Soxhlet Extraction of Lipid Biomarkers from Sediment
18,009 Views
•
•
개요
Source: Laboratory of Jeff Salacup - University of Massachusetts Amherst
Every lab needs standards that track the performance, accuracy, and precision of its instruments over time to ensure a measurement made today is the same as a measurement made a year from now (Figure 1). Because standards must test the performance of instruments over a long period of time, large volumes of the standards are often required. Many chemical standards can be purchased from retail scientific companies, like Sigma-Aldrich and Fisher. However, some compounds that occur in nature and that are relevant to paleoclimatic studies have not yet been isolated and purified for purchase. Therefore, these compounds need to be extracted from natural samples, and because of the large volumes of standards required, large volumes of sediment need to be extracted. The Accelerated Solvent Extraction (Dionex) and sonication extractions are not appropriate for the extraction of such large sediment volumes. In these circumstances, a Soxhlet extraction is used.
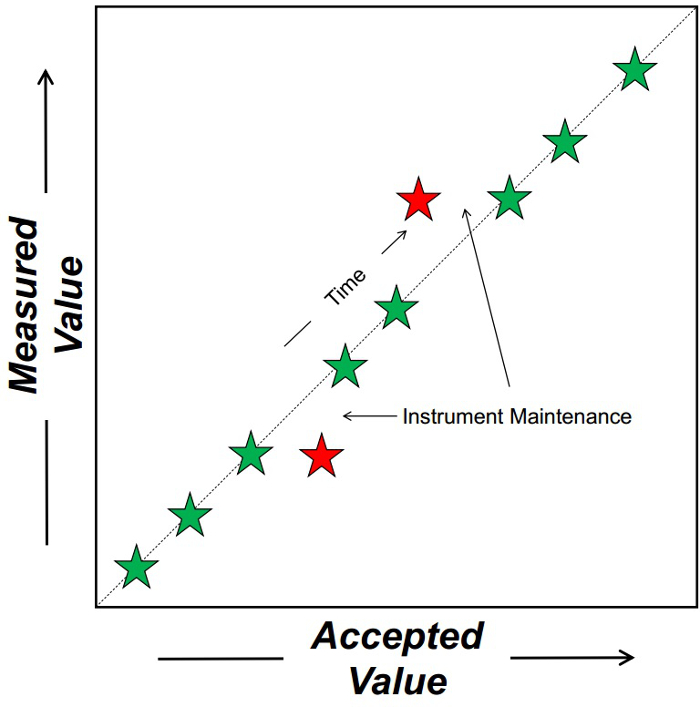
Figure 1. Schematic depicting how chemical standard tracks the performance of an instrument through time. The dashed line represents a 1:1 relationship between the accepted and measured (on the instrument) value of a variable. Each star is a weekly measurement of the chemical standard. Green stars represent standards that are accurate. Red stars reflect those that are not accurate indicating that the instrument requires corrective maintenance.
Principles
Soxhlet extraction is likely the oldest form of organic matter extraction. Archeological evidence from Mesopotamia places the use of a Soxhlet-like device that utilized hot water at ~3,500 BC1,2. Modern Soxhlets use sophisticated blown glass condensers and organic solvents in this "continuous" extraction method (Figure 2). Solvent is refluxed from a round-bottomed flask upward into a condenser with a recirculating cold-water coil. When the gaseous solvent contacts the coil, it condenses into a chamber with a glass fiber thimble holding the sample. This chamber is set with a recirculator, and when a certain volume is reached (generally a volume large enough to submerge the whole sample), the chamber is flushed back into the round-bottomed flask via a built-in siphon, where the lipid extract accumulates while the solvent becomes part of the next cycle. Hence, the term "continuous" extraction. Soxhlet extraction is often used for the extraction of larger (>10 g) samples.

Figure 2. A soxhlet apparatus.
Soxhlet extraction is a method of isolating compounds, such as lipids, from a large amount of solid material with a relatively small volume of solvent.
Many of the compounds relevant to paleoclimatic studies are not available to purchase from retail scientific companies. Standards of these compounds must therefore be prepared from natural samples.
Large quantities of standard are needed to assess the performance of an instrument over time. To obtain a suitable amount of a biomarker for standard preparation, a large volume of sediment must be extracted.
The Soxhlet extractor, invented in the 1870's by Franz von Soxhlet, allows automated, batch extraction from a solid, increasing the overall efficiency while using a small amount of solvent.
This video is part of a series on lipid extraction, purification, and analysis from sediments. It will illustrate Soxhlet extraction of lipid biomarkers from marine sediment for use in paleothermometry and will introduce a few other applications of Soxhlet extraction in Earth science and chemistry.
A typical assembly uses a round-bottomed flask, a cold water condenser, and the Soxhlet apparatus itself. The solid to be extracted is placed in a thimble in the central chamber of the apparatus. The extraction is aided by the addition of energy in the form of heat, known as refluxing. The solvent vapor rises through the distillation path in the Soxhlet apparatus to the condenser. Upon condensing, the solvent collects in the chamber, dissolving some of the organic material in the thimble. As the chamber fills, the siphon fills as well. When the siphon is full, the solution flows back into the flask. The solution level never exceeds the top of the thimble, so no solid enters the flask.
The lipid extract continually collects in the flask, whereas the solvent becomes part of the next extraction cycle. Thus, the cycle can repeat indefinitely without loss of solvent.
The conservation of the solvent, the continuous nature of the extraction, and the ability to accommodate large sample sizes makes Soxhlet extraction ideal for isolating organic compounds from large portions of insoluble material.
Now that you understand the principles of Soxhlet extraction, let's go through a procedure for Soxhlet extraction of lipid biomarkers from sediment.
For this experiment, a sample of excess marine sediment from a coring expedition is used. The sample will be freeze-dried, crushed, and homogenized. For more instruction, please reference this collection's video on Extraction by Sonication.
To prepare for the extraction, first make a 9:1 solution of dichloromethane to methanol. This solution will be used as the extraction solvent and to wash the glassware and laboratory instruments.
To remove organic contaminants, combust the round-bottomed flask, Soxhlet apparatus, glass fiber thimble, and weighing tins for 6 h at 550 °C. Wash a round-bottomed flask the DCM-methanol solution. Once ready to set up the extraction, rinse a laboratory spatula and five to ten boiling chips with the DCM-methanol solution.
To begin constructing the extraction assembly, set up a heating mantle in a fume hood. Obtain a condenser, a support stand to secure the round bottom flask, and the Soxhlet apparatus.
Tare a combusted weighing tin. With the solvent-rinsed spatula, transfer approximately 50 g of sample to the weighing tin and record the mass. Load the material into the combusted glass fiber thimble.
Next, fill the combusted and rinsed round-bottomed flask slightly more than half full of the DCM-methanol solution. Add the washed boiling chips and place the round-bottomed flask in the heating mantle.
Then, place the sample thimble open end up in the chamber of the Soxhlet apparatus. Connect the apparatus to the round-bottomed flask and clamp the apparatus in place.
Secure the condenser to the top of the Soxhlet apparatus. Connect the cold water line to the lower port of the condenser with a hose clamp or zip tie. Connect the outlet line to the upper port of the condenser and route it to the drain.
Turn on the water to the condenser and verify the flow path. Then, turn on the heating mantle and heat the solvent to reflux.
As the solvent begins condensing, ensure that the condensate is dripping into the chamber and that the extract is siphoned into the round-bottomed flask. The solvent should stay at a low boil throughout the extraction.
Monitor the extraction process and the condenser water flow until the extraction is complete. Then, stop the extraction by turning off the heating mantle. Once the extract has cooled, remove the condenser and Soxhlet apparatus. Finally, seal the round-bottomed flask containing the total lipid extract and store for further processing.
Soxhlet extraction is often used for chemical analysis of a solid sample, and can also be used for reagent preparation and purification.
Soxhlet extraction can be used to detect the presence of polychlorinated biphenyl compounds, or PCBs, in the environment. The transfer efficiency of PCBs from prey fish to predator fish was measured to gain more information about the health risks to humans and wildlife from eating contaminated fish. Soxhlet extraction of fish tissue allows preparation of samples for gas chromatography and mass spectrometry.
Compounds to be introduced to the environment in large quantities are analyzed for the presence of PCBs. Biochar is a byproduct of pyrolysis of organic matter that, when added to soil, may improve soil quality and take up pollutants. Validation of biochar production methods for widespread use includes Soxhlet extraction to test for the presence of PCBs by gas chromatography.
Soxhlet extraction can also be used to purify a solid by extraction of unwanted compounds. Long-chain fatty acids were selectively removed from tomato skins by stepwise extraction to yield the wax-free tomato cuticle. The stepwise extraction was performed with multiple solvents of varying polarities in succession. This not only provided comprehensive wax removal from the tomato skin, but allowed isolation of individual wax moieties based on solubility characteristics as well.
You've just watched JoVE's introduction to Soxhlet extraction of lipid biomarkers from geological archive sediments. You should now be familiar with the principles behind Soxhlet extraction, the procedure for Soxhlet extraction of a sediment sample, and some examples of how Soxhlet extraction may be used for analytical purposes.
Thanks for watching!
Procedure
1. Setup and Preparation of Materials
- Collect a sample of frozen, freeze-dried, crushed, and homogenized marine sediment. A sample like this contains many of the compounds needed for standards.
- Standards are often made from sediments that are left over after a coring expedition or analysis. For example, in this experiment, sediment that was obtained from the 'Mud Patch' located just south of Cape Cod is extracted. This sediment was taken as part of a coring expedition but will not be used to answer scientific questions. We can therefore use it to make a standard.
- Place a ~100 g chunk of the sediment into the freezer overnight so that it freezes through.
- Once the sediment is completely frozen, turn on the freeze dryer (available from many scientific equipment retailers like Fisher) and wait until the condenser reaches it setpoint (often ~-30 °C).
- Load the sediment sample into the freeze dryer and close the purge to begin pulling a vacuum on the sample.
- Depending on the amount of water in the sediment, and the size of the sample, it may take several days for the sample to dry.
- Once the sample is dry, turn off the freeze dryer, vent it, and remove the sample.
- Place the sample in a solvent rinsed mortar and grind to a powder using a pestle. Do this to the entire sample and store in a glass jar in the freezer until ready to extract.
- Depending on the size of the sample, use vials with volumes ranging from 4-60 mL. For this experiment, use borosilicate glass vials (40 mL) and solvent safe caps. Combust the vials, borosilicate glass pipettes, and weighing tins at 550 °C for 6 h prior to ensure removal of possible organic contaminants.
- Obtain dichloromethane and methanol (both are common in most organic geochemistry laboratories), then use them individually to rinse lab tools and glassware before use. A mixture of dichloromethane (DCM) to methanol (MeOH; 9:1) is used in many labs to efficiently extract biomarkers with a wide range of polarities. Solvents should be free of organic contaminants.
- Acquire a soxhlet apparatus to use in this experiment (these can be purchased from Fisher Scientific or other science retailer), then wash and combust it at 550 °C for 6 h prior to use.
- Obtain glass fiber thimbles (can be purchased from Whatman) and combust them at 550 °C for 6 h prior to use.
2. Preparation of Sample
- Place a combusted weighing tin on the lab scale and then tare.
- Rinse the lab spatula with solvent, then use it to transfer an appropriate mass of sample into the weighing tin, and record the mass.
- The mass of the sample varies depending on its organic matter content. Relatively organic matter lean material (marine mud) may require several grams, while organic matter rich material (leaf tissue) may require much less.
- Transfer all of the material in the weighing tin into a combusted glass fiber thimble.
3. Extraction
- Transfer ~400 mL of the DCM:MeOH (9:1) mixture into the round-bottomed flask (flask should be more than half full) and put in heating mantle. Add several (5-10) solvent-rinsed boiling chips.
- Place the sample thimble, open-end up, into the centerpiece of the Soxhlet apparatus.
- Place the centerpiece on top of the round-bottomed flask and secure with a glassware clamp.
- Install the condenser on top of the centerpiece of the Soxhlet and secure with a glassware clamp.
- Attach one of the cold water lines from the condenser to the cold water line in the hood using a hose clamp. Route the other into the drain.
- Turn on the water to ensure proper circulation and drainage.
- Turn on the heating mantle and adjust the temperature until the solvent in the round-bottomed flask is lightly boiling.
- Monitor the extraction a few times over the next hour.
- Check to make sure the temperature is properly set at a low boil, the solvent is condensing in the condenser and dripping into the center piece, the center piece is filling and emptying properly, and the water is properly draining into the hood drain.
- Monitor the extraction over the next 36 h.
- Ensure the temperature is properly set at a low boil, the solvent is condensing in the condenser and dripping into the center piece, the center piece is filling and emptying properly, the water is properly draining into the hood drain, and the solvent level in the round-bottomed flask is still about half full.
- After 36 h, stop the extraction by turning off the heating mantle.
- Label the flask "TLE".
Soxhlet extraction is a method of isolating compounds, such as lipids, from a large amount of solid material with a relatively small volume of solvent.
Many of the compounds relevant to paleoclimatic studies are not available to purchase from retail scientific companies. Standards of these compounds must therefore be prepared from natural samples.
Large quantities of standard are needed to assess the performance of an instrument over time. To obtain a suitable amount of a biomarker for standard preparation, a large volume of sediment must be extracted.
The Soxhlet extractor, invented in the 1870's by Franz von Soxhlet, allows automated, batch extraction from a solid, increasing the overall efficiency while using a small amount of solvent.
This video is part of a series on lipid extraction, purification, and analysis from sediments. It will illustrate Soxhlet extraction of lipid biomarkers from marine sediment for use in paleothermometry and will introduce a few other applications of Soxhlet extraction in Earth science and chemistry.
A typical assembly uses a round-bottomed flask, a cold water condenser, and the Soxhlet apparatus itself. The solid to be extracted is placed in a thimble in the central chamber of the apparatus. The extraction is aided by the addition of energy in the form of heat, known as refluxing. The solvent vapor rises through the distillation path in the Soxhlet apparatus to the condenser. Upon condensing, the solvent collects in the chamber, dissolving some of the organic material in the thimble. As the chamber fills, the siphon fills as well. When the siphon is full, the solution flows back into the flask. The solution level never exceeds the top of the thimble, so no solid enters the flask.
The lipid extract continually collects in the flask, whereas the solvent becomes part of the next extraction cycle. Thus, the cycle can repeat indefinitely without loss of solvent.
The conservation of the solvent, the continuous nature of the extraction, and the ability to accommodate large sample sizes makes Soxhlet extraction ideal for isolating organic compounds from large portions of insoluble material.
Now that you understand the principles of Soxhlet extraction, let's go through a procedure for Soxhlet extraction of lipid biomarkers from sediment.
For this experiment, a sample of excess marine sediment from a coring expedition is used. The sample will be freeze-dried, crushed, and homogenized. For more instruction, please reference this collection's video on Extraction by Sonication.
To prepare for the extraction, first make a 9:1 solution of dichloromethane to methanol. This solution will be used as the extraction solvent and to wash the glassware and laboratory instruments.
To remove organic contaminants, combust the round-bottomed flask, Soxhlet apparatus, glass fiber thimble, and weighing tins for 6 h at 550 °C. Wash a round-bottomed flask the DCM-methanol solution. Once ready to set up the extraction, rinse a laboratory spatula and five to ten boiling chips with the DCM-methanol solution.
To begin constructing the extraction assembly, set up a heating mantle in a fume hood. Obtain a condenser, a support stand to secure the round bottom flask, and the Soxhlet apparatus.
Tare a combusted weighing tin. With the solvent-rinsed spatula, transfer approximately 50 g of sample to the weighing tin and record the mass. Load the material into the combusted glass fiber thimble.
Next, fill the combusted and rinsed round-bottomed flask slightly more than half full of the DCM-methanol solution. Add the washed boiling chips and place the round-bottomed flask in the heating mantle.
Then, place the sample thimble open end up in the chamber of the Soxhlet apparatus. Connect the apparatus to the round-bottomed flask and clamp the apparatus in place.
Secure the condenser to the top of the Soxhlet apparatus. Connect the cold water line to the lower port of the condenser with a hose clamp or zip tie. Connect the outlet line to the upper port of the condenser and route it to the drain.
Turn on the water to the condenser and verify the flow path. Then, turn on the heating mantle and heat the solvent to reflux.
As the solvent begins condensing, ensure that the condensate is dripping into the chamber and that the extract is siphoned into the round-bottomed flask. The solvent should stay at a low boil throughout the extraction.
Monitor the extraction process and the condenser water flow until the extraction is complete. Then, stop the extraction by turning off the heating mantle. Once the extract has cooled, remove the condenser and Soxhlet apparatus. Finally, seal the round-bottomed flask containing the total lipid extract and store for further processing.
Soxhlet extraction is often used for chemical analysis of a solid sample, and can also be used for reagent preparation and purification.
Soxhlet extraction can be used to detect the presence of polychlorinated biphenyl compounds, or PCBs, in the environment. The transfer efficiency of PCBs from prey fish to predator fish was measured to gain more information about the health risks to humans and wildlife from eating contaminated fish. Soxhlet extraction of fish tissue allows preparation of samples for gas chromatography and mass spectrometry.
Compounds to be introduced to the environment in large quantities are analyzed for the presence of PCBs. Biochar is a byproduct of pyrolysis of organic matter that, when added to soil, may improve soil quality and take up pollutants. Validation of biochar production methods for widespread use includes Soxhlet extraction to test for the presence of PCBs by gas chromatography.
Soxhlet extraction can also be used to purify a solid by extraction of unwanted compounds. Long-chain fatty acids were selectively removed from tomato skins by stepwise extraction to yield the wax-free tomato cuticle. The stepwise extraction was performed with multiple solvents of varying polarities in succession. This not only provided comprehensive wax removal from the tomato skin, but allowed isolation of individual wax moieties based on solubility characteristics as well.
You've just watched JoVE's introduction to Soxhlet extraction of lipid biomarkers from geological archive sediments. You should now be familiar with the principles behind Soxhlet extraction, the procedure for Soxhlet extraction of a sediment sample, and some examples of how Soxhlet extraction may be used for analytical purposes.
Thanks for watching!
Results
At the end of extraction, a total lipid extract (TLE) for the sample is produced. The round-bottomed flask contains the extractable organic matter from the sediment sample. This TLE can now be analyzed, and its chemical constituents identified and quantified.
Applications and Summary
The extract from the marine mud contains compounds called alkenones, which are used in paleoceanography. Alkenones are long-chained alkyl-ketones produced by certain classes of haptophyte algae that live in the sunlit surface ocean3 (Figure 3). The two most common alkenones are 37 carbon atoms long and have two or three double bonds in them. The haptophytes adjust the ratio of these two alkenones in their cells according to the temperature of the water they live in. The ratio of the two alkenones defines the Uk'37 ratio:
Equation 1) Uk'37 = (C37:2) / (C37:2 + C37:3) 4,5
Culture6,7 and core-top sediment8 calibration studies led to the development of the Uk'37 Index as a quantitative SST proxy. In this work we use:
Equation 2) Uk'37 = 0.034(SST) + 0.039; ±1.4 °C from 0 to 28 °C [culture-based7]
Alkenones are preserved in sediments dating as far back as the Early Eocene (~56 million years ago)9. Knowing the distribution of alkenones in a sediment core through time relates information on the evolution of sea surface temperature at that location. However, it's necessary to first make sure the instrument accurately and precisely measures the ratio of the two alkenones, and that is why standards are needed.

Figure 3. Alkenones with 2 (C37:2) and 3 (C37:3) double bonds (left) are produced by certain haptophyte algae that live in the sunlit surface ocean (right). (Photo courtesy of Tim I. Eglinton, Woods Hole Oceanographic Institution)
References
- Jensen, W. B. The Origin of the Soxhlet Extractor J Chem Ed. 84, 1913-1914, (2007).
- Levey, M. Chemistry and Technology in Ancient Mesopotamia, Elsevier. 33-34, (1959).
- Conte, M. H., Thompson, A., Eglinton, G. Primary production of lipid biomarker compounds by Emiliania huxleyi: results from an experimental mesocosm study in fjords of southern Norway, Sarsia, 79, 319-332 (1994).
- Brassell, S. C., Eglinton, G., Marlowe, I. T., Pflaumann, U., Sarnthein, M. Molecular Stratigraphy – a New Tool for Climatic Assessment, Nature, 320 (6058), 129-133 (1986).
- Herbert, T. D. Alkenone paleotemperature determinations, in Treatise in Marine Geochemistry, edited by H. Elderfield, Elsevier 391-432 (2003).
- Prahl, F. G., Wakeham S. G., Calibration of Unsaturation Patterns in Long-Chain Ketone Compositions for Paleotemperature Assessment, Nature, 330(6146), 367-369 (1987).
- Prahl, F. G., Muehlhausen, L. A., Zahnle, D. L. Further evaluation of long-chain alkenones as indicators of paleoceanographic conditions, Geochimica et Cosmochimica Acta, 52(9), 2303-2310 (1988).
- Müller, P. J. et al. Calibration of the alkenone paleotemperature index U37K′ based on core-tops from the eastern South Atlantic and the global ocean (60°N-60°S), Geochimica et Cosmochimica Acta, 62(10), 1757-1772 (1998).
- Marlowe, I. T. et al. Long-chain Alkenones and Alkyl Alkenoates and the Fossil Coccolith Record of Marine-sediments, Chem Geol, 88(3-4), 349-375 (1990).
내레이션 대본
Soxhlet extraction is a method of isolating compounds, such as lipids, from a large amount of solid material with a relatively small volume of solvent.
Many of the compounds relevant to paleoclimatic studies are not available to purchase from retail scientific companies. Standards of these compounds must therefore be prepared from natural samples.
Large quantities of standard are needed to assess the performance of an instrument over time. To obtain a suitable amount of a biomarker for standard preparation, a large volume of sediment must be extracted.
The Soxhlet extractor, invented in the 1870’s by Franz von Soxhlet, allows automated, batch extraction from a solid, increasing the overall efficiency while using a small amount of solvent.
This video is part of a series on lipid extraction, purification, and analysis from sediments. It will illustrate Soxhlet extraction of lipid biomarkers from marine sediment for use in paleothermometry and will introduce a few other applications of Soxhlet extraction in Earth science and chemistry.
A typical assembly uses a round-bottomed flask, a cold water condenser, and the Soxhlet apparatus itself. The solid to be extracted is placed in a thimble in the central chamber of the apparatus. The extraction is aided by the addition of energy in the form of heat, known as refluxing. The solvent vapor rises through the distillation path in the Soxhlet apparatus to the condenser. Upon condensing, the solvent collects in the chamber, dissolving some of the organic material in the thimble. As the chamber fills, the siphon fills as well. When the siphon is full, the solution flows back into the flask. The solution level never exceeds the top of the thimble, so no solid enters the flask.
The lipid extract continually collects in the flask, whereas the solvent becomes part of the next extraction cycle. Thus, the cycle can repeat indefinitely without loss of solvent.
The conservation of the solvent, the continuous nature of the extraction, and the ability to accommodate large sample sizes makes Soxhlet extraction ideal for isolating organic compounds from large portions of insoluble material.
Now that you understand the principles of Soxhlet extraction, let’s go through a procedure for Soxhlet extraction of lipid biomarkers from sediment.
For this experiment, a sample of excess marine sediment from a coring expedition is used. The sample will be freeze-dried, crushed, and homogenized. For more instruction, please reference this collection’s video on Extraction by Sonication.
To prepare for the extraction, first make a 9:1 solution of dichloromethane to methanol. This solution will be used as the extraction solvent and to wash the glassware and laboratory instruments.
To remove organic contaminants, combust the round-bottomed flask, Soxhlet apparatus, glass fiber thimble, and weighing tins for 6 h at 550 °C. Wash a round-bottomed flask the DCM-methanol solution. Once ready to set up the extraction, rinse a laboratory spatula and five to ten boiling chips with the DCM-methanol solution.
To begin constructing the extraction assembly, set up a heating mantle in a fume hood. Obtain a condenser, a support stand to secure the round bottom flask, and the Soxhlet apparatus.
Tare a combusted weighing tin. With the solvent-rinsed spatula, transfer approximately 50 g of sample to the weighing tin and record the mass. Load the material into the combusted glass fiber thimble.
Next, fill the combusted and rinsed round-bottomed flask slightly more than half full of the DCM-methanol solution. Add the washed boiling chips and place the round-bottomed flask in the heating mantle.
Then, place the sample thimble open end up in the chamber of the Soxhlet apparatus. Connect the apparatus to the round-bottomed flask and clamp the apparatus in place.
Secure the condenser to the top of the Soxhlet apparatus. Connect the cold water line to the lower port of the condenser with a hose clamp or zip tie. Connect the outlet line to the upper port of the condenser and route it to the drain.
Turn on the water to the condenser and verify the flow path. Then, turn on the heating mantle and heat the solvent to reflux.
As the solvent begins condensing, ensure that the condensate is dripping into the chamber and that the extract is siphoned into the round-bottomed flask. The solvent should stay at a low boil throughout the extraction.
Monitor the extraction process and the condenser water flow until the extraction is complete. Then, stop the extraction by turning off the heating mantle. Once the extract has cooled, remove the condenser and Soxhlet apparatus. Finally, seal the round-bottomed flask containing the total lipid extract and store for further processing.
Soxhlet extraction is often used for chemical analysis of a solid sample, and can also be used for reagent preparation and purification.
Soxhlet extraction can be used to detect the presence of polychlorinated biphenyl compounds, or PCBs, in the environment. The transfer efficiency of PCBs from prey fish to predator fish was measured to gain more information about the health risks to humans and wildlife from eating contaminated fish. Soxhlet extraction of fish tissue allows preparation of samples for gas chromatography and mass spectrometry.
Compounds to be introduced to the environment in large quantities are analyzed for the presence of PCBs. Biochar is a byproduct of pyrolysis of organic matter that, when added to soil, may improve soil quality and take up pollutants. Validation of biochar production methods for widespread use includes Soxhlet extraction to test for the presence of PCBs by gas chromatography.
Soxhlet extraction can also be used to purify a solid by extraction of unwanted compounds. Long-chain fatty acids were selectively removed from tomato skins by stepwise extraction to yield the wax-free tomato cuticle. The stepwise extraction was performed with multiple solvents of varying polarities in succession. This not only provided comprehensive wax removal from the tomato skin, but allowed isolation of individual wax moieties based on solubility characteristics as well.
You’ve just watched JoVE’s introduction to Soxhlet extraction of lipid biomarkers from geological archive sediments. You should now be familiar with the principles behind Soxhlet extraction, the procedure for Soxhlet extraction of a sediment sample, and some examples of how Soxhlet extraction may be used for analytical purposes.
Thanks for watching!
