High Resolution 3D Imaging of the Human Pancreas Neuro-insular Network
Summary
Here, we present a protocol to image human pancreas sections in three dimensions (3D) using optimized passive clearing methods. This manuscript demonstrates these procedures for passive optical clearing followed by multiple immunofluorescence staining to identify key elements of the autonomic and sensory neural networks innervating human islets.
Abstract
Using traditional histological methods, researchers are hampered in their ability to image whole tissues or organs in large-scale 3D. Histological sections are generally limited to <20 µm as formalin fixed paraffin section on glass slides or <500 µm for free-floating fixed sections. Therefore, extensive efforts are required for serial sectioning and large-scale image reconstruction methods to recreate 3D for samples >500 µm using traditional methods. In addition, light scatters from macromolecules within tissues, particularly lipids, prevents imaging to a depth >150 µm with most confocal microscopes. To reduce light scatter and to allow for deep tissue imaging using simple confocal microscopy, various optical clearing methods have been developed that are relevant for rodent and human tissue samples fixed by immersion. Several methods are related and use protein crosslinking with acrylamide and tissue clearing with sodium dodecyl sulfate (SDS). Other optical clearing techniques used various solvents though each modification had various advantages and disadvantages. Here, an optimized passive optical clearing method is described for studies of the human pancreas innervation and specifically for interrogation of the innervation of human islets.
Introduction
Until recently, 3-dimensional (3D) reconstruction of large tissues or whole organs was performed through laborious serial sectioning, staining, and image reconstruction of multiple sections. These methods had several disadvantages including reliance on a large number of serial sections, poor tissue penetration with antibodies, and light scattering preventing deep imaging within tissues. To allow for greater light and antibody penetration, researchers developed chemical methods to preserve protein antigens while removing the majority of light-scattering lipids. Several related methods are Clear Lipid-Exchanged Acrylamide-hybridized Rigid Imaging Tissue hYdrogel (CLARITY), Passive Clarity Technique (PACT), and Perfusion-assisted Agent Release in situ (PARS) (reviewed in 1,2,3,4,5,6). The CLARITY method is based on stabilization of proteins using a formaldehyde, acrylamide, and bis hydrogel followed by lipid removal using electrophoresis in a SDS solution2. This approach was later modified for passive clearing and advanced imaging7. Further optimization led to the elimination of bis and varying percentages of paraformaldehyde in the hydrogel solution (1 – 4%) to obtain optimal antigen stabilization with the shortest clearing time for a technique called PACT 8. Early attempts to develop these optical clearing techniques involved organic or other compounds that were difficult to work with and quenched endogenous fluorescent proteins in transgenic mouse models. These additional methods included ScaleS, Unobstructed Brain/Body Cocktails and Computational analysis (CUBIC), SWITCH and Dimensional Imaging of Solvent-Cleared Organs (DISCO) methods, all with various advantages and disadvantages9,10,11,12. The original CLARITY method was developed in lipid-rich mouse brain tissue and optical clearing methods had limited testing in human tissues7,8,11,13,14.
Optical clearing methods are ideal for tracing nerves over long distances as in the intact mouse central nervous system. The pancreas is well innervated by both autonomic and sensory nervous systems. The pancreatic endocrine compartment, islets of Langerhans, comprise a very small portion of the entire organ (1 – 2%) and islets have known heterogeneity in sizes (50 – 250 µm), endocrine cell proportions, and density particularly in diabetes (reviewed in 15,16). In developing a protocol, several optical clearing methods were tested for human pancreas and a PACT procedure was found to provide the best balance of time to clear (~2 weeks) with excellent morphological preservation of nerves and islets. The final optimized procedure is described here in the delineation of the neuro-insular network for large-scale (millimeter distances) and high-resolution 3D imaging of multiple intact pancreatic islets. The technique is suitable for human pancreas immediately following the fixation or after storage as well as samples fixed in neutral buffered formalin and embedded in paraffin wax. The samples are suitable for imaging by confocal or lightsheet microscopy.
Protocol
All experiments were conducted in accordance with University of Florida Institutional Review Board and Federal guidelines.
Caution: Paraformaldehyde and acrylamide are toxic irritants. Handle reagents in a fume hood with appropriate personal protective equipment (lab coat, gloves, eye protection).
1. Deparaffinization of Formaldehyde-fixed Tissues (If Working with Fresh Tissues, Skip to Step 2)
- Use a new razor blade or scalpel to cut through the paraffin perpendicular to the surface of the tissue. Cut the paraffin at the edge of the tissue to finish loosening it. Use forceps or a spatula to gently loosen and remove the tissue to be optically cleared. Gently scrape excess paraffin from the tissue using a spatula (Figure 1A).
- Fill a glass container with xylene (about 30 mL for a 3 x 3 x 3 mm section of tissue) and incubate the tissue in this solution for 24 h at room temperature (RT).
- Fill another glass container with fresh xylene with the same volume as 1.2.1. Transfer and incubate the tissue in this solution for 24 h at RT.
- Place 100% ethanol into a conical tube with the same volume as 1.2.1. Transfer and incubate the tissue in this solution for 24 h at RT.
- Place 95% ethanol into a conical tube with the same volume as 1.2.1. Transfer and incubate the tissue in this solution for 24 h at RT.
- Place 70% ethanol into a conical tube with the same volume as 1.2.1. Transfer and incubate the tissue in this solution for 24 h at RT.
- Rinse the tissue in 0.01 M phosphate buffered saline (PBS) and place in 0.01 M PBS in a conical tube to equilibrate for 24 h at RT. Ensure that the tissue is free of paraffin (Figure 1B, right panel).
2. Prepare 4% Paraformaldehyde (PFA) Fixative
- Pipette 10 mL 16% PFA into a 50-mL conical tube, add 4 mL 0.1 M PBS, and add 26 mL distilled deionized water (ddH2O). Close the cap and mix briefly.
- Larger volumes of 4% PFA can be made ahead and frozen in aliquots. Aliquots are good for 1 day at room temperature (RT), one week at 4 °C, and 1 month at -20 °C.
3. Pancreas Fixation
- Fix the pancreas sample (≤ 1 x 1 x 2 cm) in freshly prepared 4% PFA at 4 °C for 48 h. If the sample is larger than 1 x 1 x 2 cm, use a scalpel or razor blade to dissect into smaller pieces no more than 1 cm thick. Wash tissue sample in three changes of 0.01 M PBS for at least 15 min each wash and store in 15 mL centrifuge tube in 0.01 % PFA/0.01 M PBS or 0.5 % sodium azide/0.01 M PBS until use.
- After fixation, section the tissue into 1 – 2 mm thick sections for further processing. Use a vibratome to assist in even sectioning.
NOTE: The final number of sections will depend on the size of the starting sample.
4. Embed Tissue in Hydrogel
- Prepare 200 mL of the 4 % acrylamide/1 % paraformaldehyde (A4P1) hydrogel monomer solution as follows:
- Place a flask on the ice in a bucket on the top of a magnetic stir plate. Make sure the flask is sitting flat and add a magnetic stir bar.
- Add the following in order: 147.8 mL cold (4 – 8 °C) ddH2O, 20 mL 0.1 M PBS, 20 mL cold (4 – 8 °C) 40 % acrylamide solution, 12.2 mL 16 % PFA solution, and 250 mg VA-044 initiator. Mix the entire hydrogel solution with a magnetic stir bar for at least 10 min and leave the solution on ice for the next step.
- Place a 15-mL conical tube in the ice next to the flask containing the hydrogel solution. Pipette 14 mL of monomer solution into the tube and add one piece of the 1 – 2 mm thick fixed tissue sample. Then cap the tube.
- Incubate the sample in monomer solution for 3 days at 4 °C and protect from light. Aliquot any remaining monomer solution and store at -20 °C for future use.
5. Degas the Monomer Solution and Polymerize the Hydrogel
- Remove oxygen from the hydrogel monomer solution using gaseous N28.
- Prepare a bucket of ice and place the sample in the hydrogel monomer solution on ice.
- Gather 2 – 3, 18-gauge hypodermic needles per sample, paraffin film, and a timer. Connect the tubing to the nitrogen tank so that the nitrogen can flow. While keeping the sample on ice, carefully pierce the cap of a conical tube containing the sample on one side and press one hypodermic needle through until it is under the surface of the liquid monomer solution.
- Use another hypodermic needle to puncture the opposite side of the cap, but do not allow it to become submerged.
NOTE: The second needle will vent the tube. - Connect the tubing from the nitrogen tank to the hypodermic needle submerged beneath the hydrogel and slowly turn on the nitrogen until it is bubbling steadily through the liquid.
- Allow the nitrogen to bubble through the liquid for 10 min.
- Once the oxygen is removed, quickly remove both needles and cover the cap with a paraffin film to prevent any further exchange of gasses between the tube and the environment. Place the degassed sample in an incubator at 37 °C for 3 h to polymerize the hydrogel.
6. Tissue Clearing
- Prepare 500 mL clearing solution (4 % SDS at pH 8.5). To ~300 mL of ddH2O, add 50 mL 0.1 M PBS and 20 g SDS powder while stirring with a magnetic stir bar. Adjust the solution using sodium hydroxide and hydrochloric acid to pH 8.5. Add ddH2O until the final volume is 500 mL.
- After polymerization, pour away excess hydrogel and discard it into a chemical waste container. Use a paper towel to gently wipe away hydrogel from the sample and discard into a chemical waste container.
- Wash the sample in 3 – 5 exchanges of 0.01 M PBS (discard wash fluid into the chemical waste) for 15 min each wash step. Transfer the sample into a 50-mL conical tube with 40 mL of clearing buffer.
- Incubate the sample in the clearing buffer at 37 °C and change sample to fresh clearing buffer every other day.
- Leave the sample in the clearing buffer for 2 – 8 weeks depending on the sample size (~8 weeks for a 3 mm x 3 mm x 3 mm sample) to ensure proper clearing.
- Monitor tissue clearing and stop when complete. Ensure that the sample is adequately transparent by holding it up to the light to check for proper clearing (usually some tan coloring will remain in the exocrine regions).
NOTE: An over-cleared sample will appear frayed at the edges and the texture will be very soft when picked up with forceps. It is common for the sample to clear unevenly. Also, the tissue will not be fully transparent until placed in mounting media (Insert, Figure 1C).
7. Multiple Immunofluorescence
- Wash the samples on a shaker at 60 rpm at RT for one day with 40 mL 0.01 M PBS changing to fresh buffer often (4 – 5 buffer changes in total, 40 mL each wash, changing every h until the final wash). Let the final wash continue overnight at RT.
- Prepare PACT staining buffer. To 500 mL 0.01 M PBS, add 50 mg sodium azide and 0.5 mL TritonX-100. Mix well.
- Incubate the sample with primary antibodies.
- Add 2% normal serum (same species as the secondary antibody) to the base PACT staining buffer in a 2-mL flat bottom tube (at least 1 mL total volume is recommended per sample/tube).
- Add primary antibody to the 2 % serum/PACT staining buffer. Use approximately 5x the amount of primary antibody for PACT staining as would be used for standard immunohistochemistry (i.e. if an antibody is diluted 1:500 for standard immunohistochemistry, use 1:100 for PACT staining).
- Use a spatula to remove the sample from the wash buffer and dab the excess buffer off onto a paper towel, then place in the tube with a primary antibody solution.
- Incubate 2-4 days at RT on a shaker at 60 rpm. Wash samples thoroughly at RT on a shaker at 60 rpm in 0.01 M PBS changing to fresh buffer 4-5 times and leaving the final wash on overnight as in step 7.1.
- Incubate samples with secondary antibodies.
- Add 2% normal serum (same species as the secondary antibody) to the base PACT staining buffer in a 2-mL flat bottom tube (1 mL total volume is recommended per sample/tube).
- Add secondary antibodies at a concentration of 1:200 (5 µl in 1 mL buffer).
NOTE: Small format antibodies are preferred, as well as highly cross-adsorbed antibodies if using more than one primary antibody. - Use a spatula to remove the sample from wash buffer and dab the excess buffer off onto a paper towel, then place in the tube with a secondary antibody solution.
- Incubate at RT on a shaker at 60 rpm for 2 days and protect the sample from light.
- Wash the samples thoroughly, as in step 7.1, at RT on a shaker at 60 rpm in 0.01 M PBS changing to the fresh buffer 4 – 5 times and leaving the final wash on overnight, protect from light during washes.
8. Mounting Samples for Imaging
- Prepare the refractive index matched solution (RIMS) buffer.
- Weigh out 11 g of non-ionic density gradient medium (e.g., Histodenz) and carefully transfer to a 50-mL conical tube.
- Add ~5 mL 0.02 M phosphate buffer (PB)8 using a spatula to release air from the powder non-ionic density gradient medium.
NOTE: The solution will be very viscous, mix well. - Bring the volume to 10 mL using more PB, mix with a spatula and scrape the excess off the spatula into the tube.
- Incubate RIMS at 37 °C until dissolved, invert and gently mix as needed.
- Transfer samples into RIMS. To do so, pipette 1 mL RIMs into a 2-mL flat-bottom tube. Use a spatula to remove the sample from wash buffer and dab the excess buffer off onto a paper towel, then place in the tube with RIMS solution.
- Gently tap the tube to submerge the sample in RIMS. Place samples in RIMS on the bench protected from light at RT for 2 – 4 days before imaging.
- To image, place a small amount of RIMs into an 8-well coverslip bottom chamber slide. Only add just enough to coat the bottom, more will cause the sample to float making it more difficult to image on an inverted scope. Add the sample to the well and cap the slide for imaging.
9. Imaging
- Confocal microscopy and imaging software
- Select appropriate lasers for the excitation and emission spectra of the fluorophores used to stain the PACT samples. Adjust the settings of the acquisition software so that any overlap between channels is eliminated. Use separate tracks if necessary (when two fluorophores have similar excitation spectra).
- Set up the image acquisition
- Choose maximum acquisition speed, 16 bit, 4 or more averages, and 1024 x 1024 resolution or better.
- Zoom into the object to be imaged.
NOTE: This will decrease acquisition time to reduce photo-bleaching and also decrease file size and downstream editing.
- Setup the z-stack.
- Select the optimal sectioning for the objective being used. If deconvolution is desired later, use a z-step smaller than optimal such as 1 µm.
- Use the z-stack correction.
- Begin nearest the surface of the tissue and increase the gain as the objective focuses through the z-plane and add corrections. Do not change the laser settings for the correction!
- Acquire a test stack with one average, 512 x 512 resolution, and maximum acquisition speed. Check the image in 3D to make sure that there is equal brightness throughout the stack for each color before acquiring the final high-resolution z-stack.
- Lightsheet Imaging
- Ensure that the samples have been equilibrated in RIMS for imaging for at least 24 h. Ideally, transfer the samples to fresh RIMS in the imaging chamber and allow to equilibrate in the chamber for at least a day.
- Mount the sample for imaging
- Select the smallest (black) capillary to mount the sample
- Use putty or a dish to hold the sample while applying super glue to the end of the capillary. Glue the tissue to the capillary touching as little a surface of the tissue as possible.
- Insert the sample for imaging.
- Image using 5X or 25X objectives suitable for optically cleared samples using the pivot scan option. If shadowing or blurring occurs, rotate the sample and try again, or let the sample continue to equilibrate in RIMS.
NOTE: A 1 mm stack can generally be acquired in less than five minutes, depending on the settings. - View and edit the image stacks using the image analysis software.
Representative Results
These staining procedures were developed to provide a large-scale examination of the human pancreas to examine islets and associated autonomic and sensory networks. Several procedures were tested including CLARITY7, iDISCO17, and PACT8 with the PACT method found best suited for human pancreas and confocal imaging.
Primary antibodies were initially tested on fixed human pancreas samples with suitable positive and negative control samples (mouse brain, other). The primary antibodies and suggested dilutions are listed in Table of Materials, though each laboratory can expect to optimize antibody dilutions depending on lot number and tissue source. Primary antibody lot-to-lot variation can impact the staining intensity and the specificity, and require validation to achieve the same degree of stain intensity as with former reagents.
In the human pancreas, islets were delineated by insulin, glucagon, and secretogranin 3 (Figure 2). Schwann cells were delineated using glial fibrillary acid protein (GFAP, Figure 2 and Figure 3A). Nerves stained with antibodies against the parasympathetic marker vasoactive intestinal peptide (VIP) were imaged on the Lightsheet (Figure 3B). Sympathetic nerves can be seen with staining for tyrosine hydroxylase (not shown).
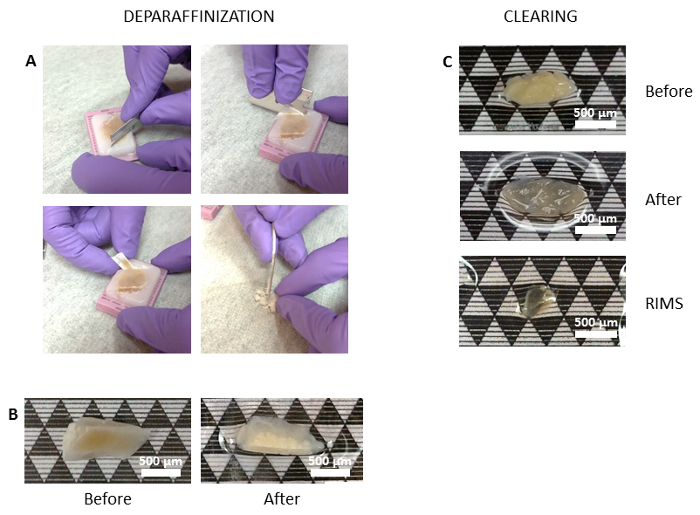
Figure 1. Human pancreas optical clearing. (A) A razor blade is used to cut and loosen a section of paraffin-embedded tissue (top panels) before removing the tissue section and scraping away excess paraffin from the tissue (bottom panels). Representative tissue samples are shown before (B left, C top) and after (B right, C middle) optical clearing of paraffin-embedded tissue, as described in the protocol section. The cleared, fixed tissue is not completely transparent after clearing (B right, C top) and often tissue swelling can be appreciated (C, middle panel). The cleared tissue becomes fully transparent after equilibration in RIMS (C, lower panel). Scale bars: 500 µm. Please click here to view a larger version of this figure.
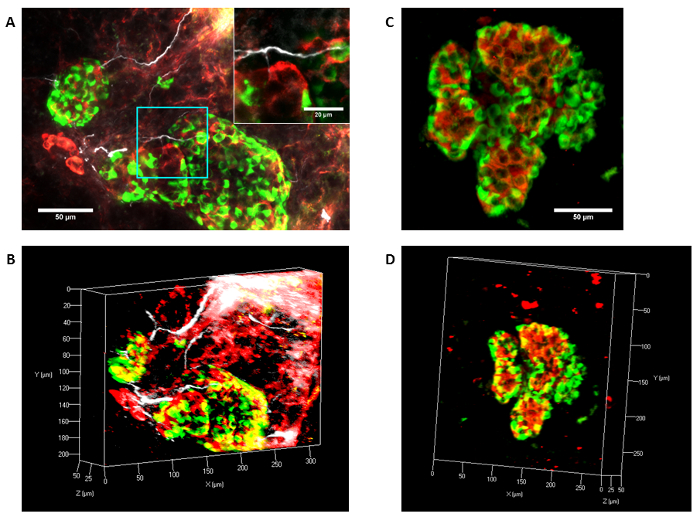
Figure 2. Human neuro-insular network. Human pancreas samples were cleared as described from nPOD organ donors or from archival paraffin-embedded tissues. (A-D) Schwann cells (GFAP+, white) and endocrine cells are shown stained by insulin (red) and glucagon (green). (A) Confocal microscopy and maximum intensity projection (MIP) shows tracing of Schwann cells (GFAP+) on nerves coursing next to blood vessels at the islet periphery and extending into the islets. The inset demonstrates high resolution obtained and shows contact between GFAP+ Schwann cells and endocrine cells. (B) A 3D image of panel (scale: x = 50 µm, y = 20 µm, z = 25 µm) A. A paraffin-PACT sample is shown imaged via confocal microscopy and presented as a max intensity projection (C) and in 3D (D), scale: x, y = 50 µm, z = 25 µm). Scale bars A, C: 50 µm. Please click here to view a larger version of this figure.
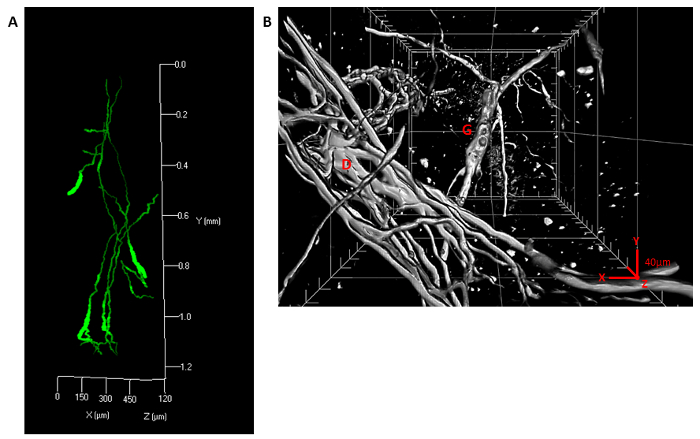
Figure 3. 3D visualization and stitching. Three stitched image stacks were acquired of Schwann cells stained using GFAP and confocal microscopy and traced using a neurite tracer plugin in the image analysis software. (A) The 3D fill of the trace is shown (Scale bars: x = 150 µm, y = 200 µm (0.2 mm), z = 120 µm) (B) A PACT sample was stained with VIP antibody and imaged using a lightsheet microscope. The stack is >1 mm in depth and nerve fibers are clearly seen in high resolution. Fibers wrapping a duct (D, foreground) and a ganglion (G, background) can be seen (Large grid: x, y, z = 200 µm; tick marks: x, y, z = 40 µm). Please click here to view a larger version of this figure.
Discussion
Availability of new optical clearing methods has permitted unprecedented large-scale examinations of the central and peripheral nervous systems in animal models. The overall innervation patterns of the human pancreas are largely unexplored due to tissue density and difficulties in acquiring high quality biospecimens. This protocol offers an optimized tissue clearing protocol for human pancreas tissue from either fixed or paraffin-embedded archival samples.
Clearing time depends on the sample size, fixative type, duration, storage duration and may require 1 – 8 weeks, so the following considerations are critical. It is best to clear tissues soon after the 4% PFA fixation if possible because long-term storage increases clearing time. To minimize clearing time, the amount of PFA in the hydrogel monomer was decreased from 4% as used in the fixation step to 1% for the human pancreas. For other tissues, especially mouse tissues, the amount of PFA necessary to provide sufficient hydrogel rigidity to preserve antigens must be determined empirically. Adequate clearing is also essential since antibody penetration is reduced in poorly cleared tissues and surface staining by secondary antibodies is increased. Changing the clearing solution every other day (or, even daily) is best to keep the pH constant and detergent fresh. Conversely, over-clearing negatively impacts cellular morphology and increases tissue friability. Since some pancreas samples clear unevenly due to inherent pathologies such as regions of fibrosis from chronic pancreatitis or other pathologies, it is important to frequently monitor this process. Use of vibratome thick sections can also be used to make uniform thicknesses yet are not required. Monitoring the clearing process, so samples are removed from detergent as soon as light passes easily through the sample, ensures that the sample is sufficiently cleared.
Antibody penetration and surface staining are important issues to consider with PACT samples. To ensure good antibody penetration, it is critical to incubate samples with the primary antibodies for at least 2 days. Various antibodies have different diffusion rates and should be optimized individually, but for most antibodies, 4 days was sufficient duration for full penetration in a 1 mm3 sample. If surface staining is a problem, especially for Lightsheet imaging, the sample may be cut in half, or the surface dissected away after staining. Small format antibodies when available would be expected to assist with tissue penetration8. Preconjugated antibodies do not appear to work as well as the same unconjugated clone and higher background from nonspecific binding and poorer tissue penetration can be observed if they work at all. For improved success with preconjugated antibodies, increase serum and TritonX-100 concentrations in the staining buffer and use longer incubations (>4 days). After staining, incubation of the sample in RIMS for several days is critical. Cleared tissues swell in PBS and RIMS incubation causes them to shrink back to original size. Especially with lightsheet imaging, the sample should be fully equilibrated before imaging.
When imaging samples on the confocal microscope, the correction must be set each time a new position is chosen. Various acquisition depths and variation in staining intensity throughout the tissue necessitates adjustments for each area of the tissue to be imaged optimally. Likewise, the secondary antibodies used must be carefully considered. Spectral unmixing is challenging in PACT samples, so fluorophores must be appropriately chosen for a low excitation or emission overlap to ensure a bright signal when filtering emissions on the confocal microscope. For each application, a balance must be struck between resolution, imaging speed, and noise reduction so that the best quality image can be acquired without photo-bleaching. Resolution greater than 1024 x 1024 is not detectable by the human eye but may be necessary for certain applications if quantification of a stain is needed.
There are many things to consider when choosing an optical clearing technique and when choosing optical clearing over other more traditional methods. Low abundance antigens may not be detectable using the PACT method thus there are limitations on antigen detection. Certain antigens such as immunological antigens (CD3, CD4, CD8, etc.) are destroyed by the PACT method and are undetectable despite high quality antibodies available to detect them. Another limitation of this method is the inherent variability between donor pancreases which are difficult to discern until after clearing staining. Each donor tissue tends to clear and stain similarly between procedures, but different donor tissues have widely varying clearing times and tissue morphology quality after clearing. The ability to use archival tissue may mitigate this limitation if one can have adequate access to a large number of patient pancreas samples though this has not been thoroughly investigated. The PACT protocol reported herein was found to be inexpensive and readily implemented with standard laboratory equipment. Cleared samples were suitable for imaging via traditional confocal microscopy, as well as 2-photon and Lightsheet technologies and provided high resolution 3D images of nerves and islets in large sections of the human pancreas. Future applications of this procedure include studies on the development of the fetal pancreas for both exocrine and endocrine compartments and islet studies in type 1 and type 2 diabetes.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Dr. Ann Fu and Joseph Canzano for technical assistance, Dr. Jennifer Treweek for invaluable advice, and Dr. Kristin Overton and Dr. Karl Deisseroth for training to MCT through the Stanford University CLARITY workshop. JDRF nPOD and the Organ Procurement Organizations provided tissue samples. This work was funded by JDRF (47-2014-1), Helmsley Charitable Trust (HCT 2015-PG-T1D052) and NIDDK 1OT2 TR001773 to MCT.
Materials
| 10x phosphate buffered saline (PBS) | Fisher | BP399-1 | Buffers |
| Sodium phosphate dibasic anhydrous | Fisher | S375-500 | PB buffer (RIMS) |
| Sodium phosphate monobasic monohydrate | Sigma | 71507-250 | PB buffer (RIMS) |
| 16% paraformaldehyde (PFA) | Electron Microscopy Sciences | 15714-5 | Immersion fixation, hydrogel, storage solution |
| 40% acrylamide | Bio-Rad | 161-0140 | Hydrogel |
| 2% bis-acrylamide | Bio-Rad | 161-0142 | Hydrogel |
| VA-044 initiator | Wako Pure Chemical Industries, Ltd. | VA044 | Hydrogel |
| Sodium dodecyl sulfate (SDS) | Fisher | BP166-5 | Clearing buffer |
| Sodium azide | Sigma | S8032 | Sample storage buffer |
| 18 gauge needles | Fisher | 14-840-91 | Degassing hydrogel solution |
| N2 tank | AirGas | various | Degassing hydrogel solution |
| Triton X-100 | Sigma-Aldrich | 100 ml | Buffers |
| Goat, normal serum | Vector | S-1000 | Use as 2% in blocking buffer |
| Histodenz | Sigma | D2158-100G | RIMS |
| 8-well chamber slides | Ibidi | 80827 | Imaging |
| Laser scanning confocal microscope | Zeiss | 710 | Imaging |
| LightSheet microscope | Zeiss | Z1 | Imaging |
| Name | Company | Catalog Number | Comments |
| Primary Antibody | |||
| CD45 | Bioss | bs-4820R-A488 | Host: Rabbit Dilution: 1:100 Comments: Did not work |
| CD45 | DAKO | M0754 | Host: Mouse Dilution: 1:200 Comments: Did not work |
| GFAP | DAKO | Z0334 | Host: Rabbit Dilution: 1:50 Comments: Worked |
| Glucagon | BD Biosciences | 565891 | Host: Mouse Dilution: 1:50 Comments: Worked |
| Glucagon | Cell Signaling | 2760S | Host: Rabbit Dilution: 1:200 Comments: Did not work |
| Glucagon | Abcam | ab10988 | Host: Mouse Dilution: 1:200 Comments: Worked |
| Insulin | DAKO | A0564 | Host: Guinea Pig Dilution: 1:200 Comments: Worked |
| NCAM (CD56) | DAKO | M730429-2 | Host: Mouse Dilution: 1:50 Comments: Did not work |
| NCAM (CD56)-FITC conjugate | DAKO | M730429-2 | Host: Mouse Dilution: 1:50 Comments: Did not work |
| Peripherin | EnCor | RPCA-Peri | Host: Rabbit Dilution: 1:100 Comments: Worked |
| PGP9.5 | DAKO | Z5116 | Host: Rabbit Dilution: 1:50 Comments: Did not work |
| Secretogranin 3 | Sigma | HPA006880 | Host: Rabbit Dilution: 1:200 Comments: Worked |
| Smooth muscle actin | Sigma | A5228; C6198 (Cy5) | Host: Mouse Dilution: 1:200; 1:200 Comments: Worked; Conjugated worked better than unconjugated |
| Substance P | BioRad | 8450-0505 | Host: Rat Dilution: 1:200 Comments: Worked |
| Tyrosine Hydroxylase | Millipore | AB152 | Host: Rabbit Dilution: 1:200 Comments: Worked |
| Tyrosine Hydroxylase | Abcam | Ab76442 | Host: Chicken Dilution: 1:100 Comments: Worked, but weak staining |
| Vasoactive Intestinal Peptide (VIP) | Immunostar | 20077 | Host: Rabbit Dilution: 1:100 Comments: Worked |
| Vesicular Acetylcholine Transporter (VAChT) | Synaptic Systems | 139103 | Host: Rabbit Dilution: 1:50 Comments: Worked |
| Secondary Antibody | |||
| Guinea pig IgG | ThermoFisher Scientific | Various | Host: Goat Dilution: 1:200 Comments: AlexaFluor conjugates |
| Mouse IgG | ThermoFisher Scientific | Various | Host: Goat Dilution: 1:200 Comments: AlexaFluor conjugates |
| Rabbit IgG | ThermoFisher Scientific | Various | Host: Goat Dilution: 1:200 Comments: AlexaFluor conjugates |
| Rat IgG | ThermoFisher Scientific | Various | Host: Goat, Donkey Dilution: 1:200 Comments: AlexaFluor conjugates |
| Chicken IgG | ThermoFisher Scientific | Various | Host: Goat Dilution: 1:200 Comments: AlexaFluor conjugates |
References
- Ariel, P. A beginner’s guide to tissue clearing. Int J Biochem Cell Biol. 84, 35-39 (2017).
- Chung, K., et al. Structural and molecular interrogation of intact biological systems. Nature. 497 (7449), 332-337 (2013).
- Richardson, D. S., Lichtman, J. W. Clarifying Tissue Clearing. Cell. 162 (2), 246-257 (2015).
- Orlich, M., Kiefer, F. A qualitative comparison of ten tissue clearing techniques. Histol Histopathol. , 11903 (2017).
- Treweek, J. B., Gradinaru, V. Extracting structural and functional features of widely distributed biological circuits with single cell resolution via tissue clearing and delivery vectors. Curr Opin Biotechnol. 40, 193-207 (2016).
- Hsueh, B., et al. Pathways to clinical CLARITY: volumetric analysis of irregular, soft, and heterogeneous tissues in development and disease. Sci Rep. 7 (1), 5899 (2017).
- Tomer, R., Ye, L., Hsueh, B., Deisseroth, K. Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat Protoc. 9 (7), 1682-1697 (2014).
- Treweek, J. B., et al. Whole-body tissue stabilization and selective extractions via tissue-hydrogel hybrids for high-resolution intact circuit mapping and phenotyping. Nat Protoc. 10 (11), 1860-1896 (2015).
- Pan, C., et al. Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat Methods. 13 (10), 859-867 (2016).
- Hama, H., et al. ScaleS: an optical clearing palette for biological imaging. Nat Neurosci. 18 (10), 1518-1529 (2015).
- Woo, J., Lee, M., Seo, J. M., Park, H. S., Cho, Y. E. Optimization of the optical transparency of rodent tissues by modified PACT-based passive clearing. Exp Mol Med. 48 (12), e274 (2016).
- Murray, E., et al. Scalable Proteomic Imaging for High-Dimensional Profiling of Intact Systems. Cell. 163 (6), 1500-1514 (2015).
- Lee, H., Park, J. H., Seo, I., Park, S. H., Kim, S. Improved application of the electrophoretic tissue clearing technology, CLARITY, to intact solid organs including brain, pancreas, liver, kidney, lung, and intestine. BMC Dev Biol. 14, 48 (2014).
- Chung, K., Deisseroth, K. CLARITY for mapping the nervous system. Nat Methods. 10 (6), 508-513 (2013).
- Campbell-Thompson, M. Organ donor specimens: What can they tell us about type 1 diabetes?. Pediatr Diabetes. 16 (5), 320-330 (2015).
- Kim, A., et al. Computer-assisted large-scale visualization and quantification of pancreatic islet mass, size distribution and architecture. J Vis Exp. (49), (2011).
- Renier, N., et al. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell. 159 (4), 896-910 (2014).

