-explored synthon in valuable synthetic transformations is merited.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by Hamilton College and the Edward and Virginia Taylor Fund for Student/Faculty Research in Chemistry.
Materials
| 2-Ethynylbenzyl alcohol, 95% | Sigma Aldrich | 520039 | |
| Copper (I) thiophene-2-carboxylate | Sigma Aldrich | 682500 | |
| Chloroform, ≥99% | Sigma Aldrich | 372978 | |
| Toluenesulfonylazide, 99.24% | Chem-Impex International | 26107 | Potentially explosive |
| Dichloromethane, ≥99.5% | Sigma Aldrich | 320269 | |
| Rhodium (II) acetate dimer, 99% | Strem Chemicals | 45-1730 | |
| Silica Gel, 32-63, 60A | MP Biomedicals Inc. | 2826 | For silica gel plugs |
| Hexanes | Sigma Aldrich | 178918 | |
| Ethyl acetate | Sigma Aldrich | 439169 | |
| Chlorofom-D | Sigma Aldrich | 151823 | |
| Ethylene glycol | Sigma Aldrich | 293237 | |
| Chlorotrimethylsilane, 98% | Acros | 11012 | |
| Sodium bicarbonate | Sigma Aldrich | S6014 | Dissolved in deionized water to prepare a saturated aqueous solution |
| Sodium sulfate | Fisher Scientific | S429 | |
| Ethyl alcohol, absolute – 200 proof | Aaper Alcohol and Chemical Co. | 82304 | |
| 10 wt% Palladium on carbon | Sigma Aldrich | 520888 | Can ignite in the presence of air, hydrogen gas, and/or a flammable solvent |
| Hydrogen gas | Praxair | UN1049 | |
| Diethyl ether | Sigma Aldrich | 309966 | |
| 60 wt% sodium bis(2-methoxyethoxy)aluminum hydride solution in toluene | Sigma Aldrich | 196193 | Reacts violently with water |
| Methanol | Sigma Aldrich | 34966 | |
| Ammonium chloride | Fisher Scientific | A661 | Dissolved in deionized water to prepare a saturated aqueous solution |
| Hydrochloric acid, 37% | Sigma Aldrich | 258148 | Dissolved in deionized water to prepare a 1M solution |
| Sodium Chloride | Sigma Aldrich | S25541 | Dissolved in deionized water to prepare a saturated aqueous solution |
| 2-5 mL Microwave vials | Biotage | 355630 | |
| Microwave vial caps | Biotage | 352298 | |
| RediSep Rf Gold Normal Phase, Silica Columns, 20 – 40 micron | Teledyne Isco | 69-2203-345 | For column chromatography |
| Balloons | CTI Industries Corp. | 912100 | For hydrogenation |
| Biotage Initiator+ Microwave Reactor | Biotage | 356007 |
References
- Horneff, T., Chuprakov, S., Chernyak, N., Gevorgyan, V., Fokin, V. V. Rhodium-Catalyzed Transannulation of 1,2,3-Triazoles with Nitriles. J. Am. Chem. Soc. 130 (45), 14972-14974 (2008).
- Cuprakov, S., Kwok, S. W., Zhang, L., Lercher, L., Fokin, V. V. Rhodium-Catalyzed Enantioselective Cyclopropanation of Olefins with N-Sulfonyl 1,2,3-Triazoles. J. Am. Chem. Soc. 131 (50), 18034-18035 (2009).
- Grimster, N., Zhang, L., Fokin, V. V. Synthesis and Reactivity of Rhodium(II) N-Triflyl Azavinyl Carbenes. J. Am. Chem. Soc. 132 (8), 2510-2511 (2010).
- Chattopadhyay, B., Gevorgyan, V. Transition-Metal-Catalyzed Denitrogenative Transannulation: Converting Triazoles into Other Heterocyclic Systems. Angew. Chem. Int. Ed. 51 (4), 862-872 (2012).
- Davies, H. M. L., Alford, J. S. Reactions of metallocarbenes derived from N-sulfonyl-1,2,3-triazoles. Chem. Soc. Rev. 43 (15), 5151-5162 (2014).
- Anbarasan, P., Yadagiri, D., Rajasekar, S. Recent Advances in Transition-Metal-Catalyzed Denitrogenative Transformations of 1,2,3-Triazoles and Related Compounds. Synthesis. 46 (22), 3004-3023 (2014).
- Hockey, S. C., Henderson, L. C. Rhodium(II) Azavinyl Carbenes and their Recent Application to Organic Synthesis. Aust. J. Chem. 68 (12), 1796-1800 (2015).
- Jia, M., Ma, S. New Approaches to the Synthesis of Metal Carbenes. Angew. Chem. Int. Ed. 55 (32), 9134-9166 (2016).
- Volkova, Y. A., Gorbatov, S. A. 1-Sulfonyl-1,2,3-triazoles as promising reagents in the synthesis of nitrogen-containing linear and heterocyclic structures. Chem. Heterocylc. Compd. 52 (4), 216-218 (2016).
- Jiang, Y., Sun, R., Tang, X. -. Y., Shi, M. Recent Advances in the Synthesis of Heterocycles and Related Substances Based on α-Imino Rhodium Carbene Complexes Derived from N-Sulfonyl-1,2,3-triazoles. Chem Eur. J. 22 (50), 17910-17924 (2016).
- Bennett, J. M., et al. Synthesis of phthalan and phenethylamine derivatives via addition of alcohols to rhodium(II)-azavinyl carbenoids. Tetrahedron Lett. 58 (12), 1117-1122 (2017).
- Miura, T., Biyajima, T., Fujii, T., Murakami, M. Synthesis of α-Amino Ketones from Terminal Alkynes via Rhodium-Catalyzed Denitrogenative Hydration of N-Sulfonyl-1,2,3-triazoles. J. Am. Chem. Soc. 134 (1), 194-196 (2012).
- Chuprakov, S., Worrell, B. T., Selander, N., Sit, R. K., Fokin, V. V. Stereoselective 1,3-Insertions of Rhodium(II) Azavinyl Carbenes. J. Am. Chem. Soc. 136 (1), 195-202 (2014).
- Shen, H., Fu, J., Gong, J., Yang, Z. Tunable and Chemoselective Syntheses of Dihydroisobenzofurans and Indanones via Rhodium-Catalyzed Tandem Reactions of 2-Triazole-benzaldehydes and 2-Triazole-alkylaryl Ketones. Org. Lett. 16 (21), 5588-5591 (2014).
- Yuan, H., Gong, J., Yang, Z. Stereoselective Synthesis of Oxabicyclo[2.2.1]heptenes via a Tandem Dirhodium(II)-Catalyzed Triazole Denitrogenation and [3 + 2] Cycloaddition. Org. Lett. 18 (21), 5500-5503 (2016).
- Yu, Y., Zhu, L., Liao, Y., Mao, Z., Huang, X. Rhodium(II)-Catalysed Skeletal Rearrangement of Ether Tethered N-Sulfonyl 1,2,3-Triazoles: a Rapid Approach to 2-Aminoindanone and Dihydroisoquinoline Derivatives. Adv. Synth. Catal. 358 (7), 1059-1064 (2016).
- Sun, R., Jiang, Y., Tang, X. -. Y., Shi, M. RhII-Catalyzed Cyclization of Ester/Thioester-Containing N-Sulfonyl-1,2,3-triazoles: Facile Synthesis of Alkylidenephthalans and Alkylidenethiophthalans. Asian J. Org. Chem. 6 (1), 83-87 (2017).
- Miura, T., Tanaka, T., Biyajima, T., Yada, A., Murakami, M. One-Pot Procedure for the Introduction of Three Different Bonds onto Terminal Alkynes through N-Sulfonyl-1,2,3-Triazole Intermediates. Angew. Chem. Int. Ed. 52 (14), 3883-3886 (2013).
- Medina, F., Besnard, C., Lacour, J. One-Step Synthesis of Nitrogen-Containing Medium-Sized Rings via α-Imino Diazo Intermediates. Org. Lett. 16 (12), 3232-3235 (2014).
- Alford, J. S., Davies, H. M. L. Mild Aminoacylation of Indoles and Pyrroles through a Three-Component Reaction with Ynol Ethers and Sulfonyl Azides. J. Am. Chem. Soc. 136 (29), 10266-10269 (2014).
- Miura, T., Tanaka, T., Matsumoto, K., Murakami, M. One-Pot Synthesis of 2,5-Dihydropyrroles from Terminal Alkynes, Azides, and Propargylic Alcohols by Relay Actions of Copper, Rhodium, and Gold. Chem. Eur. J. 20 (49), 16078-16082 (2014).
- Jung, D. J., Jeon, J. J., Lee, J. H., Lee, S. CuI/RhII-Catalyzed Tandem Convergent Multicomponent Reaction for the Regio- and Stereocontrolled Synthesis of γ-Oxo-β-amino Esters. Org. Lett. 17 (14), 3498-3501 (2015).
- Meng, J., Ding, X., Yu, X., Deng, W. -. P. Synthesis of 2,5-epoxy-1,4-benzoxazepines via rhodium(II)-catalyzed reaction of 1-tosyl-1,2,3-triazoles and salicylaldehydes. Tetrahedron. 72 (1), 176-183 (2016).
- Cheng, X., Yu, Y., Mao, Z., Chen, J., Huang, X. Facile synthesis of substituted 3-aminofurans through a tandem reaction of N-sulfonyl-1,2,3-triazoles with propargyl alcohols. Org. Biomol. Chem. 14 (16), 3878-3882 (2016).
- Mi, P., Kumar, R. K., Liao, P., Bi, X. Tandem O-H Insertion/[1,3]-Alkyl Shift of Rhodium Azavinyl Carbenoids with Benzylic Alcohols: A Route To Convert C-OH Bonds into C-C Bonds. Org. Lett. 18 (19), 4998-5001 (2016).
- Seo, B., et al. Sequential Functionalization of the O-H and C(sp2)-O Bonds of Tropolones by Alkynes and N-Sulfonyl Azides. Adv. Synth. Catal. 358 (7), 1078-1087 (2016).
- Miura, T., Nakamuro, T., Kiraga, K., Murakami, M. The stereoselective synthesis of α-amino aldols starting from terminal alkynes. Chem. Commun. 50 (72), 10474-10477 (2014).
- Hazen, G. G., Weinstock, L. M., Connell, R., Bollinger, F. W. A Safer Diazotransfer Reagent. Synth. Commun. 11 (12), 947-956 (1981).
- Easton, N. R., Bartron, L. R., Meinhofer, F. L., Fish, V. B. Synthesis of Some Substituted 3-Piperidones. J. Am. Chem. Soc. 75 (9), 2086-2089 (1953).
- Van den Branden, S., Compernolle, F., Hoornaert, G. J. Synthesis of lactam and ketone precursors of 2,7-substituted octahydro-pyrrolo[1,2-a]pyrazines and octahydro-2H-pyrido[1,2-a]pyrazines. J. Chem. Soc., Perkin Trans 1. 1 (8), 1035-1042 (1992).
- Aszodi, J., Rowlands, D. A., Mauvais, P., Collette, P., Bonnefoy, A., Lampilas, M. Design and synthesis of bridged γ-lactams as analogues of β-lactam antibiotics. Bioorg. Med. Chem. Lett. 14 (10), 2489-2492 (2004).
- D’hooghe, M., Baele, J., Contreras, J., Boelens, M., De Kimpe, N. Reduction of 5-(bromomethyl)-1-pyrrolinium bromides to 2-(bromomethyl)pyrrolidines and their transformation into piperidin-3-ones through an unprecedented ring expansion-oxidation protocol. Tetrahedron Lett. 49 (42), 6039-6042 (2008).

