Induction of Cerebral Arterial Gas Embolism in Rat
Summary
This protocol provides a detailed description of inducing cerebral air emboli in rats. It compares direct injection into the common carotid artery and introduction through the external carotid artery. It provides a technical description of the air bubble generator, the effect of different air volumes, and procedural challenges.
Abstract
We present a methodological approach for preclinical research of cerebral arterial gas embolism (CAGE), a condition characterized by gas bubbles within the cerebral circulation causing multifocal ischemia. The current work describes two surgical methods to induce CAGE in the rat: one with injection of air via the external carotid artery (ECA), thereby sacrificing the vessel, and one via direct injection into the common carotid artery (CCA). Male Wistar rats were used and divided into groups (n=5) to undergo either the ECA- or CCA-entry method with injection of different air emboli volumes (6000, 7000 and 8000 nL) or sham surgery. A custom-made bubble generator was used to produce gas emboli with consistent size, and open-source software was developed for real-time bubble analysis. The comparison between the two methods revealed the CCA approach to be superior in terms of consistent bubble production within the bubble generator, reduced embolization time and fewer complications.
Introduction
Cerebral arterial gas embolism (CAGE) is characterized by the lodging of gas bubbles within the cerebral arterial circulation, leading to a spectrum of neurological impairments. This condition is predominantly known as a complication in diving, where overexpansion of the lungs during ascent results in barotrauma and air entry into the pulmonary veins, which then flow to the cerebral arteries1. In addition to this well-known occurrence in diving, medical professionals are increasingly recognizing CAGE as a complication of invasive medical procedures. Iatrogenic air embolism can occur during the placement, handling, or removal of central venous catheters and chest drains, as well as throughout the course of open and endovascular procedures, including heart valve interventions, thoracic endovascular aortic repairs and endovascular thrombectomy in ischemic stroke2,3. Despite its clinical significance, the research on CAGE, particularly using animal models, remains sparse and fragmented4.
Since the pioneering study by Rosengren et al. in 1977 with rats, animal models for CAGE have undergone significant refinement5. The approach employed by Rosengren involved cannulation of the common carotid artery (CCA) to introduce a total volume of 10 µL of air. This technique was not without limitations, including altered hemodynamics due to arterial ligation and the uncontrolled size and excessive volume of the air embolus6. Furlow's method, described in 1982, improved the precision of air embolization by advancing a catheter into the internal carotid artery and administering a total air volume of 5 µL. However, although its importance was recognized early, the concept of uniform bubble size was only implemented decades later. Gerriets et al. were able to produce a consistent number of bubbles with a uniform diameter, initially 160 µm, later reduced to 45 µm7,8. The surgical method used here required sacrificing the external carotid artery (ECA). Recently, Schaefer et al. introduced a less invasive method by inserting a microcatheter into the CCA through the femoral artery, more accurately mimicking air embolism scenarios seen during endovascular procedures9. Their method had the limitation of not ligating the arterial branches of the CCA (for example, ECA and pterygopalatine artery (PPA)), thereby allowing bubbles to not only flow to the desired cerebral arteries but also to non-cerebral territories. This may result in inconsistent cerebral ischemic damage, complicating the reproducibility of experiments.
Despite the advancements in preclinical CAGE models, challenges remain in replicating bubble generation techniques, standardizing surgical methods, and acquiring consistent cerebral lesions. The current study introduces both a conventional surgical approach that requires sacrificing the ECA and an alternative method in which air bubbles are injected directly into the CCA. We report detailed procedures, challenges, and open-source software for real-time bubble analysis. We also include the technical details needed to build a bubble generator.
Protocol
All procedures involving animals were conducted in accordance with the Guide for Use and Care of Laboratory Animals. We obtained full approval from the Central Committee on Animal Experiments of The Netherlands (AVD11800202114839). Male Wistar rats with a weight range of 300 – 350 g were used. Animals were housed in pairs with food and water ad libitum and 12 h light-dark cycles. Upon arrival, animals underwent a 7-day acclimatization period before any experimental procedure was initiated.
NOTE: We used two surgical methods, the ECA-entry method and the CCA-entry method. For both techniques, rats were randomized into Vehicle or 8000 nL CAGE groups using a statistical analysis tool. Vehicle treatment included saline injection only; CAGE treatment used air bubbles in saline to a volume of 8000 nL, with each bubble having a target diameter of 160 µm. After the completion of this series, further refinement of the model with 6000 nL and 7000 nL (both with 160 µm bubble diameter) was done only through the CCA-entry method. In case of procedural failure, replacement rats were added to obtain final group sizes of n=5.
1. Air bubble generator
NOTE: The air bubble generator (Figure 1, Figure 2, and Supplementary Figure 1A-C) consists of several custom-made components that generate and detect equally sized-gas bubbles.
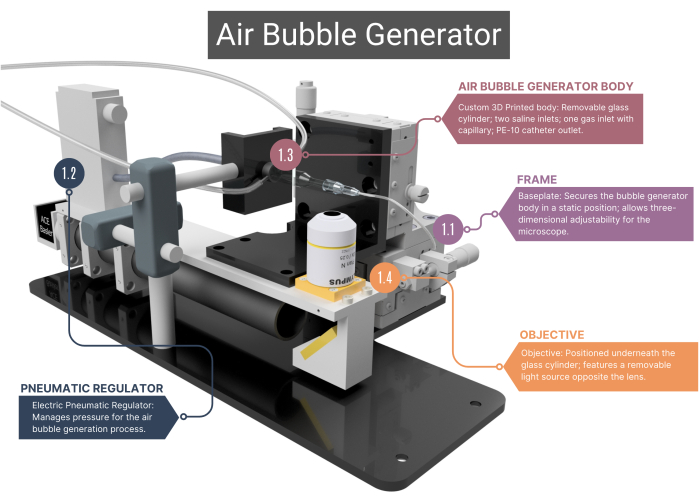
Figure 1: Overview of the air bubble generator. The bubble generator comprises a supporting frame (1.1) and a pneumatic regulator for airflow management (1.2). It includes a 3D-printed main body housing a glass capillary (1.3), linked to an objective and high-speed camera (1.4). See Supplementary Figure 1A-C for more images. Numbers correspond with the methodological steps in the main text. Please click here to view a larger version of this figure.
- Frame
- Use a frame with a custom-made stainless-steel baseplate that ensures the static positioning of the bubble generator's main body, while the microscope objective positioned underneath it can be moved in three dimensions. This adjustment capability is facilitated by XYZ assemblies that enable alterations in the field of view and allow focus on the tip of the microchannel and air bubbles.
- Pneumatic regulator
- For compressed air supply, use an electric pneumatic regulator, which has a pressure range of 0.001 to 0.1 MPa. Ensure the regulator is connected to the glass capillary through intravenous (IV) tubing, air-sealed with heat shrink-sleeving. Regulate pressure by changing the regulator's voltage.
- Air bubble generator body
NOTE: The main body of the bubble generator consists of a glass chamber, plastic inner housing, and a glass capillary. All components of the body need to be airtight, ensuring that no leakage of air or saline is possible.- Ensure that the custom-made cylindrical glass chamber has an inner diameter (ID) of 1000 µm that merges into a microchannel with an ID of 400 µm. Flatten the top side of this cylindrical shaped microchannel to obtain an optimal view through the objective.
- Fit the outer end of the glass cylinder to a 21G needle with the tip removed. Inside this needle, glue a second needle (27G, ID 210 µm). Fit this smaller diameter needle into the microchannel and attach it to a polyethylene (PE-10) catheter (ID 280 µm, length 125 mm).
- Fit a plastic body onto the back of the glass chamber, which features two stainless steel inlets; here, the IV tubing for the entry of saline can be attached. The rear of this body has an inlet for gas through a custom glass capillary that is positioned with O-ring rubbers and locked in place by screwing on a cap.
- For the CCA-entry method, attach the end of the catheter to a needle (30G, ID 159 µm) with the plastic Luer-lock housing removed and carefully bend it to an angle of 45°.
- Microscope adjustment
- Position the objective (4x) underneath the bubble generator body and connect it to a high-speed camera. Opposite the lens, place an adjustable LED light source.
- Before the start of each experiment, make sure the objective lens is clean. Dirt will result in incorrect bubble analyses.
- Creation of the glass capillary
NOTE: The process of creating capillaries involves a significant amount of finetuning. While the settings provided serve as a baseline, adjustments may be necessary to achieve the desired bubble diameter.- Make the glass capillary using a micro-pipette puller (Supplementary Figure 1A-C). To create the pipettes, set up the micropipette puller heaters: set the left heater (no. 1) to adjustment = 70 and the right heater (no 2) to adjustment = 980.
- Place the glass capillary into the puller and add two blocks of 100 g weight to ensure the correct shape after the pulling process. Press Start. Once the pulling process is completed, two capillaries are formed.
- Cut or break each capillary diagonally at the tip using a bright field microscope. The shape and diameter of the tip are critical as they influence the diameter of the gas bubbles. To ensure consistency in bubble size across experiments, use the same capillary for the desired bubble diameter.
- Insert the glass capillary tip into the cylindrical channel (ID 400 µm) of the bubble generator body.
- Setting up the microbubble generator
- Connect the bubble generator to a syringe pump with sterile saline (50 mL) and start a continuous flow at 20 mL/h. Make sure there is no air trapped in the system. In case air is trapped in the system, hold the bubble generator vertically so the air can escape through the catheter.
- Turn on the light source, camera, and software.
- When the system is at the correct pressure (after approximately 2 min), check the system for gas or fluid leakage.
- To create bubbles, slowly increase pressure by increasing the voltage, starting from 2.1 V and continuing until gas fills up the glass capillary. This may take some time, but it is important not to rush this part. When the pressure in the glass capillary is equal to that in the channel, the production of gas bubbles can start. By simply increasing the voltage up to 2.6 V, bubbles will be produced.
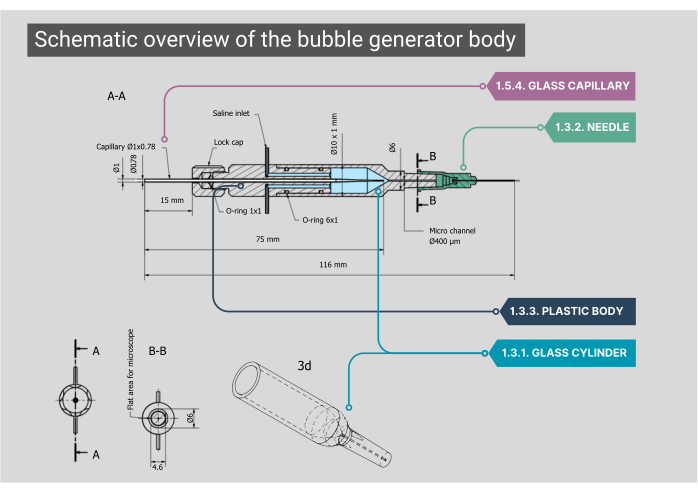
Figure 2: Schematic overview of the bubble generator body. A detailed blueprint of the bubble generator body, including dimensions and annotations for each component. Numbers correspond with the methodological steps in the main text. Please click here to view a larger version of this figure.
2. Gas bubble detection and analysis
NOTE: The Python code (Supplementary File 1) is designed to track and calculate the number, diameter, and volume of each gas bubble in real time. During the production of gas bubbles, the code processes each detected bubble as it moves through the field of view. This data is continuously displayed as the bubble count, cumulative volume of air, average bubble diameter, and total duration of the recording. After every recording, data is exported into a spreadsheet and .mp4 video file.
- Camera settings (Supplementary Figure 2)
- When the microbubble generator is set up correctly, turn on the camera in the Pylon Viewer. Select the correct gain and threshold.
- Find the correct region of interest by moving the X- and Y-axis of the bubble generator and enhance the sharpness by moving the Z-axis.
- To optimize the frame rate (171 fps), set the camera to 2480 x 400 pixels (width x height). When the camera setup is finished, make sure to turn off the camera in Pylon Viewer.
- Real-time bubble analysis (Supplementary Figure 2)
- Before starting an experiment, perform a test run of the script while producing gas bubbles. Check the recording and adjust the threshold and gain when necessary.
- To start the analysis of the bubbles, run the Python code. The code will automatically display the live video, bubble count, diameter, and volume.
3. CAGE surgery
- Preparation for the surgery
- Prepare the surgical area with a sterile cloth under the rat and lay out all surgical instruments and sutures on a second sterile cloth. Prepare necessary medication.
- Ensure the heating pad, with feedback system, is set to 37 °C. Adjust the microscope to the correct height.
- Remove the rat from its home cage and prepare for anesthesia.
- Anesthesia and pre-surgical care
- Administer 0.05 mg/kg buprenorphine subcutaneously 30 min before surgery.
- Anesthetize the rat using 4% isoflurane and 1 L/min air mixture (30% oxygen and 70% air) in the induction chamber. Once fully anesthetized, weigh the rat and shave the surgical neck area using an electric razor.
- Move the rat to the surgical table and switch the isoflurane to the facial mask to maintain anesthesia with 2%-2.5% isoflurane in 70%/30% air mix.
- Apply artificial tears to the eyes to prevent drying. Lubricate the temperature probe and insert it rectally.
- Position the rat in a supine position and place a 10 mL syringe under the neck for easy access to the surgical area.
- Secure the front paws to the surgery table using tape. Clean the surgical neck area thoroughly with betadine to ensure sterility. Confirm depth of anesthesia by toe pinch.
- Incision
- Make a midline incision with fine scissors from the sternal notch to below the mandible (approximately 1.5 cm).
- Use two tweezers to dissect the connective tissue (superficial cervical fascia), exposing the underlying muscles.
- Separate the left and right sternohyoid muscles by gently tearing the connective tissue in between.
- Identify and retract the following muscles underneath: right sternomastoid muscle (retract using a tissue-hook laterally) and right omohyoid muscle (retract using a tissue-hook caudal-medially). The right-sided carotid triangle (CCA, ECA, internal carotid artery (ICA)) should be visible.
- Surgical preparation of the CCA
- Prepare the CCA by removing the overlying fascia and adipose tissue. Carefully separate the CCA from the vagus nerve without damaging the nerve. Do not grab or pinch the nerve or its branches. Only gently push it away from the CCA. Do not touch or press against the trachea while preparing the vessels.
- Place a 3-0 thread around the CCA using curved tweezers and secure the thread with hemostats. This will later serve to elevate the CCA.
- Temporary ligation of the ECA, ICA, and their branches
- Locate and expose the ECA and the ICA bifurcation. Clear surrounding fascia and adipose tissue if needed. Temporarily ligate the ECA with 3-0 suture.
- Locate the occipital artery (OA, mainly branching from the ICA, in the middle between the Y-shaped bifurcation of ICA and ECA origin) and the superior thyroid artery (branching from the medial side of the ECA). These small branches rupture easily. Temporarily ligate the OA with a 3-0 suture proximally.
- Following the ICA distally, the next branch moving laterally is the pterygopalatine artery (PPA). Clear its surrounding fascia and adipose tissue while being very careful not to damage the vagus nerve. Temporarily ligate the PPA with a 3-0 suture.
NOTE: It is recommended to approach the PPA from a cranial direction; tilting the rat slightly sideways can facilitate easier access and visualization during surgery.
- ECA-entry method (Figure 3A)
- Ligate the arteries as follows: use the suture from step 3.4.2. to gently and temporarily ligate the CCA, ensuring the flow is stopped but minimizing the risk of creating thrombotic cloths. Then, use a 3-0 suture or vascular clip to temporarily ligate the ICA and OA.
- Apply two sutures around the ECA: one proximal and one distal. Ensure the distal suture includes the superior thyroid artery and is tightened to stop blood flow permanently, while the proximal suture is loosely tied upon further use.
- Create an arteriotomy in between the two sutures of the ECA using vascular scissors. There should be no bleeding at this stage.
- Insert the de-aired catheter from the bubble generator until the tip reaches the bifurcation of the CCA. Tighten the proximal suture and then ligate and excise the ECA distal to the arteriotomy site.
- Loosen the ligatures on the CCA and ICA to restore blood flow and, at the same time, start the flow of saline through the bubble generator into the catheter (step 1.6.3.). Confirm the stability of the catheter placement and the absence of leakage of blood or saline. Rotate the ECA stump with the inserted catheter about 90° counterclockwise so the flow from the catheter goes into the direction of the ICA.
- Start the embolization process. Start the Python code and start the creation of air bubbles as described in step 1.6.4.
- After the embolization is finished, temporarily stop the blood flow by tightening the ICA suture/ vascular clamp.
- Remove the catheter carefully and permanently close the proximal end of the ECA by firmly tying the existing suture.
- Gently remove the 3-0 sutures and/or clamp from the PPA, OA, ICA, and CCA to allow reperfusion. Clean any residual blood around the surgery area.
- CCA-entry method (Figure 3B)
- Continue after step 3.5.3; temporarily ligate the ECA proximal from the superior thyroid branch, close to the bifurcation.
- Start the run of saline through the air bubble generator into the catheter, but without starting the production of air bubbles yet.
- Gently elevate the CCA using the hemostats from step 3.4.2. and proceed to insert the needle attached to the catheter of the bubble generator into the lumen of the CCA in the flow direction. Ensure the needle opening is faced upwards and that the needle does not perforate through the opposite vessel wall of the CCA.
- After insertion, slowly lower the hemostats and accompanying CCA again to allow reperfusion of the CCA. Ensure there is no leakage of blood or saline around the insertion site.
- Start the embolization process by starting the Python code and the creation of air bubbles as described in step 1.6.4.
- After completing the embolization, elevate the CCA again and carefully retract the needle. Apply pressure with a cotton tip to the insertion site while maintaining elevation of the CCA to stop the bleeding.
- Continue applying pressure on the puncture site. Gently remove the cotton tip by rolling it away distally along the CCA, using minimal pressure to ensure the clot remains undisturbed and in place. It may take between 30 s to 5 min to achieve hemostasis.
NOTE: In female rats, closing the puncture site can take a few minutes longer than in male rats. - Carefully retrieve the 3-0 sutures from the ECA, ICA, and CCA to allow reperfusion. Again, confirm the bleeding has permanently stopped and clean any residual blood around the surgery area if necessary.
- Closure and postsurgical care
- Separate the skin from the subcutaneous tissue of the neck to facilitate suturing. Suture the skin with a curved needle and 4-0 suture.
- Administer subcutaneous bupivacaine 2.5 mg/mL (0.01 – 0.02 mL/kg) around the incision site.
- Carefully transfer the animal to a recovery cage (33 – 35 °C) for 30-120 min. During the recovery period, the animal is not left unattended until it has regained sufficient consciousness to maintain a sternal recumbency position. When the animal is fully recovered, transfer it back to the home cage. Place wet chow within easy reach inside the cage.
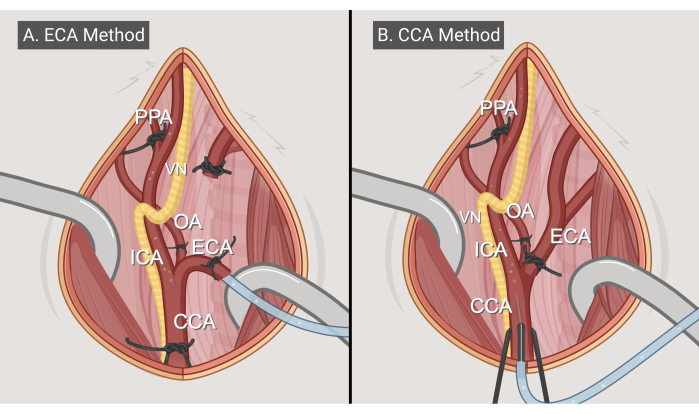
Figure 3: Surgery methods. Illustrations of the two surgical approaches, (A) the ECA-entry method and (B) the CCA-entry method. Abbreviations: CCA = common carotid artery; ECA = external carotid artery; ICA = internal carotid artery; OA = occipital artery; PPA = pterygopalatine; VN = vagus nerve. Figure made with BioRender.com. Please click here to view a larger version of this figure.
4. Follow-up
- Monitoring and humane endpoints
- Monitor animals for a 7-day postoperative period. Euthanize rat earlier if humane endpoint is reached according to the directive 2010/63/EU of the European Parliament.
- Clinical neurological deficits
- Assess clinical neurological deficits (CND) on postoperative days (for example, days 1, 3, and 7). Our definition of CND includes decreased left forelimb flexion (for instance, the inhibition of extension of the fore limb while raising the rat), the inability to walk straight, or circling behavior, similar to the Bederson score10. Use any standardized neurological scoring or tests to evaluate motor function and neurological status.
- Magnetic resonance imaging
- Perform postoperative magnetic resonance imaging (MRI) (for example, on days 1, 3, 5, and 7) on a 7 T small animal MRI system with the rat anesthetized with 1.2% – 2.5% isoflurane in a mixture of 0.5 L/min medical air and 0.5 L/min oxygen. Monitor respiration and temperature continuously.
- Obtain T2-weighted scans using the following sequence parameters: repetition time 2500 ms, echo time 9 ms, echo spacing 9 ms, and 10 echoes. Set the field of view to 35 x 35 mm with an acquisition matrix of 128 x 128. The slice thickness is 1.1 mm with a total of 15 slices acquired, maintaining an aspect ratio of 1.00 and using a single dynamic acquisition.
- Histological analysis
- After completion of the MRI on the 7th day (or when the experimental endpoint is reached), maintain the rat under anesthesia for euthanasia using cardiac perfusion with 30 mL of 0.1 M phosphate-buffered saline followed by 30 mL of paraformaldehyde 4%.
- Extract the brain and post-fixate it overnight in 4% paraformaldehyde. After a 24 h fixation period, embed the brain in paraffin, slice in coronal sections, and stain with hematoxylin and eosin (H&E) using the standard method11.
Representative Results
Neurological outcome
Table 1 gives an overview of all inclusions and exclusions across the different experimental groups. None of the sham-operated rats showed any CND. In the ECA-CAGE group (8000 nL), two out of five rats did not exhibit CND, while the three remaining rats experienced CND, of which two died within 24 h. In the CCA-CAGE group (8000 nL) all animals showed CND, three of five did not survive up to 48 h. In the CCA-CAGE groups with lower volumes of air, all rats survived postoperatively. All five rats in the 7000 nL group demonstrated CND, whereas in the 6000 nL group, two out of five rats showed CND.
| ECA | CCA | |||||||
| sham | 8000 nL | sham | 8000 nL | 7000 nL | 6000 nL | |||
| Included | 5 | 5 | 5 | 5 | 5 | 5 | ||
| Exhibited clinical neurological deficits | 0 | 3 | 0 | 5 | 5 | 2 | ||
| Mortality < 24 h | 0 | 2 | 0 | 2 | 0 | 0 | ||
| Mortality 24-48 h | 0 | 0 | 0 | 1 | 0 | 0 | ||
| Excluded total | 3 | 4 | 1 | 0 | 2 | 1 | ||
| Excluded due to bleeding complication | 2 | 3 | 0 | 0 | 0 | 0 | ||
| Excluded due to thrombotic complication | 0 | 1 | 0 | 0 | 0 | 1 | ||
| Intraoperative death due to vagal nerve compression | 1 | 0 | 1 | 0 | 2 | 0 | ||
Table 1: Animal group inclusions and exclusion. The number of rats in each group, inclusions and exclusions, deaths, and survival with clinical neurological deficits.
MRI
Figure 4 shows a representative T2-weighted image of a rat that received 7000 nL air bubbles through the CCA-entry method, showing cortical hyperintensities. Similar abnormalities were seen in all animals in the CCA-CAGE group that received 7000 or 8000 nL, and to a lesser extent in the 6000 nL group and the ECA-CAGE group. Notably, while none of the animals in the sham groups showed any CND, one ECA-sham rat exhibited an area of hyperintensity on MRI; in the CCA-sham group, no rats displayed abnormalities on MRI.
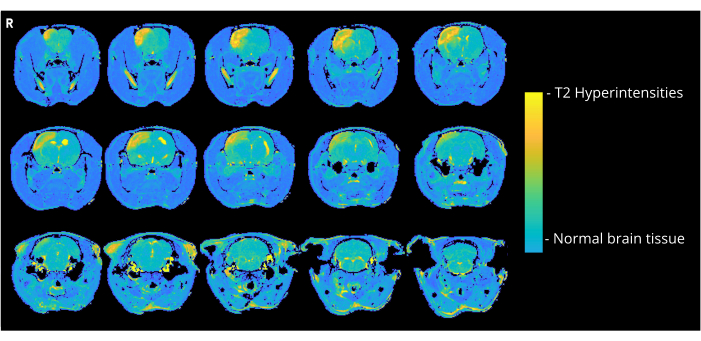
Figure 4: MRI example images. Representative T2-weighted MRI images (3 days post CAGE surgery) showing cortical hyperintensities due to CAGE in a rat of the CCA-CAGE 7000 nL group. Please click here to view a larger version of this figure.
Histology
Figure 5 shows a representative H&E-stained brain section of the 7000 nL CCA-CAGE rat from Figure 4, demonstrating cortical ischemic brain damage with neuronal cell loss and reactive gliosis, including reactive astrogliosis and microglial activation.
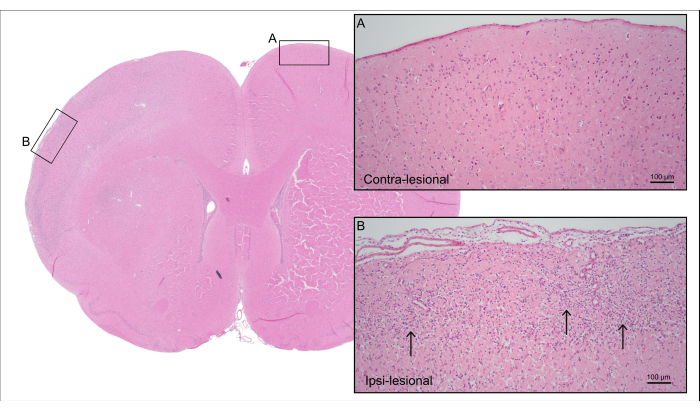
Figure 5: Post-mortem histology. Representative H&E staining of the rat from Figure 4 showing cortical tissue of the contra-lesional side with (A) intact neurons and (B) the ipsi-lesional side with ischemic cortical tissue with neuronal cell loss and reactive gliosis (arrows). Please click here to view a larger version of this figure.
Technical challenges
Due to technical challenges, the ECA-entry method had a substantially lower success rate compared to the CCA-entry method (Table 1). This predominantly stemmed from the short catheter length required in the ECA-entry method, which frequently resulted in catheter dislocation and bleeding. Additionally, the ECA-entry method also resulted in an approximately 20 min longer surgery time, as well as a larger variation in bubble diameter.
Supplementary Figure 1: Details of the air bubble generator. (A) Images of the body of the bubble generator and the capillary puller highlight their assembly and functional aspects. (B) Lateral view of the bubble generator, showing its design and structural features from a side perspective. (C) Frontal view of the bubble generator, illustrating the key frontal aspects and features. Please click here to download this File.
Supplementary Figure 2: Steps for running the software. This file provides a detailed guide on the procedures and steps to be followed for effectively running and utilizing the software associated with the air bubble generator. Please click here to download this File.
Supplementary File 1: Python code. Code consists of the two scripts (A and B) that should be saved within the same folder. Please click here to download this File.
Discussion
We have described how to introduce air emboli in the rat cerebral arteries using two methods and have shown that introduction through a needle inserted in the CCA has multiple advantages over a method that involves embolization through a catheter into the ECA. Specifically, we observed fewer complications with the CCA-entry method, as well as a more consistent bubble diameter and reduced surgical time. The CCA-entry method results in dose-dependent CND, and abnormalities on MRI indicative of cerebral infarction as confirmed with histology.
The initial choice of the ECA-entry method was inspired by Gerriets et al.7. However, we identified several difficulties with this approach, including substantial variations in bubble size and a higher surgical complication rate compared to the CCA-entry method. A primary source of these complications is related to the catheter length. In our model, using a short catheter (125 mm) helped maintain the stability of the bubbles because the longer the catheter, the higher the probability that bubbles merge while flowing through the catheter9. However, in the ECA-entry method, a longer catheter facilitates easier placement and leverage for movement. Using a short catheter in the ECA-entry method results in frequent dislocation and deterioration of the ECA stump due to excessive manipulation.
A second difficulty encountered in the ECA-entry method regarded the creation of consistently sized bubbles (Figure 6). In the ECA-entry method, the flow of saline through the catheter has to be temporarily halted while the catheter is inserted into the ECA. When the arterial flow is restored and the embolization can start, the catheter is suddenly subjected to the blood pressure of the rat. As a result, this leads to blood retrogradely entering the catheter and bubble generator. The effect of the fluctuating blood pressure on the pressure inside the bubble generator leads to a higher variation in bubble size, sometimes leading to cylindrical-shaped bubbles filling up the channel (Figure 6B). This can be avoided by increasing the pressure within the system before positioning the catheter in the ECA. This is best done by a second person for precise timing. Furthermore, since this method is more time-consuming, it leads to a larger amount of saline being infused into the rat than the CCA-entry method. In the CCA-entry method, saline is continuously flowing through the catheter, and the needle is inserted in the direction of the blood flow through the CCA, thereby solving the pressure gradient issue described above. This results in the absence of backflow into the catheter and a more uniform bubble size.
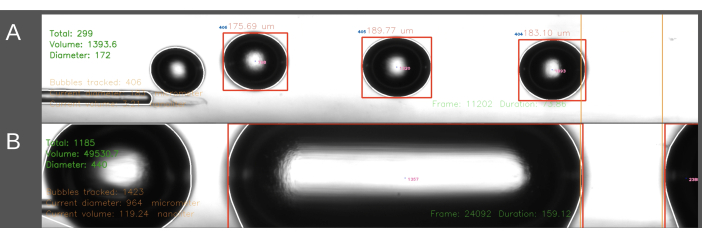
Figure 6: Example recording and analysis of bubble production. Images display screen grabs showing the real-time analysis of the total number of bubbles, total volume, and average diameter in the measurement area (green, upper left corner). The measurement area is highlighted between the orange lines on the right. The bubble diameter and volume are calculated based on the horizontal diameter. Images include the formation of (A) successfully generated air bubbles and (B) unsuccessfully generated cylindrical bubbles. Please click here to view a larger version of this figure.
Despite the CCA-entry method being a favorite, we still encountered various technical difficulties. Firstly, preparation of the PPA is challenging, since accidental compression of the vagus nerve can result in depression of breathing and subsequent death of the animal12. To reduce this risk, one should tilt the rat slightly sideways and approach the PPA from the cranial direction. In addition, due to the challenging anatomy of the ICA and PPA bifurcation, there is a risk of vascular damage and uncontrollable bleeding. This can only be circumvented by improving surgical skills. These challenges highlight that mastering the CAGE model in rats is complex and requires significant practice and precision13.
The proposed technical setup of the air bubble generator has its limitations, particularly related to the custom-made glass capillaries, because of their fragility. The tubing that connects the pneumatic regulator to the capillary is prone to breakage when adjusting the three-way valve for pressure release after embolization. Furthermore, replacing a capillary requires destroying the existing capillary due to the heat-shrink rubber being permanently attached to it. Additionally, each capillary has unique bubble characteristics due to minor differences in tip diameter and shape. Lastly, the manual operation of the bubble generation via the pneumatic regulator required significant experience. Inexperienced handling can result in the production of excessively large bubbles. Automated pressure regulation with a feedback loop from the Python code could enhance automated precision in future studies.
Our thorough documentation, encompassing both the technical specification of the bubble generator, a detailed surgical protocol, and providing the software, makes an important contribution to this field of research. Our CCA technique ensures minimal disruption to physiological cerebral perfusion, by maintaining CCA flow throughout the procedure and abolishing the need to sacrifice the ECA. Our study provides a reliable and reproducible experimental model to investigate CAGE and its potential treatments.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was funded by the Netherlands Military Healthcare Insurance Foundation (Stichting Ziektekosten Verzekering Krijgsmacht) under grant number 20-0232 and the Dutch Heart Foundation 2021 E. Dekker Grant (03-006-2021-T019 to IAM). We also thank Lindy Alles, Paul Bloemen, and Ed van Bavel for their outstanding assistance.
Materials
| Aluminum Crossed Roller XYZ Stage Center Drive Metric Threads with Fine Pitch Screw | Optosigma | TAM-405CLFP | Part of frame (Step 1.1.) |
| Basler Ace – acA2440-35um | Basler AG | 107208 | High speed camera (Step 1.4.) |
| Bupivacaine 2.5 mg/ml | Aurobindo Pharma B.V. | RVG20949 | Medication perioperative (Step 3.8.3.) |
| Buprenorfine 0.3 mg/ml | Indivior | 112515 | Medication perioperative (Step 3.2.1.) |
| Custom glass chamber | Technoglas Lab. App. B.V. | – | Custom made (Step 1.3.1.) |
| Duratears | Alcon | – | Artificial tears (Step 3.2.5.) |
| Electric razor | Aesculap | GT416-VR | |
| Electro-Pneumatic Regulator – ITV0010-3L | SMC | ITV0010-3L | Pneumatic regulator of bubble generator (Step 1.2.) |
| GC100T-15 thin wall W/O filament 1.0mmOD | Multi Channel Systems | 300036 | Borosilicate glass capillaries (Step 1.5.) |
| Graphpad Tool: www.graphpad.com/quickscalcs/randomize1/ | Dotmatics | – | Randomly assign subjects to treatment groups |
| Heatshrink rubber | Pro-POWER | 1190988 | Holds capillary and pneumatic tubing in place (Step 1.2.) |
| Isoflurane 1000 mg/g | Laboratorios Karizoo S.A. | 118938 | |
| Laptop | Dell | – | 12th Gen Intel® Core™ i5-1235U 1.30 GHz, 16.0 GB ram, Windows 10 |
| Light source station with two dual white LED and goosenecks | Euromex Microscopen B.V. | LE.5212 | Led light source (Step 1.4.) |
| Micro forceps bent | Aesculap | BD329R | (Step 3.3.2.) |
| Micro needle holder | Silber | GU1870 | For inserting needle in CCA (Step 3.7.3.) |
| Micro scissors | HEBU medical | HB7384 | Vascular scissor (Step 3.6.3) |
| Micro vascular clip | Biemer | FD562R | (Step 3.6.1.) |
| Microlance 3 (21G, 27G and 30G) | BD Medical | 304000 | (Step 1.3.2.) |
| Mosquito artery clamp | Aesculap | BH105R | (Step 3.4.3.) |
| NexiusZoom | Euromex Microscopen B.V. | NZ.1903-B | Microscope for surgery (Step 3.3.) |
| Narishige PB-7 | Narishige Group | – | Micropipette puller (Step 1.5.1.) |
| Optomechanical mounts, adapter and post assemblies | Thor Labs | – | Various parts to hold the bubble generator body in static position (Step 1.1.) |
| PE-10 tubing | Intramedic | 427401 | Catheter (Step 1.3.2.) |
| Perfusor Space | B.Braun | 8713030 | Syringe pump (Step 1.6.1.) |
| Plan Achromat Objective, 0.10 NA, 18.5 mm WD 4X | Olympus | RMS4X | Magnification lens (Step 1.4.) |
| Python | Python Software Foundation | – | Version 3.11.2 (Step 2.2.1.) |
| Pylon viewer | Basler AG | – | Version 7.4.0 (Step 2.1.1.) |
| Rubber O-RING 1 x 1 mm silicone | Op den Velde Industrie B.V. | 99002887 | Prevents leakage of saline (Step 1.3.3.) |
| Rubber O-RING 6 x 1 mm silicone | Op den Velde Industrie B.V. | 99002886 | Holds glass chamber in place (Figure 2.) |
| Rodent Warmer X1 with Rat Heating Pad and Rectal Probe | Stoelting | 53800R | Heating pad (Step 3.1.2.) |
| Skeleton Fine Forceps | Hoskins | 2710-B-2074 | (Step 3.3.2.) |
| Wistar rats | Charles River Laboratory | – |
References
- Mitchell, S. J., Bennett, M. H., Moon, R. E. Decompression sickness and arterial gas embolism. N Engl J Med. 386 (13), 1254-1264 (2022).
- Bessereau, J., et al. Long-term outcome of iatrogenic gas embolism. Intensive Care Med. 36 (7), 1180-1187 (2010).
- Brown, A. E., Rabinstein, A. A., Braksick, S. A. Clinical characteristics, imaging findings, and outcomes of cerebral air embolism. Neurocrit Care. 38 (1), 158-164 (2023).
- Weenink, R. P., Hollmann, M. W., Van Hulst, R. A. Animal models of cerebral arterial gas embolism. J Neurosci Methods. 205 (2), 233-245 (2012).
- Rosengren, L., Persson, L. Enhanced blood-brain barrier leakage to Evans blue-labelled albumin after air embolism in ethanol-intoxicated rats. Acta neuropathologica. 38 (2), 149-152 (1977).
- Furlow, T. W. Experimental air embolism of the brain: An analysis of the technique in the rat. Stroke. 13 (6), 847-852 (1982).
- Gerriets, T., et al. A rat model for cerebral air microembolisation. J Neurosci Methods. 190 (1), 10-13 (2010).
- Juenemann, M., et al. Impact of bubble size in a rat model of cerebral air microembolization. J Cardiothorac Surg. 8, 198 (2013).
- C Schaefer, T., et al. Investigation of experimental endovascular air embolisms using a new model for the generation and detection of highly calibrated micro air bubbles. J Endovasc Ther. 30 (3), 461-470 (2022).
- Bederson, J. B., et al. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke. 17 (3), 472-476 (1986).
- Schulte, E. K. Standardization of biological dyes and stains: Pitfalls and possibilities. Histochemistry. 95 (4), 319-328 (1991).
- Wayman, C., et al. Performing permanent distal middle cerebral with common carotid artery occlusion in aged rats to study cortical ischemia with sustained disability. J Vis Exp. (108), e53106 (2016).
- Themistoklis, K. M., et al. Transient intraluminal filament middle cerebral artery occlusion stroke model in rats: A step-by-step guide and technical considerations. World Neurosurg. 168, 43-50 (2022).

.
