Recording Brain Electrical Activity in Behaving Mice Using Multi-Shank Linear Silicon Probes
Abstract
Source: Sauer, J., et al. Recording Spatially Restricted Oscillations in the Hippocampus of Behaving Mice. J. Vis. Exp. (2018).
This video outlines a protocol for implanting a multi-shank linear silicon probe into the mouse brain to measure local field potentials. The procedure involves securing the probe to a stereotaxic unit, positioning it accurately over the target brain region, inserting it into the brain, and securing it with dental cement. After implantation, the assembly is protected with a casing. Following recovery, the setup allows for recording local field potentials, providing insights into neuronal activity.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Preparations
- Design and build an appropriate insertion tool transiently carrying the silicon probe and the electrode connector during the process of implantation. See Figure 1 for an example custom built insertion tool.
- Carefully release the silicon probe and electrode connector from its packaging using ceramic-tipped forceps.
- Lift the connector board and securely fix it with a crocodile clamp attached to a stand.
- Using a stereoscope, align the probe with the insertion tool with ceramic-tipped forceps. Apply a ~2 mm layer of paraffin wax melted with a cauterizer to glue the probe to the insertion tool. Take care not to touch the probe shanks during this procedure.
- Fix the electrode connector to the shaft of the insertion tool using standard adhesive tape. Note that depending on the manufacturer, ground wires might need to be soldered to the electrode connector board prior to implantation. Remove the insulation from two short pieces of varnish-insulated copper wire using tin-solder applied with a soldering iron (400 °C). Solder the ground wires to the appropriate slots in the electrode connector board.
- Remove the insulation of two additional pieces of copper wire. Wrap each bare copper wire three times around a stainless steel screw (1 mm diameter, 2 mm length). Apply flux suitable for soldering steel and solder the copper wire to the bottom of the screw cap. Make sure that the bottom half of the screw thread remains free of tin-solder.
- Use a standard multimeter to check for electrical contact between wire and screw.
- Disinfect the shanks of the silicon probe and the ground screws by immersion in 70% ethanol (10 s).
- Prepare a protective cover for the probe implant by cutting the head of a plastic Pasteur pipette in half.
2. Implantation Surgery
- Sterilize surgical instruments (scissors, fine-tipped forceps, surgical clamps) with a hot bead sterilizer. Wipe all surfaces with 70% ethanol.
- Induce anesthesia with 3% isoflurane in oxygen delivered at ~1 L/min.
- For maintenance, use 1 – 1.5% isoflurane. Note that the isoflurane concentration required to achieve surgical tolerance can vary from animal to animal.
- Stable surgical tolerance is achieved when the animal fails to respond to toe-pinching. Monitor the breathing rate of the mouse and adjust the concentration of isoflurane if necessary.
- Apply ointment to the animal's eyes to prevent drying out.
- Mount the mouse in a stereotaxic frame by gently inserting ear bars into the ear canal. Once the head of the mouse is stabilized by the ear bars, place a mouth piece over the snout for continuous isoflurane delivery. Place the mouse on towel or pad over a heating pad and inject buprenorphine subcutaneously (0.05 – 0.1 mg/kg body weight) to ensure postoperative analgesia.
- Shave the head with a standard shaver and disinfect the skin with 70% ethanol. Using surgical scissors, make an incision into the skin along the midline of the skull and open the skin using surgical clamps.
- Align the head of the animal with the aid of a stereotaxic alignment tool to level bregma and lambda. There should be less than 50 µm of height offset between bregma and lambda. Furthermore, level the head along the mediolateral axis by measuring the depth from bregma at the skull surface at defined distances left and right (e.g., 1 mm left and right of bregma). Adjust the tilt of the head if necessary.
- Clean the head with 3% hydrogen peroxide and wipe dry with sterile cotton wipes.
- Determine the location of the craniotomy relative to bregma using an appropriate stereotaxic atlas.
- Using a 0.9 mm drill head, drill two screw holes in the bone over the cerebellum to place ground and reference screws. Additionally, 1 – 3 holes for anchoring screws are desirable to stabilize the implant. The location of anchor screws will depend on the location of the craniotomy. For implantation into the hippocampus, place anchor screws over the contralateral parietal and ipsilateral frontal cortex. Insert the screws in the bone using a suitable screwdriver. Take care not to penetrate into the brain.
- Perform the craniotomy by slowly thinning the skull with the drill in a rectangular area around the implantation side. Frequently moisten the bone with sterilized phosphate buffer (PB). The remaining thinned skull can be gently pierced and removed with the aid of a fine (27G) injection needle and a pair of tweezers.
- Carefully pierce the dura mater with a thin (27G) injection needle. Form a small hook by bending the tip of the needle with a pair of tweezers and pull the dura for removal. Apply PB to prevent the brain surface from drying out.
- Mount the electrode insertion tool on a stereotaxic holder, zero the probe on bregma, and move the probe to the stereotaxic coordinates over the craniotomy. Slowly penetrate the brain surface. Make sure the probe shafts do not bend. Avoid implanting through blood vessels.
- Slowly lower the probe until ~200 µm above the desired depth. Cover the craniotomy and shanks of the silicon probe with sterilized Vaseline for protection. Apply dental cement to fix the base of the probe to the anchoring screw in the skull.
- Right after cement application, slowly move the probe to the target depth. Advancing the last ~200 µm after application of the cement reduces lateral movement of the probe and ensures minimal tissue damage in the target area. Note that the curing time of the cement used can affect this step of the protocol. With quickly curing cement, omit this step and directly implant the probe to the target depth in order to avoid damage to the silicon probe.
- After the cement has cured, release the probe from the insertion tool by melting the wax with a cauterizer.
- Release the connector board from the insertion device and position it at a suitable place on the skull using a crocodile clamp attached to the insertion handle. In case of probe implantation into the hippocampus, place the connector board on the contralateral parietal bone. Fix the connector board to the skull using dental cement.
- Solder the ground and reference wires of the connector board to the wires attached to the two screws over the cerebellum.
- Trim the protective cover to the correct height and place it over the silicon probe. Fix the cover to the connector board and skull using dental cement, avoiding the skin around the exposed skull. Suturing the skin around the implantation site is usually not required.
3. Recovery After Surgery
- Apply appropriate analgesic treatment for at least 2 days (e.g. subcutaneous injections of buprenorphine every 6 h during daytime and in the drinking water overnight combined with carprofen (4 – 5 mg/kg body weight) subcutaneously every 24 h). Single housing is recommended to prevent damage to the implant.
- Allow at least one week for recovery. Consult with local animal welfare guidelines.
4. Data Acquisition
- Record LFPs from freely moving mice using a suitable data acquisition system connected through a commutator. To acquire LFPs, use a sampling frequency of 1 – 5 kHz. Higher sampling rates (20 – 30 kHz) are required if single-unit discharges are to be recorded along with the LFP.
- Store raw recording files of the individual channels for offline analysis.
Representative Results
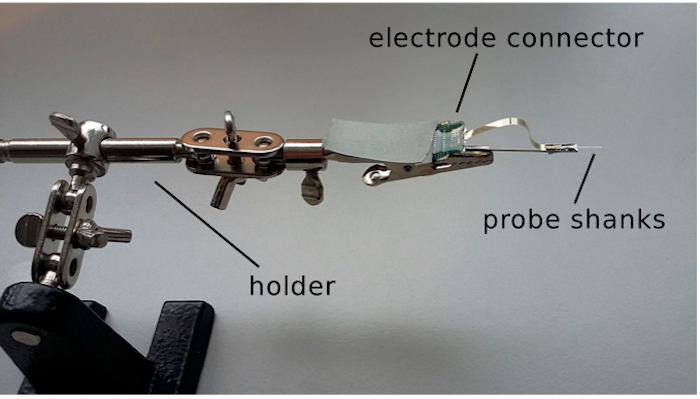
Figure 1: Image of the insertion tool. The silicon probe is attached to an insect pin glued to the base of a crocodile clamp. The shaft of the holder can be inserted in a stereotaxic micro manipulator for implantation.
Disclosures
The authors have nothing to disclose.
Materials
| Crocodile clamp with stand | Reichelt Elektronik | HALTER ZD-10D | |
| Silicon probe | Cambridge Neurotech | P-series 32 | |
| Stereoscope | Olympus | SZ51 | |
| Varnish-insulated copper wire | Bürklin Elektronik | 89 F 232 | |
| Ground screws | Screws & More GmbH (screwsandmore.de) | DIN 84 A2 M1x2 | |
| Flux | Stannol | 114018 | |
| Ceramic-tipped forceps | Fine Science Tools | 11210-60 | |
| Paraffine Wax | Sigma-Aldrich | 327204 | |
| Cauterizer | Fine Science Tools | 18010-00 | |
| Soldering iron | Kurtz Ersa | OIC1300 | |
| Ethanol | Carl Roth | 9065.1 | |
| Pasteur pipettes | Carl Roth | EA65.1 | |
| Heat sterilizer | Fine Science Tools | 18000-45 | |
| Stereotaxic frame | David Kopf | Model 1900 | |
| Stereotaxic electrode holder | David Kopf | Model 1900 | |
| Isoflurane | Abbvie | B506 | |
| Oxygen concentrator | Respironix | 1020007 | |
| Buprenorphine | Indivior UK Limited | ||
| Electrical shaver | Tondeo | Eco-XS | |
| Heating pad | Thermolux | 463265/-67 | |
| Surgical clamps | Fine Science Tools | 18050-28 | |
| Hydrogen peroxide | Sigma-Aldrich | H1009 | |
| Sterile cotton wipes | Carl Roth | EH12.1 | |
| Drill | Proxxon | Micromot 230/E | |
| 21G injection needle | B. Braun | 4657527 | |
| Phosphate buffer/phosphate buffered saline | |||
| Stereotaxic atlas | Elsevier | 9.78E+12 | |
| Surgical scissors | Fine Science Tools | 14094-11 | |
| Surgical forceps | Fine Science Tools | 11272-40 | |
| 27G injection needles | B. Braun | 4657705 | |
| Vaseline | |||
| Dental cement | Sun Medical | SuperBond T&M | |
| Carprofen | Zoetis | Rimadyl 50mg/ml | |
| Recording amplifier | Intan Technologies | C3323 | |
| USB acquisition board | Intan Technologies | C3004 | |
| Recording cables | Intan Technologies | C3216 | |
| Electrical commutator | Doric lenses | HRJ-OE_FC_12_HARW |

