Single Molecule Analysis of Laser Localized Psoralen Adducts
Summary
Lasers are frequently used in studies of the cellular response to DNA damage. However, they generate lesions whose spacing, frequency, and collisions with replication forks are rarely characterized. Here, we describe an approach that enables the determination of these parameters with laser localized interstrand crosslinks.
Abstract
The DNA Damage Response (DDR) has been extensively characterized in studies of double strand breaks (DSBs) induced by laser micro beam irradiation in live cells. The DDR to helix distorting covalent DNA modifications, including interstrand DNA crosslinks (ICLs), is not as well defined. We have studied the DDR stimulated by ICLs, localized by laser photoactivation of immunotagged psoralens, in the nuclei of live cells. In order to address fundamental questions about adduct distribution and replication fork encounters, we combined laser localization with two other technologies. DNA fibers are often used to display the progress of replication forks by immunofluorescence of nucleoside analogues incorporated during short pulses. Immunoquantum dots have been widely employed for single molecule imaging. In the new approach, DNA fibers from cells carrying laser localized ICLs are spread onto microscope slides. The tagged ICLs are displayed with immunoquantum dots and the inter-lesion distances determined. Replication fork collisions with ICLs can be visualized and different encounter patterns identified and quantitated.
Introduction
DNA is under constant assault from exogenous agents such as radiation, ultraviolet light, environmental toxins, combustion products, etc. Additionally, it is also attacked by endogenous radical species produced by oxidative metabolism. All of these have the potential to chemically or physically disrupt the integrity of DNA 1. Perturbations in the genome can activate the DNA Damage Response (DDR), a recruitment and post translational modification cascade with hundreds, if not thousands, of proteins and microRNAs involved in lesion repair, regulation of the cell cycle, apoptosis, senescence, and inflammatory pathways 2.
Most of our information about the DDR comes from studies with DSBs. This is in large part due to the availability of technologies for introducing breaks, including sequence specific breaks, in genomic DNA in living cells 3. In addition, the propensity of breaks to induce foci of DDR proteins, which can be displayed by immunofluorescence, has been very helpful for identifying the kinetics and requirements of responding proteins. One of the key technologies for studying the DDR was introduced by Bonner and colleagues, who used a laser beam to direct a stripe of DSBs in a "Region of Interest" (ROI) in the nuclei of living cells 4. In effect, they created a lengthy focus in which proteins of the DDR could be identified by immunofluorescence. This was illustrated by their demonstration of the strong stripe of phosphorylated histone H2AX (γ-H2AX) in the laser exposed cells. Since then, the laser approach has been employed in numerous studies of the DDR induced by DSBs. Although powerful and popular, and the source of dramatic immunofluorescence images, it should be noted that in most experiments the laser intensity is adjusted so as to produce observable results, without concern for lesion identity, density, or spacing. Indeed, it can be difficult to make these estimates. Thus they are largely ignored, despite the multiplicity of lesions introduced into DNA by lasers 5. This contributes to the many contradictions in the literature 6.
In contrast to DSBs, most chemical modifications of DNA do not stimulate the formation of discrete foci of DDR proteins. This is important in the light of our current understanding of lesion frequencies. It has been estimated that human cells in culture incur as many as 50 DSBs per cell cycle, formed largely during S phase 7,8,9. Fewer are formed in non-proliferating cells. This contrasts with the number of nucleobase losses or modification events, which are in the tens of thousands per cell/day 1,10. Thus, we know most about the DDR induced by events that are relatively rare, and much less about those induced by helix distorting lesions, which in aggregate are far more common.
In order to address questions about the cellular response to covalent modifications of genomic DNA, we wanted to work with a helix distorting DNA adduct that had inherent DDR induction activity. Furthermore, to facilitate experimental design and interpretation we were interested in a structure whose introduction could be controlled with respect to time and was amenable to visualization. Accordingly, we developed a strategy based on psoralen. Psoralens are well characterized photoactive DNA intercalators favoring 5' TA:AT sites. Unlike other crosslinking agents such as nitrogen mustards and mitomycin C (MMC) they are not DNA reactive unless exposed to long wave UV (UVA) light. The intercalated molecules react with thymine bases on opposite strands to produce helix distorting interstrand crosslinks (ICLs) 11. With the trimethyl psoralen used in our experiments most products are ICLs, relatively few monoadducts are generated (less than 10%) 12, and intrastrand crosslinks between adjacent bases on one strand are not formed. Because they are powerful blocks to replication and transcription, psoralen and other crosslinking agents, like cis-platinum and MMC, are commonly used in chemotherapy. Thus psoralen enabled studies that followed the activation of the DDR by a helix distorting structure, and also provided insight into the cellular response to a compound with clinical importance.
We synthesized a reagent in which trimethyl psoralen was linked to digoxigenin (Dig), a plant sterol not found in mammalian cells and frequently used as an immunotag. The requirement for photoactivation permits localization by laser light (365 nm) of psoralen ICLs in defined ROI in nuclei in living cells. These can be displayed by immunofluorescence against the Dig tag. DNA repair and DDR proteins appeared in the stripes of laser localized ICLs 13,14.
The DDR activated by the high laser intensities used to produce DSBs could be due to isolated or clustered damage 15,16. Consequently, the relevance of results from these experiments to naturally occurring lesions, present at much lower concentration, is uncertain. To address similar questions about psoralen adduct frequency and spacing in DNA, we took advantage of DNA fiber technology 17 and immunoquantum dots. Quantum dots are much brighter than fluorescent dyes and are not bleached by exposure to light. Thus they are frequently used for single molecule imaging 18, an application for which fluorescent dyes are insufficiently bright. Individual DNA fibers can be stretched on glass slides and can be displayed by immunofluorescence against nucleoside analogues incorporated during incubations prior to cell harvest. We treated cells with Dig-psoralen and exposed the ROI to laser micro irradiation. Fibers were prepared from the cells and individual Dig-psoralen adducts could be visualized with the immunoquantum dots. Exposing the cells to nucleoside analogues for relatively short times (20-60 min) permits the display of replication tracts in the vicinity of the laser localized ICLs.
Protocol
1. Preparation of Dig-TMP
- Mix 50 mg (0.18 mmoles) of 4'-chloromethyl-4,5',8-trimethylpsoralen and 590 mg (2.7 mmoles) of 4,7,10-trioxa-1,13-tridecanedi-amine in a dry 25 mL round-bottom flask under nitrogen. Add 10 mL toluene and reflux for 12 h. Remove solvent in a rotary evaporator under reduced pressure.
- Purify the residue by flash column chromatography over silica gel. Elute the column with chloroform, methanol, and 28% ammonia solution (9:1:0.5). Evaporate the solvent in a rotary evaporator under reduced pressure, and recover the pure 4'-[N-(13-amino-4,7,10-trioxatrideca)]aminomethyl-4,5',8-trimethylpsoralen as a pale yellow viscous liquid.
- Mix 5.5 mg (0.012 mmoles) of 4'-[N-(13-amino-4,7,10-trioxatrideca)]aminomethyl-4,5',8-trimethylpsoralen with 5 mg (0.008 mmoles) of digoxigenin NHS ester in a dry round-bottom flask under nitrogen. Add 0.5 mL anhydrous dimethyl formamide (DMF) and 3.4 µL of trimethylamine and stir at 50 °C for 18 h.
- Remove solvent in a rotary evaporator under reduced pressure.
- Dissolve the residue in minimum amount of dichloromethane. Apply the solution in 2 µL spots horizontally across the bottom of a preparative thin layer plate with silica gel as a stationary phase. Run the preparative TLC in chloroform: methanol: 28% ammonium hydroxide (8:1:0.1).
- Mask the plate except for the vertical edges and identify the band of product with short wavelength UV 254 nm lamp. Scrape the product from the plate with a spatula and isolate the pure product using chloroform: methanol: 28% ammonium hydroxide (8:1:0.1) mixture.
- Remove solvents in a rotary evaporator under reduced pressure. Dissolve the residual pellets in 1 mL of 50% EtOH:H2O, dispense into 50 µL aliquots in 1.5 mL tubes (~20 aliquots) and dry in a centrifugal evaporator until all solvent is evaporated (~3 h).
- Redissolve each aliquot in 200 µL of 50% EtOH:H2O and dry again in a centrifugal evaporator. Dissolve each aliquot again in 200 µL of 50% EtOH:H2O, dry in a centrifugal evaporator and store pellets at -20 °C for long term storage.
- For use, dissolve the pellet in 50 µL of 50% EtOH:H2O. Prepare a 1:100 dilution of the dissolved Dig-TMP in H2O and measure OD at 250 nm. The extinction coefficient of Dig-TMP is 25,000. The solution is stable for about a month if stored at -20 °C. Verify the concentration by measuring OD at 250 nm before each use.
- Calculate stock concentration: Abs x 100 x106/25,000 = Concentration (in µM). Typically the stock solution is about 3 mM. It is important to be in this range in order to minimize the volume of EtOH added to the medium.
2. Laser Localized Dig-TMP ICLs
- Perform laser localization with a confocal microscope with a SRS nitrogen laser (337 nm) pumped through a dye cell emitting a line of 365 nm and firing 3 ns pulses at 10 Hz, with a power of 0.7 nW.
- Make a vertical mark on the side of a 35 mm glass bottomed cell culture dish with a marking pen. Scratch a cross in the center of the glass on the culture surface with a diamond pen such that the black stripe on the side of the dish is at the top of one arm of the cross. The cross is marked on the growth surface of the glass so that it and the cells will be in the same focal plane.
- Sterilize the plate after marking with a rinse of 70% EtOH. Dry before plating cells.
- Plate cells (standard laboratory strains such as HeLa or U2OS) in the cross marked culture dishes one or two days before. Cell should be actively dividing and 50-70% confluent on the day of the experiment. Incubate 24 h with 1 µM 5-chloro-2-deoxyuridine (CldU) to uniformly label DNA.
- Add Dig-TMP in 50% EtOH: H2O to cell culture medium to a final concentration of 20 µM. Bring the medium to 37 °C. Change the medium over the cells and place in incubator (37 °C, 5% CO2) for 30 min to allow the Dig-TMP to equilibrate.
- While the cells are incubating, establish the x/y coordinates of the field of view in which the laser is active. Follow the manufacturer's calibration procedure in which the laser is directed by the software to etch a mirrored glass slide along a horizontal and diagonal line. The two lines define the boundaries of the field in which the laser can strike a target.
- Place the plate in the environmental chamber, at 37 °C with controlled CO2 and humidity, on the microscope stage and focus with the 60X oil objective on the intersection of the cross.
- Direct the 365 nm laser beam to an ROI, established by the investigator with the shape tool in the software, to form a stripe of 3 µm x 0.6 µm. The outline of the ROI is placed by the cursor in nuclei of cells located in the immediate vicinity of the intersection of the cross. Adjust the z focal plane to target the laser midway through the nucleus. Use a laser in the range of 350-370 nm. 405 nm lasers cannot induce crosslinks 19.
NOTE: We locate the ROI in nucleoplasmic areas outside the nucleoli, which can be distinguished from nucleoplasm in differential interference contrast (DIC) imaging. - Verify the focus and activity of the laser by increasing the power setting to a level sufficient to obliterate a cell (the cell will darken and then disappear). This is an important diagnostic for an unimpeded light path for the laser through the microscope and then through the coverslip and into the cells.
- Lower the power setting to just enough to activate the psoralen and the ICL induced DDR (this must be determined empirically for each laser/microscope combination). Use a laser intensity setting (1.7% here) that does not activate the DDR in the absence of psoralen (monitored by the formation of γ-H2AX).
NOTE: This is an important determination and the settings may require adjustment if the laser source or a component in the light path is changed. Follow the accumulation of GFP tagged repair proteins such as XPC or FAN1, both of which are rapidly recruited (in seconds) to the laser psoralen stripes, but not to ROI exposed to the laser alone. Some proteins of the DDR appear within seconds, while several minutes may be required for others. It is necessary to perform time course determinations for each protein of interest. We also note that in cells that were not exposed to the laser there are no stripes of responding proteins.
- Lower the power setting to just enough to activate the psoralen and the ICL induced DDR (this must be determined empirically for each laser/microscope combination). Use a laser intensity setting (1.7% here) that does not activate the DDR in the absence of psoralen (monitored by the formation of γ-H2AX).
- After all cells in a field have been targeted move the plate to a new field, staying close to the intersection of the cross.
- In experiments designed to determine the distribution of inter lesion distances expose cells to the laser in 20-25 fields (4-5 cells/field) around the intersection. In order to analyze replication patterns in the vicinity of the localized ICLs incubate cells with 10 µM 5-Iodo-2-deoxyuridine (IdU) after laser firing.
NOTE: The time of incubation with IdU is somewhat arbitrary. We have used as short as 20 min and as long as 60 min (see below). Longer pulses (a few hours) often result in multiple replication tracts coalescing, thus losing the opportunity to image the progress of single forks. In some experiments ces are labeled with CldU for 24 h to uniformly label DNA prior to exposure to Dig-TMP followed by pulse labeling of replication tracts.
3. Harvest of Cells and Stretching of DNA Fibers
- Remove the plate from the stage, remove the medium, and wash with phosphate buffered saline (PBS). Remove the PBS solution. Place a 10 µL drop of a commercial trypsin/EDTA solution on the intersection of the cross in the middle of the glass surface.
- Incubate for 3-4 min at room temperature (RT) and then draw the trypsin solution with the detached cells into the pipet tip. There is no need to neutralize the trypsin.
- Place the drop of trypsin/EDTA with the cells at one end of a silanized glass microscope slide. Lyse the cells and release the DNA by addition of 10 µL of 0.5% SDS solution and incubate for 3-4 min at RT mixing gently with the pipet tip, allowing the periphery of the pool to dry.
- Tilt the slide 20º and allow the liquid to run down to the end. The DNA fibers are extended/stretched by the hydrodynamic forces of the flowing liquid. Let the slides air dry (~10 min) and fix in 3:1 methanol/acetic acid for 10 min. Remove the slides from the fix solution and air dry again. At this point they can be stored indefinitely in 70% EtOH at -20 °C. On removal from the 70 % EtOH allow to air dry before the next step.
- Wash slides in PBS, and then denature in 2.5 M HCl for 1 h at RT. This treatment depurinates DNA making the incorporated halogenated nucleobases accessible to antibodies.
- Neutralize slides in 0.4 M Tris-HCl, pH 7.4. Wash twice in PBS/0.5% Tween-20 (PBST) for 5 min each.
- Block slides with 5% bovine serum albumin (BSA) and 10% goat serum in PBS. Cover the slides gently with a parafilm to spread the blocking solution evenly over the slide and incubate for 1 h at RT.
- Pipet 100 µL of 1:200 dilution in blocking solution of rat anti-bromodeoxyuridine (specifically detects CldU) primary antibody to each slide. Cover the slides gently with a parafilm to spread the dilution evenly over the slide and incubate in a humidified chamber for 1 h at RT. Remove the parafilm and wash the slides three times in PBST for 5 min each.
- Pipet 100 µL of diluted (1:100) goat anti-rat Alexa Fluor 647 in blocking solution to each slide. Cover the slides gently with a parafilm and incubate for 45 min at RT. Wash the slides three times in PBST for 5 min each.
- Pipet 100 µL of diluted (1:40) mouse anti-bromodeoxyuridine (detects ldU and CldU. This dual specificity necessitates the blocking of CldU by the rat anti BrdU in the prior incubation) primary antibody and rabbit anti-DIG antibody (1:200) in blocking solution to each slide. Cover the slides gently with a parafilm and incubate in a humidified chamber for 1 h at RT. Wash the slides three times in PBST for 5 min each.
- Pipet 100 µL of diluted (1:100) goat anti-mouse Alexa Fluor 488 andc(1:5,000) goat anti-rabbit probe (e.g., Qdot 655) in blocking solution to each slide. Cover the slides gently with parafilm and incubate for 45 min at RT. Wash the slides three times in PBST for 5 min each.
- Drain excess PBST onto a paper towel. Add 50 µL of antifade mounting medium onto each slide and cover with a coverslip. Image or store for no more than 48 h at -20 °C.
4. Fiber Imaging by Fluorescence Microscopy
- Perform image acquisition through a 63X objective on an inverted microscope with an attached fully motorized filter wheel with FITC, Cy5 and Q Dot 655 detection. The excitation filter is 425 nm, and the emission filter is 655 nm with 20 nm centered at the emission peak 20.
Representative Results
Laser localized Dig-TMP (Figure 1A) ICLs can be displayed by immunofluorescence against the Dig-tag linked to the psoralen. Although the laser can be directed to strike in an area of any contour, stripes are not "natural" shapes in cells, and legitimate signals can be easily distinguished from artifacts due to non-specific binding by primary or secondary antibodies. This feature is helpful when using antibodies of less than perfect specificity. An example of the well known marker of the DDR, γ-H2AX, in a stripe of Dig-TMP ICLs is shown in Figure 1B.
The immunofluorescence of the Dig tagged lesions in the laser stripes does not allow any conclusions as to the frequency and spacing of adducts. In order to make this determination cells were incubated with CldU for 24 h prior to introduction of the ICLs. The staining against the CldU permits the visualization of the Dig tagged lesions on long fibers and yields clearer fiber patterns than direct stains such as DAPI (4′,6-diamidino-2-phenylindole) or YOYO {1,1'-(4,4,8,8-tetramethyl-4,8-diazaundecamethylene)bis[4-[(3-methylbenzo-1,3-oxazol-2-yl)methylidene]-l,4-dihydroquinolinium] tetraiodide}. After introduction of ICLs by laser photoactivation, fibers were spread from the targeted cells. The analysis of the Dig signals on the fibers such as those shown in Figure 2 revealed that the inter ICL distances ranged from less than 10 kb to greater than 160 kb, as described in our recent publication 21. There was no evidence for clustered adducts. Thus the stripe of the Dig tag, and the accumulation of the DDR proteins in the stripe, reflected the presence of well separated lesions, at least as measured along the extended DNA. Considered in terms of cellular chromatin if the ICLs formed in nucleosomal arrays, with a 6 fold compression of extended DNA length, they would still be separated by the equivalent of approximately 1.6 kb of DNA.
Experiments featuring laser induced DNA damage generally follow the induction of the DDR. These experiments are rarely concerned with questions regarding DNA replication. On the other hand, DNA fiber assays are typically used for studies of replication. Exposure of cells to short pulses of halogenated nucleoside analogues results in their incorporation into DNA. Immunofluorescence analysis of DNA fibers reveals tracts of incorporated analogues representing recent DNA synthesis. It was of interest to ask if DNA replication occurred in the laser localized ICL stripes, and if so, what would be the pattern of replication in the vicinity of the ICLs? Cells were incubated for 24 h with CldU to facilitate display of long fibers. Laser localized ICLs were introduced into cells followed by a 1 h pulse of IdU. DNA fibers were prepared from the targeted cells and the Dig tagged ICLs and the replication tracts displayed. The results indicated that laser activation of the psoralen does not shut down replication or affect the overall frequency of replication patterns. Replication occurred on one side, and also on both sides of the ICLs, with the double sided patterns in a 4:1 majority as we have recently shown 21.

Figure 1: Generation of Laser Localized Dig-TMP ICLs. (A) Structure of Dig-trimethyl psoralen. (B) Accumulation of γ-H2AX (green) in ROI containing laser localized ICLs (Dig, red). The nucleus is stained with DAPI (blue). The brightfield photo shows a cell partially on the mark made by the diamond pen. Note the nucleoli, and the placement of the ROI outside them. Please click here to view a larger version of this figure.
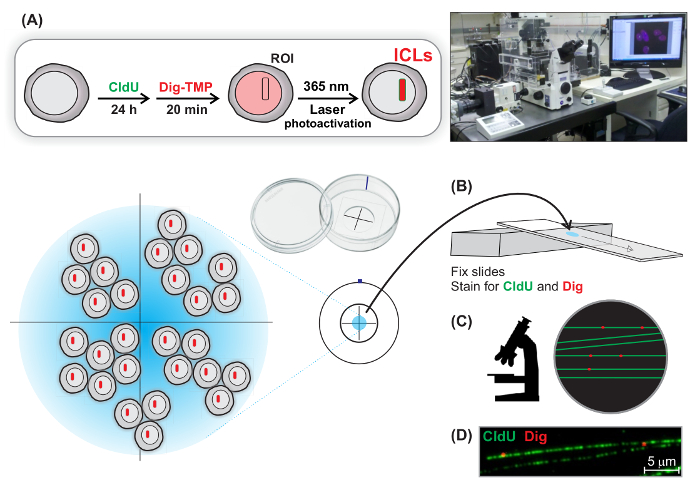
Figure 2: DNA Fibers with Laser Localized Dig-TMP Signals. (A) Cells were incubated for 24 h with CldU to label DNA. Then laser localized ICLs were introduced and (B) the cells were harvested and fibers spread. (C, D) The Dig signals were displayed with an immunoquantum dot (red) and the CldU by immunofluorescence (green). Please click here to view a larger version of this figure.
Discussion
The laser localization technology requires the use of adherent cells with nuclei that are visible in bright field microscopy. We have tried to attach nonadherent cells, such as primary lymphocytes, or loosely adherent cultured cells such as AD293, to the glass surface with cell adhesive preparations such as polylysine or collagen, or more complex mixtures. Although these treatments may bind the cells to the surface, we find that they generally stay rounded making it very difficult to focus into the nuclei. Furthermore, the cells often fail to survive the initial wash after the removal of the growth medium. However, within the requirement for adherent cells, we have successfully used a broad range of standard cell lines as well as primary cells for these studies. It is useful to test cells for mycoplasma contamination. We find that cell responses are altered by these infections.
The focal plane of the laser is important. The goal is to place the ICLs within the nucleus. Too high and the photoactivation will be above the nucleus; too low and it will be on the glass. We find it useful to sharpen the focus of the DIC image at the intersection of the cytoplasm and the nucleus.
The fiber assays are not difficult, but require some practice before interpretable fields are recovered. It is important to implement this technique properly. The number of cells recovered from the laser experiments is low and each sample is used in its entirety on a single slide. Consequently it is essential to maximize the yield from each experiment, as samples cannot be split, with aliquots saved for later analysis. However, hundreds of events can be recovered from a single sample. Two features of the procedure are of central importance. One is the rate of flow of the cell lysate down the slide. If too fast, much of the DNA will run off the slide. The quality of the glass slides is also critical. We occasionally receive lots that are ineffective. Consequently each new lot must be tested prior to use.
As is true for all laboratory manipulations the reliability of commercial reagents-antibodies, immunoquantum dots, etc., is always a concern. These are not always stable and experimental failure is usually traced to problems with one or another reagent. The immunoquantum dots are particularly problematic and more than one lot may need to be tested in order to identify one that is acceptable. Ineffective lots are marked by high background signals. These are not associated with fibers and will be seen on regions of the slide that have no DNA.
The use of lasers to introduce local DNA damage has become quite popular since it was introduced by the Bonner group 4. In those, and most subsequent experiments the laser power settings are set so as to induce the DDR directly. We have used a power setting sufficiently low such that the activation of the DDR is dependent on both laser and psoralen. As discussed above, the separation between psoralen adducts argues that we are looking at the response to individual events. While the number of ICLs that are formed by endogenous crosslinking agents is not known, we note that the localization of the psoralen adducts does not kill the cells, as they are able to remove the psoralen adducts within 6-8 h (unpublished data) and are present and healthy 24 h later. Thus this approach is much less toxic to cells than protocols that require exposure to agents that introduce DNA damage throughout the nucleus and mitochondria.
The laser/psoralen combination introduce a helix distorting structure that activate the DDR. In this regard the results obtained from this approach are applicable to a range of helix distorting lesions, given the cascading nature of the DDR, following the activation of the ATM/DNAPK/ATR kinases 22. In this regard the DNA fiber approach described here could be extended to any DNA structure that can be detected by immunologic or chemical or biochemical means that can be displayed by fluorescence. These could include UV photoproducts 23, bulky alkylators 24, G quadruplexes 25, and oxidative lesions 5.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging (Z01 AG000746-08) and the Fanconi Anemia Research Fund.
Materials
| Digoxigenin NHS ester | Sigma-Aldrich | 11333054001 | |
| Chloro-psoralen | Berry and Associates | PS 5000 | |
| diaminoglycol | Sigma-Aldrich | 369519 | 4,7,10-Trioxa-1,13-tridecanediamine |
| Chloroform | Acros Organics | 423550040 | |
| Methanol | Fisher Scientific | A4524 | |
| Ammonium solution | Sigma-Aldrich | 5002 | |
| TLC plates | Analtech, Inc. | P02511 | |
| Flass glass column 24/40, 100ml | Chemglass Life Sciences | CG-1196-02 | |
| Nikon T2000_E2 spinning disk confocal microscope, equipped with automated stage and environmental control chamber and plate holder | Perkin Elmer | With Volocity Software | |
| Micropoint Galvo | Andor Technologies | with a Nitrogen pulsed laser | |
| dye cell | Andor Technologies | MP-2250-2-365 | |
| 365 dye | Andor Technologies | MP-27-365-DYE | |
| IdU | Sigma-Aldrich | 17125 | |
| 35mm glass botomm plates 1.5 coverslip, 10mm glass diameter, uncoated | Matek | P35G-1.5-10-C | |
| microscope slides | New Comer Supply | Part # 5070 | New Silane Slides |
| Mouse anti BrdU antibody (IdU) | BD Biosciences | 347580 | 1 in 40 |
| Rat anti BrdU Antibody (CldU) | Abcam | ab6326 | 1 in 200 |
| Rabbit anti Dig antibody | ThermoFisher Scientific | 710019 | 1 in 200 |
| Q-dot 655 goat anti Rabbit IgG | ThermoFisher Scientific | Q-11421MP | 1 in 5000 |
| AF647- goat anti Rat IgG | Jackson Immunoresearch | 112-605-167 | 1 in 100 |
| AF488-goat anti mouse IgG | Jackson Immunoresearch | 115-545-166 | 1 in 100 |
| Zeiss epifluorescent microscope A200 | Zeiss | with Axiovision software | |
| Q-dot 655 filter | Chroma | 39107 |
References
- Lindahl, T. The Intrinsic Fragility of DNA (Nobel Lecture). Angew. Chem. Int. Ed Engl. 55 (30), 8528-8534 (2016).
- Sirbu, B. M., Cortez, D. DNA damage response: three levels of DNA repair regulation. Cold Spring Harb. Perspect. Biol. 5, a01272 (2013).
- Berkovich, E., Monnat, R. J., Kastan, M. B. Assessment of protein dynamics and DNA repair following generation of DNA double-strand breaks at defined genomic sites. Nat. Protoc. 3 (5), 915-922 (2008).
- Rogakou, E. P., Boon, C., Redon, C., Bonner, W. M. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 146, 905-916 (1999).
- Lan, L., et al. In situ analysis of repair processes for oxidative DNA damage in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 101, 13738-13743 (2004).
- Reynolds, P., Botchway, S. W., Parker, A. W., O’Neill, P. Spatiotemporal dynamics of DNA repair proteins following laser microbeam induced DNA damage – when is a DSB not a DSB?. Mutat. Res. 756, 14-20 (2013).
- Vilenchik, M. M., Knudson, A. G. Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc. Natl. Acad. Sci. U.S.A. 100, 12871-12876 (2003).
- Vilenchik, M. M., Knudson, A. G. Radiation dose-rate effects, endogenous DNA damage, and signaling resonance. Proc. Natl. Acad. Sci. U.S.A. 103, 17874-17879 (2006).
- Aguilera, A., Garcia-Muse, T. Causes of genome instability. Annu. Rev. Genet. 47, 1-32 (2013).
- Swenberg, J. A., et al. Endogenous versus exogenous DNA adducts: their role in carcinogenesis, epidemiology, and risk assessment. Toxicol. Sci. 120 (1), S130-S145 (2011).
- Eichman, B. F., Mooers, B. H., Alberti, M., Hearst, J. E., Ho, P. S. The crystal structures of psoralen cross-linked DNAs: drug-dependent formation of holliday junctions. J. Mol. Biol. 308, 15-26 (2001).
- Lai, C., et al. Quantitative analysis of DNA interstrand cross-links and monoadducts formed in human cells induced by psoralens and UVA irradiation. Anal. Chem. 80, 8790-8798 (2008).
- Thazhathveetil, A. K., Liu, S. T., Indig, F. E., Seidman, M. M. Psoralen conjugates for visualization of genomic interstrand cross-links localized by laser photoactivation. Bioconjug. Chem. 18, 431-437 (2007).
- Muniandy, P. A., Thapa, D., Thazhathveetil, A. K., Liu, S. T., Seidman, M. M. Repair of laser-localized DNA interstrand cross-links in G1 phase mammalian cells. J Biol. Chem. 284 (41), 27908-27917 (2009).
- Nowsheen, S., et al. Accumulation of oxidatively induced clustered DNA lesions in human tumor tissues. Mutat. Res. 674, 131-136 (2009).
- Meyer, B., et al. Clustered DNA damage induces pan-nuclear H2AX phosphorylation mediated by ATM and DNA-PK. Nucl Acids Res. 41, 6109-6118 (2013).
- Schwab, R. A., Niedzwiedz, W. Visualization of DNA replication in the vertebrate model system DT40 using the DNA fiber technique. J Vis. Exp. (56), e3255 (2011).
- Kad, N. M., Wang, H., Kennedy, G. G., Warshaw, D. M., Van, H. B. Collaborative dynamic DNA scanning by nucleotide excision repair proteins investigated by single- molecule imaging of quantum-dot-labeled proteins. Mol. Cell. 37 (5), 702-713 (2010).
- Spielmann, H. P., Sastry, S. S., Hearst, J. E. Methods for the large-scale synthesis of psoralen furan-side monoadducts and diadducts. Proc. Natl. Acad. Sci. U.S.A. 89 (10), 4514-4518 (1992).
- Huang, J., et al. The DNA translocase FANCM/MHF promotes replication traverse of DNA interstrand crosslinks. Mol. Cell. 52, 434-446 (2013).
- Huang, J., et al. Single Molecule Analysis of Laser Localized Interstrand Crosslinks. Front Genet. 7, 84 (2016).
- Ciccia, A., Elledge, S. J. The DNA damage response: making it safe to play with knives. Mol. Cell. 40, 179-204 (2010).
- Mori, T., Matsunaga, T., Hirose, T., Nikaido, O. Establishment of a monoclonal antibody recognizing ultraviolet light-induced (6-4) photoproducts. Mutat. Res. 194, 263-270 (1988).
- Poirier, M. C. Antisera specific for carcinogen-DNA adducts and carcinogen-modified DNA: applications for detection of xenobiotics in biological samples. Mutat. Res. 288, 31-38 (1993).
- Henderson, A., et al. Detection of G-quadruplex DNA in mammalian cells. Nucleic Acids Res. 42, 860-869 (2014).

