An Integrated Method for Crafting Flexible and Convenient Electrophysiological Optrodes for Multi-Region In Vivo Recording
Summary
We have developed a simplified and cost-effective approach for electrode fabrication and conducted recordings of signals across multiple regions in freely moving mice. Utilizing optogenetics, alongside multi-region electrophysiology and calcium signal recording, enabled the revelation of neuronal activities across regions in the seizure kindling model.
Abstract
Epilepsy is a neurological disorder characterized by synchronized abnormal discharges involving multiple brain regions. Focal lesions facilitate the propagation of epileptic signals through associated neural circuits. Therefore, in vivo recording of local field potential (LFP) from the critical brain regions is essential for deciphering the circuits involved in seizure propagation. However, current methods for electrode fabrication and implantation lack flexibility. Here, we present a handy device designed for electrophysiological recordings (LFPs and electroencephalography [EEG]) across multiple regions. Additionally, we seamlessly integrated optogenetic manipulation and calcium signaling recording with LFP recording. Robust after-discharges were observed in several separate regions during epileptic seizures, accompanied by increasing calcium signaling. The approach used in this study offers a convenient and flexible strategy for synchronous neural recordings across diverse regions of the brain. It holds the potential for advancing research on neurological disorders by providing insights into the neural profiles of multiple regions involved in these disorders.
Introduction
Epilepsy is a common neurological condition characterized by recurrent seizures, which manifest as convulsions, sensory disturbances, and loss of consciousness1. The pathophysiological mechanisms underlying epilepsy are complex and involve multiple interconnected brain regions2,3. Recent advances in neuroimaging have shed light on the large-scale networks involved in epilepsy4,5. However, understanding of the intricate circuitry and network mechanisms underlying the generation and propagation of epilepsy remains limited, partly due to the insufficient application of multi-region neural recording techniques6. Therefore, developing a flexible, integrated method capable of simultaneously monitoring neural activity across disparate brain regions is imperative.
Electrophysiological recordings are conducted to capture seizures and determine the presence of epilepsy7. Except for recording electrophysiological activity, there is a growing emphasis on the precise calcium activity of specific neural populations in epilepsy studies8,9. Advancements in calcium indicator synthesis and various probe designs have prompted researchers to adopt fiber photometry for capturing changes in neuronal activity and neural substances within the brain10,11. The two independent methods of detecting neuronal activity, namely electrophysiology and fiber photometry recording, complement each other, facilitating a more comprehensive understanding of dynamic neuronal processes.
Additionally, synchronous recording and modulating neural activity are pivotal for gaining insights into the brain functioning at both network and cellular levels. This approach enables researchers to observe and manipulate the brain's complex processes in real-time. Optogenetics has emerged as an indispensable tool for probing neural signaling, owing to its distinctive ability for selective stimulation or inhibition12. Despite widespread applications of multi-site electrophysiological recording in neuroscience13, the integration of multi-region electrophysiological recording with fiber photometry and optogenetic manipulation remains limited. More importantly, current methods for fabricating and implanting multi-region electrodes lack flexibility14. These limitations hinder our capacity to dissect specific circuit functions and interactions across multiple regions. Here, we present a cost-effective and convenient multi-region in vivo recording approach that sheds light on neuronal processes across regions in kindling-induced seizures and other neuropsychiatric disorders.
Protocol
This protocol received approval from the Animal Care and Use Committee at Fudan University and was conducted following the guidelines and regulations designed by the National Institutes of Health Guide for Care and Use of Laboratory Animals. All possible measures were implemented to minimize the number of animals utilized in this study. The time required to perform each step is included in the respective steps.
1. Preparation of electrodes (Figure 1)
- Cut an appropriate length of tungsten wire (~20 mm) and burn off the insulation layer at one end to expose the tungsten wire (1.6-2 mm). Bend the other end of the wire into an "L" shape (see Figure 1A, ~1 min)
- Carefully place the "L" end of the tungsten wire onto the optical fiber (see Figure 1B, ~1 min).
NOTE: The tungsten wire should be 0.2-0.4 mm longer than the tip of optical fiber. - Dip a drop of adhesive and apply it to the interface of the optical fiber and the tungsten wire (see Figure 1C, ~1 min). Due to the surface tension of the liquid, the tungsten wire will attach to the optical fiber.
- Apply the adhesive once and allow it to dry. Then, apply a second coat. This will further tighten the bond between the tungsten wire and the optical fiber (see Figure 1C, ~1 min).
NOTE: During the application process, care should be taken to prevent the adhesive from flowing to the tip of the optical fiber, as this may affect its transmittance. - At the corner of the "L" shape, use adhesive to closely bond the base of the optical fiber and the tungsten wire together (~1 min).
NOTE: The increased contact area and the spherical solidification of the adhesive droplet ensure a firm bond between the optical fiber and the tungsten wire. - Use soldering to establish a connection between the tungsten wire and the pin of the female connector (see Figure 1D, ~3 min for each)
NOTE: Since tungsten wire is not sensitive to heat, the success rate of soldering can be increased to nearly 100% by a metal sleeve fashioned by cutting the pin slot location to physically unite the two components. Subsequently, solder filling the sleeve ensures a stable connection between the inserted tungsten wire and the pin. - According to the experimental design, solder the required number of optrodes (see Figure 1E, ~10 min)
NOTE: Three optrodes were utilized in this study. - Cut the enameled wire to the appropriate length (~30 mm) and burn off the insulation on both ends. Wrap one end of the enameled wire around the base of the screw and solder it. Connect the other end of the enameled wire to the pins of the female connector via a soldering iron, serving as the EEG recording and ground connection for the skull electrode (see Figure 1F, ~5 min)
NOTE: Due to the flexibility of the enameled wire, it is convenient to connect the skull and the pins of the female connector. Soldering after winding the enameled wire around a screw is more reliable than surface-mount soldering. - Apply hot-melt adhesive to the female connector's pin array to encapsulate the female connector and ensure the solder joints are fully enveloped. During the solidification of the hot-melt adhesive, shape the adhesive using a plastic board to minimize space occupancy during implantation (see Figure 1F, ~3 min)
NOTE: Due to the potential burn risks associated with using hot-melt glue, AB adhesive is considered a safer alternative. - Next, use a light intensity meter and digital multimeter to measure luminousness and conductivity. Upon passing these tests, the setup is ready for use (see Figure 1G, ~2 min)
NOTE: When working with lasers, always wear protective eyewear suitable for the specific wavelength and power of the laser light. High-quality fibers are required to transmit at least 80% of the light from the end of the patch cord to the tip of the implantable fiber. Additionally, the resistance from the tip of the tungsten wire to the pin header slot was measured with an acceptable range of 1-5 Ω. - Before use, immerse the prepared electrodes in 75% alcohol for 15 min, then transfer them to sterile saline (~16 min).
NOTE: The total weight of the encapsulated electrode device (0.6701 ± 0.01123 g, n = 4) does not affect the activity of the mice.
2. Protocol for electrode implantation (Figure 2)
- Anesthetize the mice with isoflurane (1%-1.5%) and secure them in a stereotaxic apparatus (see Figure 2A, ~2 min). Use eye ointment on mouse eyes to prevent dryness while under anesthesia.
NOTE: Proper anesthetization is confirmed through the absence of the righting reflex and the loss of response to toe pinch. For prolonged surgical procedures, using a liquid anesthetic is more appropriate. - After securing the animal in the stereotaxic apparatus, trim away the scalp along the edge of the skull and wipe the skull surface with 75% alcohol to expose the positions of the bregma and lambda (see Figure 2B, ~3 min).
- Level the brain and drill holes (~0.8 mm) above the regions of interest (ROI) using a microdrill. Inject the relevant viruses with a syringe pump (see Figure 2C – F, ~20 min).
NOTE: Detailed information about the viruses and ROIs in this study is shown in the Table of Materials and Figure 3. - Hold the optrodes with a holder and move their tip to the bregma point, setting this position as 'zero'. Using the bregma point as the reference coordinate, move the optrodes to the target regions (see Figure 2G, ~3 min). Position the optrodes 0.3 mm above the virus injection site.
NOTE: In the vertical implantation strategy, the minimum distance between the two electrodes is approximately 2 mm, depending on the thickness of the fiber optic sleeve and the outer diameter of the ferrule. An angled insertion method can be used for simultaneous recording from closely located regions. - After inserting the optic fibers into the corresponding brain areas, apply a small amount of tissue adhesive around them to cover the exposed tissue. Then, sequentially apply additional adhesive and a little dental cement. After the dental cement solidifies, release the holder carefully (see Figure 2H, ~5 min).
- Before each new optrode implantation, perform recalibration, which involves repeating step 2.4 (see Figure 2I).
NOTE: For each implantation, position the optrode at bregma as the 'zero' point. - After implanting all optrodes, drill the holes behind the lambda point for 'Ground' and above the neocortex for 'EEG' using a microdrill (~1 min).
NOTE: The size of the drilled hole (~1.1 mm) needs to match the diameter of the screw. - Insert skull screws into the hole above the cortex and behind the lambda point (see Figure 2J, ~5 min).
NOTE: Skull screws are used for EEG recording and grounding, respectively. - Organize the tungsten wires and enameled wires so they are positioned above the implantation area and not exposed outside the skull. Secure the position of the female connector using a holder (see Figure 2K, ~2 min).
- Fill the implantation site with dental cement to ensure a stable connection between the electrode device and the skull. Encapsulate the internal wires, leaving the female connector's insertion holes exposed for connection to the headstage (see Figure 2L, ~6 min).
- After dental cement hardening, apply iodine to the wound and then place the animal on a warming pad (~1 min).
NOTE: Ensure that the animal is not left unattended until it has regained sufficient consciousness to maintain sternal recumbency. Sterile conditions should be maintained throughout the survival surgery. - Administer antibiotics and analgesics to support post-surgical recovery.
NOTE: Animals that have undergone surgery are not reintroduced to the company of other animals until they have fully recovered.Animals were administered intraperitoneal injections of ceftriaxone sodium (180 mg/kg), and lidocaine gel was applied to the surgical wounds daily for 3 days.
3. Protocol for recording in vivo brain
- Before conducting signal recording and behavioral testing (open field test)15, ensure that the mouse undergoes a 3-day period of gentle handling to acclimatize them to the environment.
NOTE: In this study, the mice were placed in an open field (42 cm × 42 cm) to move around freely, allowing us to observe their seizure behavior. - After anesthetizing the mice with isoflurane (1%-1.5%), connect the optical fibers sequentially in the order of the recording site before inserting the headstage.
- Set the 635 nm light pulse with a 10% duty cycle at 20 Hz. Set up the light stimulation for 10 s (200 pulses) during recording. Set the intensity at the optical fiber tips at 3 mW.
- Use a fiber photometry system to record Gcamp6m signals behavioral testing period at a sampling rate of 30 Hz by 470 nm LED channel.
- Simultaneously record calcium signals, LFPs, and EEG under optogenetic stimulation, along with corresponding behavioral performances (see Figure 3).
- Employ transistor-transistor logic (TTL) signal synchronization tagging to ensure temporal consistency in recorded data.
4. Data processing
- Load photometry data to MATLAB software. Then, use custom coding (Supplementary File 1) to detrend the calcium signal.
- Define the changes in fluorescence intensity with equation (1):
ΔF/F = (Ft - F0)/ F0 (1)
Where Ft represents the real-time calcium signal fluorescence values, and F0 denotes the averaged baseline fluorescence intensity before the event. - Import the electrophysiological data, sampled rate of 1000 Hz, into MATLAB (Supplementary File 2).
- Coordinate the display of calcium signaling and electrophysiological data in alignment with synchronized markers.
Representative Results
We combined optogenetics with multiregional electrophysiological recording and calcium imaging to observe neuronal activity across various brain regions during optogenetic seizures. For this purpose, an adeno-associated virus (AAV) expressing ChrimsonR under the control of the CaMKIIα promoter (AAV-CaMKIIα-ChrimsonR-mcherry)16 was injected into a classical epileptogenic site, the piriform cortex (ROI 1)17, in rodents. Additionally, AAV-hsyn-Gcamp6m18 was injected into three regions (ROI 1-3, as shown in Figure 3A). Subsequently, custom-made optrodes were implanted in these regions, and EEG electrodes were implanted in the contralateral skull (Figure 3B).
ChrimsonR and Gcamp6m were highly expressed in the target brain regions (Figure 3C) 4 weeks later. Mice exhibited generalized seizure behaviors following a brief 10-s photostimulation at 20 Hz to activate ChrimsonR-positive neurons. We collected the calcium activities and LFP signals from the ROIs in freely moving mice by employing simultaneous multi-fiber photometry and electrophysiology techniques (Figure 3D). Robust after-discharges, along with remarkable increases in Ca2+ activities, were elicited in these regions by brief optogenetic stimulation (Figure 3E). A 30 Hz response was observed in the opto-stimulating region, induced by the activation of optogenetic proteins via a blue light LED at a sampling frequency of 30 Hz. No 50 Hz powerline interference was observed in the recording channels. These results demonstrated the reliable recording of synchronized electrophysiological and calcium signaling responses across multiple regions under optogenetic stimulation.
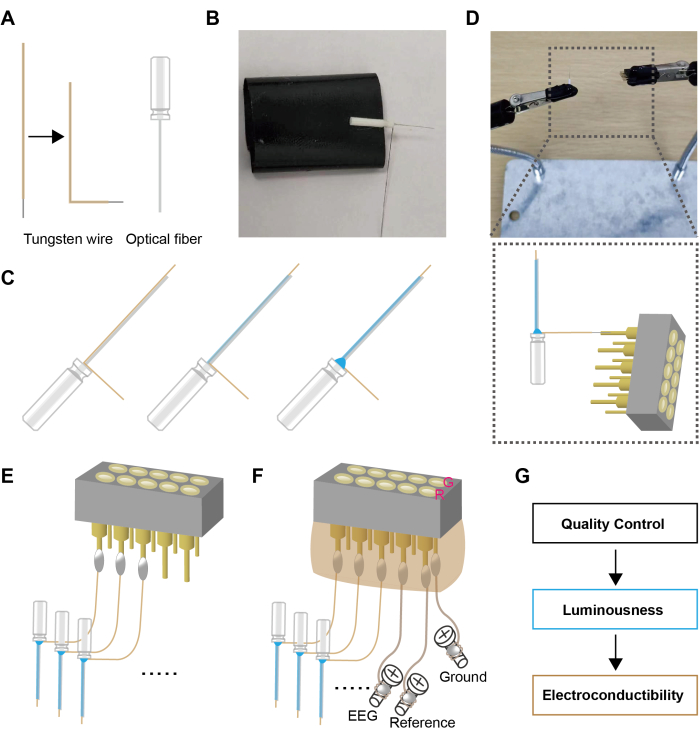
Figure 1: Schematic design of flexible optrode. (A) Preparation of the "L" shaped tungsten wires and optical fibers. (B) The optical fibers and the tungsten wires were positioned on the adhesive tape. (C) The optical fiber and the tungsten wire were stuck together using glue. (D,E) The tungsten wires were welded to the connector. (F) The screws were welded to the connector, and the connector was encapsulated with hot melt adhesive. (G) Quality inspections were conducted on the optrode assembly. G represents the pinhole for the ground electrode; R represents pinhole for the reference electrode. Please click here to view a larger version of this figure.
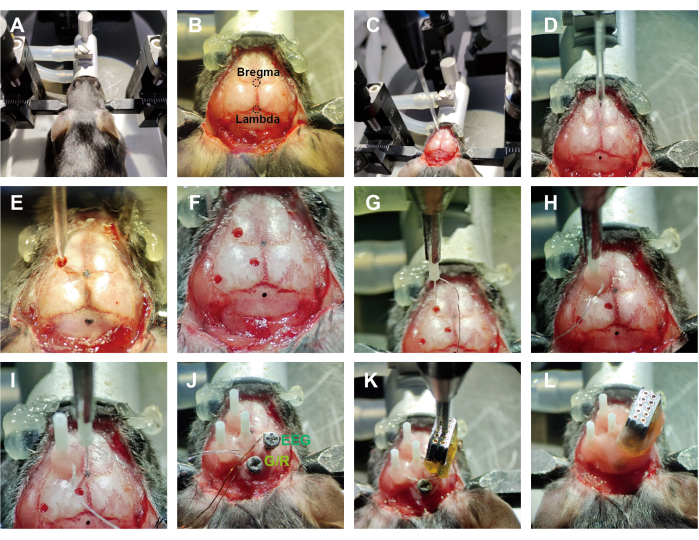
Figure 2: Workflow of implantation surgery. (A) Mouse was secured on a stereotaxic apparatus. (B) The scalp was removed, and the skull was adequately exposed. (C) The injection glass pipe was connected to the pump. (D) The mouse's head was adjusted to a horizontal position. (E,F) Holes were drilled, and the virus was injected. (G) The optrode was grasped by the holder. (H) The optrode was implanted in a related area and fastened with dental cement. (I) Readjusting zero position before each implantation. (J) EEG electrodes were implanted after optrode implantation. G/R: the ground electrode and reference electrode were shorted at the pin positions. (K) The wires were arranged in order, and the connector was fixed. (L) The electrode device was wrapped and fixed with dental cement. Please click here to view a larger version of this figure.
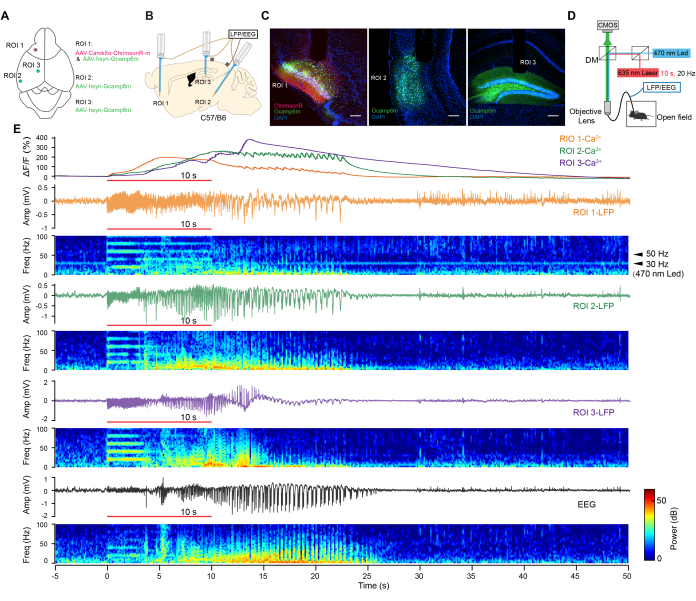
Figure 3: In vivo synchronous electrophysiological and calcium recording. (A) Experimental design for viral injections. (B) Experimental scheme of optrode implantation. (C) Confocal images show virus expression in target areas. Scale bar = 200 µm. (D) Schematic of optogenetic stimulation, synchronous electrophysiological, and calcium recording in a freely moving state. (E) Representative traces of calcium signals (top), LFP (middle) signals, EEG (bottom), and corresponding power spectrograms. Red line: 10 s light. Please click here to view a larger version of this figure.
Supplementary File 1: Coding files for calcium signal. Please click here to download this File.
Supplementary File 2: Coding files for LFP signal. Please click here to download this File.
Discussion
Here, we employed a self-made optrode device for in vivo neural signal recording across multiple regions. The feasibility of this system for simultaneous optogenetic stimulation, calcium signal recording, and electrophysiological recording has been validated. The electrode preparation method described herein is efficient and cost-effective. According to the experimental design, we could record signals from relevant brain regions. The strategic arrangement of optrodes allows for the potential extraction of abundant information from multiple regions across the brain. The integration of multimodal detection paradigms provides opportunities to dissect intricate neural circuits, elucidating the relationships between brain regions.
In the representative data of the present study, calcium signals and electrophysiological signals from broad-spectrum neurons of the entire local brain region were simultaneously recorded. Following advancements in transgenic animal construction technology19,20, the combination of Cre-mice and FlpO-mice with expressing-Gcamp virus allows for the recording of neural activity from specific neurons. Hence, this method allows us to decode the responses of specific neurons across different brain regions through these integrated approaches. Additionally, diverse analytical methods will be beneficial in analyzing the electrophysiological and calcium signals gathered from multiple brain regions. For instance, coherence analysis and Granger causality analysis could be employed to obtain novel theoretical communication characteristics among different brain regions21,22,23. Besides, advancements in circuit tracing techniques aid in unraveling the structural connections between brain regions24. These integrated technologies enhanced the comprehension of the associated responses across various brain areas and revolutionized the understanding of connection and interaction in circuits and networks.
In this study, we employed optogenetic kindling modeling of epilepsy. Optogenetics offers not only higher temporal resolution but also enhanced cell specificity compared to electrical stimulation in the kindling model of epilepsy25,26. Additionally, it enables light delivery and calcium recording to be conducted through the same optical fiber. The multi-region activity detections and optogenetic modulations will complement closed-loop epilepsy intervention methods, allowing for regulatory interventions across multiple brain regions via inhibitory modulation, thereby enhancing the anti-epileptic effects27,28. It is also important for these devices to be combined with other existing neuromodulation technologies for the detection and closed-loop intervention of chronic pain29,30. Besides, this approach may extend to other neurological disorders such as sleep disorders31 and Alzheimer's disease, where phenomena like 40 Hz oscillations and information processing following sensory input require elucidation via multi-region in vivo recording32. Therefore, adeptly applying this multifaceted approach will deepen our understanding of interactions among multiple regions and provide valuable insights for precision therapies in various diseases.
Limitations exist in this optrode detection method, which could be improved in future research. While LFP signals were readily acquired, the detection of spike activities remained challenging with the current method33. We aim to replace tungsten wire with multi-channel flexible electrodes. The thickness of the fiber and the safety of the glue adhesive should be carefully considered.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (31871085), the Natural Science Foundation of Shanghai (21ZR1407300), the Shanghai Municipal Science and Technology Major Project (2018SHZDZX01), ZJ Lab, and Shanghai Center for Brain Science and Brain-Inspired Technology.
Materials
| 8-32 adapter | Plexon | Custom ordered | Connect the female connector and headstage |
| AAV-CaMKIIα-ChrimsonR-mcherry | Taitool Bioscience | S0371-9 | 4 x 1012 VG/mL |
| AAV-hsyn-Gcamp6m | Taitool Bioscience | S0471-9 | 4 x 1012 VG/mL |
| DAPI | Sigma | 236276 | Titered 1:500 |
| Dental Cement | New Century Dental | 430205 | |
| Electrophysiological recordings system | Plexon | Omniplex | |
| Enameled wire | N/A | Custom ordered | Diameter = 0.2 mm |
| Female connector | N/A | Custom ordered | 1.25 mm pitch |
| Glue | Loctite | 45282 | |
| Laser | Changchun New Industries | BH81563 | 635 nm |
| MATLAB | MathWorks | R2021b | |
| Microdrill | RWD | 78001 | |
| Multichannel fiber photometry | ThinkerTech | FPS-SS-MC-LED | |
| Optical fiber | Xi'an Bogao | L-200UM | Select the appropriate fiber length based on the depth of the targeted brain regions. |
| PFA-Coated Tungsten wire | A-M System | 795500 | Bare 0.002"; Coated 0.0040" |
| Power meter | Thorlabs | PM100D | |
| Stereotaxic Fxrame | RWD | 68807 | |
| Tissue adhesive | 3M | 1469SB |
References
- Devinsky, O., et al. Epilepsy. Nat Rev Dis Primers. 4, 18024 (2018).
- Piper, R. J., et al. Towards network-guided neuromodulation for epilepsy. Brain. 145 (10), 3347-3362 (2022).
- Bertram, E. H. Neuronal circuits in epilepsy: Do they matter. Exp Neurol. 244, 67-74 (2013).
- Goodman, A. M., Szaflarski, J. P. Recent advances in neuroimaging of epilepsy. Neurotherapeutics. 18 (2), 811-826 (2021).
- Caciagli, L., Bernhardt, B. C., Hong, S. J., Bernasconi, A., Bernasconi, N. Functional network alterations and their structural substrate in drug-resistant epilepsy. Front Neurosci. 8, 411 (2014).
- Dai, Y., et al. In vivo microelectrode arrays for detecting multi-region epileptic activities in the hippocampus in the latent period of rat model of temporal lobe epilepsy. Micromachines (Basel). 12 (6), 659 (2021).
- Staba, R. J., Stead, M., Worrell, G. A. Electrophysiological biomarkers of epilepsy. Neurotherapeutics. 11 (2), 334-346 (2014).
- Grienberger, C., Giovannucci, A., Zeiger, W., Portera-Cailliau, C. Two-photon calcium imaging of neuronal activity. Nat Rev Methods Primers. 2 (1), 67 (2022).
- Akerboom, J., et al. Optimization of a gcamp calcium indicator for neural activity imaging. J Neurosci. 32 (40), 13819-13840 (2012).
- Zhang, Y., et al. Fast and sensitive gcamp calcium indicators for imaging neural populations. Nature. 615 (7954), 884-891 (2023).
- Wu, Z., Lin, D., Li, Y. Pushing the frontiers: Tools for monitoring neurotransmitters and neuromodulators. Nat Rev Neurosci. 23 (5), 257-274 (2022).
- Vierock, J., et al. Bipoles is an optogenetic tool developed for bidirectional dual-color control of neurons. Nat Commun. 12 (1), 4527 (2021).
- Luo, W., et al. Acquiring new memories in neocortex of hippocampal-lesioned mice. Nat Commun. 13 (1), 1601 (2022).
- Armstrong, C., Krook-Magnuson, E., Oijala, M., Soltesz, I. Closed-loop optogenetic intervention in mice. Nat Protoc. 8 (8), 1475-1493 (2013).
- Kraeuter, A. K., Guest, P. C., Sarnyai, Z. The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol Biol. 1916, 99-103 (2019).
- Klapoetke, N. C., et al. Independent optical excitation of distinct neural populations. Nat Methods. 11 (3), 338-346 (2014).
- Vismer, M. S., Forcelli, P. A., Skopin, M. D., Gale, K., Koubeissi, M. Z. The piriform, perirhinal, and entorhinal cortex in seizure generation. Front Neural Circuits. 9, 27 (2015).
- Chen, T. W., et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 499 (7458), 295-300 (2013).
- Birling, M. C., Dierich, A., Jacquot, S., Herault, Y., Pavlovic, G. Highly-efficient, fluorescent, locus directed cre and flpo deleter mice on a pure c57bl/6n genetic background. Genesis. 50 (6), 482-489 (2012).
- Huang, T., et al. Identifying the pathways required for coping behaviours associated with sustained pain. Nature. 565 (7737), 86-90 (2019).
- Likhtik, E., Stujenske, J. M., Topiwala, M. A., Harris, A. Z., Gordon, J. A. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 17 (1), 106-113 (2014).
- Tavares, L. C. S., Tort, A. B. L. Hippocampal-prefrontal interactions during spatial decision-making. Hippocampus. 32 (1), 38-54 (2022).
- Lu, Y., et al. Optogenetic dissection of ictal propagation in the hippocampal-entorhinal cortex structures. Nat Commun. 7, 10962 (2016).
- Liu, Q., Wu, Y., Wang, H., Jia, F., Xu, F. Viral tools for neural circuit tracing. Neurosci Bull. 38 (12), 1508-1518 (2022).
- Christenson Wick, Z., Krook-Magnuson, E. Specificity, versatility, and continual development: The power of optogenetics for epilepsy research. Front Cell Neurosci. 12, 151 (2018).
- Paschen, E., et al. Hippocampal low-frequency stimulation prevents seizure generation in a mouse model of mesial temporal lobe epilepsy. Elife. 9, e54518 (2020).
- Gummadavelli, A., Zaveri, H. P., Spencer, D. D., Gerrard, J. L. Expanding brain-computer interfaces for controlling epilepsy networks: Novel thalamic responsive neurostimulation in refractory epilepsy. Front Neurosci. 12, 474 (2018).
- Vandekerckhove, B., et al. Technological challenges in the development of optogenetic closed-loop therapy approaches in epilepsy and related network disorders of the brain. Micromachines (Basel). 12 (1), 38 (2020).
- Li, Z. J., Zhang, L. B., Chen, Y. X., Hu, L. Advancements and challenges in neuromodulation technology: Interdisciplinary opportunities and collaborative endeavors. Sci Bull (Beijing). 68 (18), 1978-1982 (2023).
- Zhang, Q., et al. A prototype closed-loop brain-machine interface for the study and treatment of pain. Nat Biomed Eng. 7 (4), 533-545 (2023).
- Varela, C., Wilson, M. A. Mpfc spindle cycles organize sparse thalamic activation and recently active ca1 cells during non-rem sleep. Elife. 9, e48881 (2020).
- Adaikkan, C., et al. Gamma entrainment binds higher-order brain regions and offers neuroprotection. Neuron. 102 (5), 929-943.e8 (2019).
- Muthmann, J. O., et al. Spike detection for large neural populations using high density multielectrode arrays. Front Neuroinform. 9, 28 (2015).
Tags

.
