Electrophysiology on Isolated Brainstem-spinal Cord Preparations from Newborn Rodents Allows Neural Respiratory Network Output Recording
Summary
The central respiratory drive is located in the brainstem. Spontaneous respiratory motor output from an isolated brainstem-spinal cord is recorded by placing an electrode on the fourth ventral root. This experimental approach is valuable for pharmacological investigations or the assessment of respiratory challenges and genetic manipulations on rhythmic motor behavior.
Abstract
While it is well known that the central respiratory drive is located in the brainstem, several aspects of its basic function, development, and response to stimuli remain to be fully understood. To overcome the difficulty of accessing the brainstem in the whole animal, isolation of the brainstem and part of the spinal cord is performed. This preparation is maintained in artificial cerebro-spinal fluid where gases, concentrations, and temperature are controlled and monitored. The output signal from the respiratory network is recorded by a suction electrode placed on the fourth ventral root. In this manner, stimuli can be directly applied onto the brainstem, and the effect can be recorded directly. The signal recorded is linked to the inspiratory signal sent to the diaphragm via the phrenic nerve, and can be described as bursts (around 8 bursts per minute). Analysis of these bursts (frequency, amplitude, length, and area under the curve) allows precise characterization of the stimulus effect on the respiratory network. The main limitation of this method is the viability of the preparation beyond the early post-natal stages. Thus, this method greatly focuses on the study of the whole network without the peripheral inputs in the newborn rat.
Introduction
Breathing is a complex and vital activity controlled by the brain, allowing dioxygen (O2) uptake and carbon dioxide (CO2) elimination. The central respiratory drive is generated by a complex network located in the brainstem in both mammals 1, amphibians 2, reptiles 3, birds 4 and fishes 5. Even if the study of breathing can be processed in vivo, precise mechanistic investigations require direct access to the respiratory control network. To this end, Adrian and Buytendijk developed a reduced goldfish preparation, in which electrodes placed on the brainstem surface record the generated rhythm associated with gill ventilation 5. This approach was subsequently adapted by Suzue in 1984 6 for use in newborn rodents. The advent of this preparation has led to significant advances in respiratory neurobiology. Since it is relatively simple, the technique presented here is amenable to a broad range of basic investigations of rhythmic motor behaviors and their origins in newborn rodents.
The overall goal of this method is to record the neural correlate of inspiratory activity, a respiratory-like rhythm called fictive breathing, produced by the respiratory network. This method can be employed in a wide range of research objectives, targeting inspiratory responses to respiratory variations or pharmacology in both wild type 7 and transgenic 8 animals. Given that experiments are performed at a low temperature, without sensory afferents, and under conditions where the concentrations of glucose and O2 within the aCSF are high, questions have been raised regarding the physiological relevance of the signal recorded. While there are clear differences between in vivo and in vitro conditions (e.g., the frequency of inspiratory bursts) the fact remains that the presence of the core elements of the respiratory network 6 make it possible to study a robust rhythm associated to a vital homeostatic function 9,10.
The rationale behind the development and the use of this technique is to facilitate direct access to the brainstem elements of the respiratory network, which are hardly accessible in vivo, especially in newborns. The brainstem is placed under strictly controlled conditions: the recorded rhythm is not modulated by peripheral afferent inputs from the lungs or the carotid bodies, allowing the study to focus on the central respiratory drive itself 11. Thus, this access is utilized to apply stimuli and record the output signal. In contrast to plethysmography recordings, the respiratory rhythm is modulated by all of its components throughout the body (e.g., lung distension, peripheral chemosensors), making it difficult to apply precise stimuli.
In a newborn rat, the protocol consists of recording the fourth ventral root signal on an isolated brainstem and a truncated spinal cord, maintained in artificial cerebro-spinal fluid (aCSF). The rhythm generated by brainstem-spinal cord preparations is composed of individual slow bursts that are linked to the inspiratory signal 9. Isolated brainstem-spinal cord preparations are easily recordable in rats from post-natal day 0 to 4 (P0 – P4) 7. This approach is commonly used to evaluate the hypoxic response of the respiratory network, and also the response to hypercapnia, acidosis or drugs. An acute hypoxia protocol is presented here. This stimulation is obtained by withdrawal of O2 in the aCSF; this approach is commonly used to assess the tolerance and responsiveness to hypoxic insults. The protocol induces a rhythm depression from the first minute until the end of the hypoxia exposure (Figure 1)12. This depression is reversed during post-hypoxic recovery 12. Concerning the experimental design, it is important to notice that the pons, located at the rostral part of the brainstem, has an inhibitory action on the rhythm generator 8. Thus, preparations of full brainstem and rostral spinal cord display a lower rhythm. Inclusion of the pons in the isolated sample for the recording is determined according to the goal of the experiment 13; the study of pontine influence on the medulla oblongata network would require recordings with and without the pons to compare the results 14. Moreover, one of the advantages of this technique is the possibility of extending the rostral part of the preparation to include mesencephalic and/or diencephalic regions 15,16, making it possible to assess the effect of these regions on the ponto-medullary respiratory network.
Protocol
This method required the use of animal subjects, allowed by Laval University Animal Ethics Committee (protocol # 2012-170).
1. Setup and Preparation
- Solutions
- Prepare aCSF stock solutions according to the following recipes 7,17. Other recipes with concentration variations are available in the literature. Store stock solutions at 4 °C for up to one month.
- Salt solution: add 75.39 g of NaCl (129 mM final); 2.5 g of KCl (3.35 mM final); 0.81 g of NaH2PO4 (0.58 mM final); 2.33 g of MgCl2 (1.15 mM final); 1.85 g of CaCl2 (1.26 mM). Dissolve in 800 ml of distilled water then fill to 1 L with distillated water.
- Bicarbonate solution: add 17.65 g of NaHCO3 (21 mM final). Dissolve in 900 ml of distillated water then fill to 1 L with distilled water. Variations of the bicarbonate concentration will result in pH variations.
- Glucose solution: add 54.06 g of glucose (30 mM final). Dissolve in 400 ml of distillated water then fill to 500 ml with distilled water.
- Prepare aCSF by diluting 100 ml of salt solution, 100 ml of bicarbonate solution, and 50 ml of glucose solution in 1 L of distilled water. Store at 4 °C until use.
- Prepare a glass electrode by warming and stretching a glass tube until it breaks. Sand down the tip before use. The electrode can be reused many time as long as it stays clean.
- Prepare aCSF stock solutions according to the following recipes 7,17. Other recipes with concentration variations are available in the literature. Store stock solutions at 4 °C for up to one month.
- Experimental Setup
Note: The set-up details are presented in Figure 2.- Turn on the amplifier, moving averager, data acquisition system, temperature controller and pump. Check the signal amplification (gain = 10,000), the filtration (low threshold, 10 Hz; high threshold, 5 kHz), and the sampling rate of the analog to digital conversion of the raw signal (2.5 kHz).
- Fill a bottle with aCSF and bubble it with carbogen (95% O2, 5% CO2). Induce a bubbled- and warmed-aCSF flow in the recording chamber (volume 5 ml) at 4 ml/min. Hold the temperature in the recording chamber at 26 (± 1) °C. pH should be 7.4 in these standard conditions.
- Keep 50 ml of RT and carbogen-bubbled aCSF in a 50 ml syringe near the dissection chamber.
- Turn on the computer and start the recording software.
2. Dissection
- Weigh and visually determine the sex of the animal (males have a longer ano-genital distance, while females have a short ano-genital distance; males genitals are often dark while female genitals are pink).
- Anesthetize the newborn rodents by one of the following options: cryoanesthesia (completely immerse the animal in ice for 4 – 5 min 18), injection (equitensine at 4 ml/kg) 19 or inhalation of volatile anesthetics (isoflurane 20 or ether 21). Confirm an adequate plane of anesthesia by the absence of a paw withdrawal reflex.
- Place the animal on the bench, ventral face down. Section the rostral part of the head coronally with a scalpel at the bregma level (visible through the skin and skull). Perform this step immediately after the animal is anesthetized.
- Section the body coronally with a scalpel under the anterior members .
- Remove skin, muscles, fatty tissues and viscus with surgical and thin scissors and pliers. Since bones are soft at this age, be cautious to not damage the nervous system. Perform this step on the bench.
- Place the preparation in the dissection chamber. Use the aCSF stored in the syringe to oxygenate the preparation. From this point to the end of the recording, use a microscope.
- On the dorsal face of the preparation, cut the skull and vertebras from the rostral to the caudal part along the medium axis with surgical and thin scissors and pliers. Open the cut skull and vertebras in order to expose the nervous tissue. Use the aCSF strored in the syringe to oxygenate the preparation.
- With the pliers, remove the Arachnoid membrane, a thin tissue covering the surface of the nervous tissue. Retain the pia mater and blood vessels against the nervous tissue. Use the aCSF stored in the syringe to oxygenate the preparation.
- Place the preparation with the dorsal face down, and carefully bring down the brainstem and spinal cord by cutting the nerves and connective tissue with the scissors while holding the preparation in place. A dorsal approach is also possible. Keep the roots and nerves as long as possible. Remove the bones to isolate the brainstem and spinal cord. Use the aCSF stored in the syringe to oxygenate the preparation.
- Remove the cerebellum and remaining rostral structures by sectioning them with the scalpel. Use the aCSF stored in the needle to oxygenate the preparation.
- Optionally depending on experimental design, remove the pons with the scalpel by a coronal section anterior to the inferior cerebellar artery 22. Note that keeping the whole pons will slow down the rhythm in rats and fully inhibit it in mice. Preparations that include only the caudal part of the pons display a respiratory-like activity with a stable frequency at the C4 root.
3. Recording
- Place the preparation in the recording chamber, ventral face up. Fix the preparation with pins in the lowest part of the spinal cord and rostral-most part of the brainstem.
- Using a syringe linked to the electrode by its needle, induce a depression in the electrode (glass tip diameter: 150 – 225 µm) by pulling out the syringe piston, in order to partially fill the electrode with aCSF.
- Using a micromanipulator, carefully place the electrode close to one of the fourth ventral roots. Other ventral roots could also present a respiratory-like activity (e.g., XII cranial nerve, C1 ventral root).
- Induce a depression in the electrode tank via the syringe by pulling out the piston in order to gently aspirate the nerve rootlet. Then carefully move the electrode to apply it against the spinal cord.
Note: Ideally the size of the rootlet matches the size of the electrode opening and thus creates a seal between the internal and external compartments of the electrode. Since differential amplifiers are commonly used for such recordings, any opening between the inside of the electrode and the recording chamber reduces the quality of the signal and makes it difficult to eliminate background noise. - Start the recording.
- Record the rhythm produced by the preparation under normoxic conditions (i.e., aCSF bubbled with carbogen: 95% O2 and 5% CO2) for at least 20 min to determine the baseline parameters of the preparation.
- Switch the perfusion from carbogen-bubbled aCSF to stimulus aCSF (i.e., bubbled with 95% N2 and 5% CO2 for hypoxia stimulus) for 15 min. Alter exposure duration depending of the experimental protocol. Record the exposure duration on the recording and animal datasheet. Use stainless steel tubes between the aCSF bottle and the chamber whenever possible to avoid gas diffusion to the outside of the tube.
- Switch the perfusion back to standard carbogen-bubbled aCSF for at least 15 min for a recovery recording. Record this on the recording and animal datasheet.
- End the recording.
4. Statistical Analysis
- From the integrated signal, calculate the frequency as the number of bursts per minute (expressed in bursts/min), the amplitude as the difference between the baseline and the peak of the burst (expressed in mV), the burst duration as the duration from the beginning to the end of the burst (expressed in s), the burst area as the area under the curve of the burst on the integrated signal (expressed in mV·s) (Figure 3).
- Calculate the frequency, amplitude, burst duration and burst area under normoxic (baseline), hypoxic (stimulus) and post-hypoxic (post-stimulus) recovery conditions as the average of the last 5 min of the recordings for each condition. Do not analyze the first portion of each condition because there is a delay between switching the perfusion and aCSF homogenization in the recording chamber.
- Express then stimulus (i.e., hypoxia) and post-stimulus (i.e., post-hypoxic) recovery values as a percentage of the baseline values for the corresponding recording. Express the results as means ± S.D.
Representative Results
As mentioned in the introduction, one of the most important advantages of this technique is the direct access to the brainstem to apply various stimuli. As an example, hypoxia was applied here. Figure 1.A.B. displays a full protocol recording, with both normoxic and hypoxic conditions. Figure 1.C.E. displays the rhythm recorded in normoxic conditions (i.e., aCSF bubbled with 95% O2 and 5% CO2 at 26 °C). As previously demonstrated in this exact preparation 11, the frequency is about 8 bursts/min, and the amplitude is about 0.800 mV. Note that the amplitude is only indicative because of its important inter-preparation variations. The upper trace represents the integrated signal while the lower trace represents the raw signal. Figure 1.D.F. displays the rhythm recorded in hypoxic conditions (i.e., aCSF bubbled with 95% N2 and 5% CO2 at 26 °C). As previously characterized23, the frequency dramatically decreased during hypoxia. Figure 1.G.H. displays a single burst showing typical patterns in normoxic and hypoxic conditions.
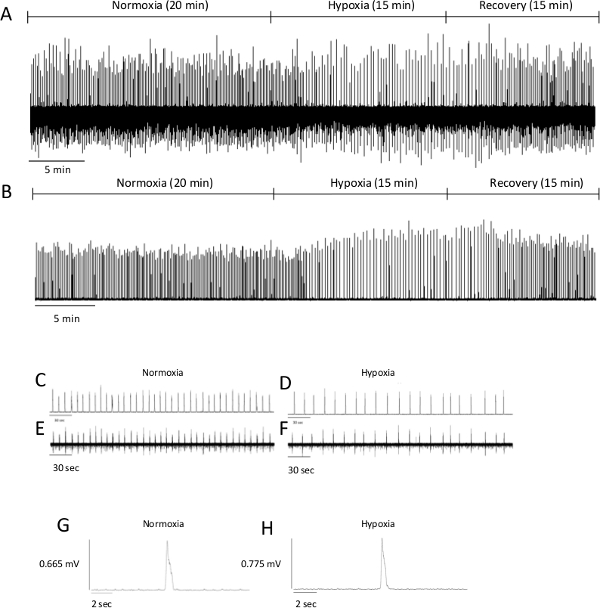
Figure 1. Examples of Recordings Obtained from Brainstem-spinal Cord Preparations. Respiratory output recorded from the C4 ventral root of preparations from P4 rats under normoxic, hypoxic and post-hypoxic recovery conditions ((A) integrated signal and (B) raw signal). For each condition, enlarged recordings ((C) integrated normoxic signal; (D) integrated hypoxic signal; (E) raw normoxic signal; (F) raw hypoxic signal) and single burst ((G) normoxic burst and (H) hypoxic burst) are shown. As mentioned in the text, please note that amplitude has to be interpreted carefully. Please click here to view a larger version of this figure.
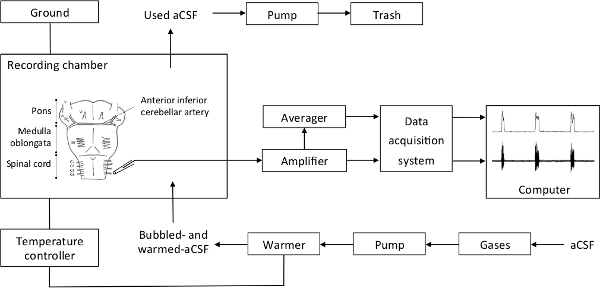
Figure 2. Recapitulative Schema of the Setup. The bubbled aCSF is warmed and sent by a pump in the recording chamber. aCSF excess in the recording chamber is aspirated by a pump and disposed of. The recording chamber is linked to the ground and the temperature controller. The electrode output is directly relayed to the amplifier, then to the moving averager and the data acquisition system, and finally the computer. Please click here to view a larger version of this figure.
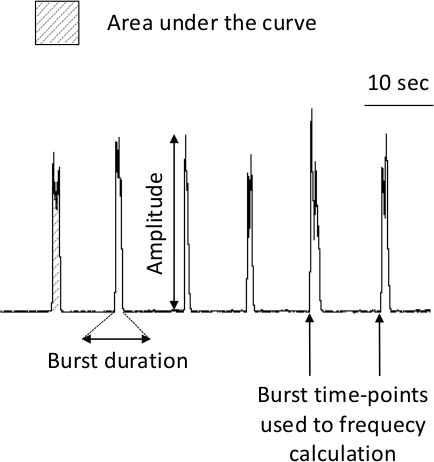
Figure 3. Analyzed Burst Parameters. The frequency is calculated as the number of bursts per minute (expressed in bursts/min), the amplitude as the difference between the baseline and the peak of the burst (expressed in mV), the burst duration as the duration from the beginning to the end of the burst (expressed in s), the burst area as the area under the curve of the burst on integrated signal (expressed in mV·s). Please click here to view a larger version of this figure.
Discussion
Accurate quantification of respiratory activity can be challenging. Indeed, breathing is a function that can be both automatic and voluntary, and that is modulated according to the environment, the body’s needs, the emotional state and the behavior. The advantage of this technique is the isolation of the neural elements responsible for producing the respiratory command. Thus, electrophysiological recordings of brainstem-spinal cord preparations and plethysmography are complementary techniques to study the whole neuronal respiratory network in vitro and in vivo, respectively. Patch-clamp recordings in the brainstem-spinal cord preparation (e.g., approaching from the ventral surface) are also conceivable and allow the study of a particular neuron in a preserved respiratory network 24.
This protocol includes some critical steps, mainly in the dissection. In order to avoid nervous tissue damage, all the dissection steps should be executed swiftly and precisely. The nervous tissue should not be damaged and be carefully conserved by constant immersion in aCSF. As mentioned previously, the quality of the connection between the nerve rootlet and the electrode is a second critical step. Aspiration of the rootlet can bring the electrode closer to the preparation. While the contact between the electrode and the brainstem can improve the quality of the seal, one has to avoid making a depression or physically damaging the preparation.
The integrated signal should always be used for analysis, but raw signals can be useful to differentiate proper bursts from background noise. Moreover, a loudspeaker can be added to the setup to listen to the rhythm and determine signal quality and background noise present by audition. Indeed, some variations in the background baseline could be present due to electric interference and cause to an unstable baseline. Such interferences should be prevented by checking cable connections and root suctioning by the electrode. Moreover, because burst amplitude is dependent on both background noise and the connection between the root and the electrode, frequency is a much of a reliable parameter. Thus, the burst amplitude should always be expressed as percentages of baseline values to avoid inter-individual variations due to setup.
Limitations of the technique are determined by the age of the animal from which the preparation can be realized. This allows a precise study of the setting up of the central respiratory drive at birth, which is very interesting and clinically relevant, but it focuses on these early ages and cannot be extended to later ages. Older ages can be used in arterially perfused working heart-brainstem preparations 25, but with substantial protocol modifications (see below). An isolated brainstem-spinal cord preparation from adult guinea pigs has been developed by Morin-Surun et al.26, in which the brainstem is perfused via the basilar artery and the rhythm is recorded in the XII cranial nerve. Another limitation is the duration of the experiment. The preparation cannot be kept for more than 7 hr in the previously described conditions 6. Therefore, this technique is not appropriate for long-term experiments, even if preparations from tadpoles (see below) have been kept 24 hr in the laboratory.
Various modifications can be applied to this technique. Here, hypoxic challenge has been performed, but other gas variations are also conceivable (e.g., hypercapnia 27 as well as pH variations 28). In the same way, the preparation can be perfused with aCSF in which drugs have been dissolved and applied onto the brainstem as a pre-treatment 29 or during 30 the recording. In this case, the rhythm can be accelerated 29 or slowed down 30 according to the experimental drug. Moreover, with a compartmented recording chamber, different aCSF can be applied on selective parts of the preparation 6 and temperature variations 9 can be employed. This compartmentalization allows the study of the implication of selected brainstem parts in the stimulus-induced effects. While the technique presented uses newborn rats, it is also applicable on other animals models. Using a protocol similar to the one applied for rats, mice can be used from gestational day 16 (E16) until P4 12. The main difference is the need to warm and carbogen-bubble the aCSF during the dissection. Turtles, tadpoles and adult frogs can also be used; however, these species require different dissection techniques as well as adjustments to the composition of the aCSF.
This technique can also be coupled with patch-clamp recordings on the rostral face of the preparation 31, allowing simultaneous visualization of the network output signal and the activity of one particular cell of this network. Voltage sensitive dye imaging can also be applied in newborn rat brainstem-spinal cord preparations to localize the activated areas on the ventral face of the brainstem 32. c-fos analysis in the preparations can also be realized in order to identify the neural populations involved in specific respiratory responses. Finally, the preparation can be modified to keep other organs such as the heart 25, ribs 6, or physiological artery perfusion with other physiological rhythms 33. However, these very important modifications would require other dissection protocols.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors sincerely thank the Canadian Institutes of Health Research MOP 130258 and the Star Foundation for Children’s Health Research, along with the Molly Towell Foundation, for the provision of the research facility and financial support. The authors also sincerely thank Dr. Kinkead Richard for manuscript proofreading and advice.
Materials
| Sylgard | Sigma Aldrich | 761036-5EA | Use under hood |
| NaCl | Bioshop | SOD002 | |
| KCl | Bioshop | POC888 | |
| CaCl2 | Bioshop | CCL444 | |
| MgCl2 | Bioshop | MAG510 | |
| NaHCO3 | Bioshop | SOB999 | |
| NaH2PO4 | Bioshop | SPM306 | |
| D-glucose | Bioshop | GLU501 | |
| Carbogen | Linde | 343-02-0006 | |
| Temperature Controller | Warner Instruments, Hamden, CT, USA | TC-324B | |
| Suction electrode | A-M Systems, Everett, WA, USA | model 573000 | |
| Differential AC amplifier | A-M Systems, Everett, WA, USA | model 1700 | |
| Moving averager | CWE, Ardmore, PA, USA | model MA-821 | |
| Data acquisition system | Dataq Instruments, Akron, OH, USA | model DI-720 | |
| LabChart software | ADInstruments, Colorado Springs, CO, USA | ||
| Prism sofware | Graphpad, La Jolla, CA, USA | ||
| Dissection chamber | Plastic box (e.g. petri box) will do | ||
| Recording chamber | Home made | ||
| Base | Kanetec, Bensenville, IL, USA | MB | |
| Micromanipulator | World Precision Instrument Inc, Sarasota, FL, USA | KITE-R | |
| Base | Kanetec, Bensenville, IL, USA | MB | |
| Peristaltic pump | Gilson, Middleton, WI, USA | MINIPULS 3 | |
| Faraday Cage | Home made | ||
| Computer |
References
- Feldman, J. L., Del Negro, ., A, C., Gray, P. A. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol. 75, 423-452 (2013).
- Taylor, A. C., Kollros, J. J. Stages in the normal development of Rana pipiens larvae. Anat Rec (Hoboken). 94, 7-13 (1946).
- Takeda, R., Remmers, J. E., Baker, J. P., Madden, K. P., Farber, J. P. Postsynaptic potentials of bulbar respiratory neurons of the turtle. Respir Physiol. 64, 149-160 (1986).
- Bouverot, P. Control of breathing in birds compared with mammals. Physiol Rev. 58, 604-655 (1978).
- Adrian, E. D., Buytendijk, F. J. Potential changes in the isolated brain stem of the goldfish. J Physiol. 71, 121-135 (1931).
- Suzue, T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 354, 173-183 (1984).
- Fournier, S., et al. Gestational stress promotes pathological apneas and sex-specific disruption of respiratory control development in newborn rat. J Neurosci. 33, 563-573 (2013).
- Caravagna, C., Kinkead, R., Soliz, J. Post-natal hypoxic activity of the central respiratory command is improved in transgenic mice overexpressing Epo in the brain. Respir Physiol Neurobiol. 200, 64-71 (2014).
- Onimaru, H., Arata, A., Homma, I. Neuronal mechanisms of respiratory rhythm generation: an approach using in vitro preparation. Jpn J Physiol. 47, 385-403 (1997).
- Onimaru, H. Studies of the respiratory center using isolated brainstem-spinal cord preparations. Neurosci Res. 21, 183-190 (1995).
- Ballanyi, K., Onimaru, H., Homma, I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog Neurobiol. 59, 583-634 (1999).
- Viemari, J. C., Burnet, H., Bevengut, M., Hilaire, G. Perinatal maturation of the mouse respiratory rhythm-generator: in vivo and in vitro studies. Eur J Neurosci. 17, 1233-1244 (2003).
- Rybak, I. A., Abdala, A. P., Markin, S. N., Paton, J. F., Smith, J. C. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog Brain Res. , 165-201 (2007).
- Hilaire, G., Viemari, J. C., Coulon, P., Simonneau, M., Bevengut, M. Modulation of the respiratory rhythm generator by the pontine noradrenergic A5 and A6 groups in rodents. Respir Physiol Neurobiol. 143, 187-197 (2004).
- Okada, Y., Kawai, A., Muckenhoff, K., Scheid, P. Role of the pons in hypoxic respiratory depression in the neonatal rat. Respir Physiol. 111, 55-63 (1998).
- Voituron, N., Frugiere, A., Gros, F., Macron, J. M., Bodineau, L. Diencephalic and mesencephalic influences on ponto-medullary respiratory control in normoxic and hypoxic conditions: an in vitro study on central nervous system preparations from newborn rat. 신경과학. 132, 843-854 (2005).
- Somjen, G. G. Ion regulation in the brain: implications for pathophysiology. Neuroscientist. 8, 254-267 (2002).
- Danneman, P. J., Mandrell, T. D. Evaluation of five agents/methods for anesthesia of neonatal rats. Lab Anim Sci. 47, 386-395 (1997).
- Formenti, A., Zocchi, L. Error signals as powerful stimuli for the operant conditioning-like process of the fictive respiratory output in a brainstem-spinal cord preparation from rats. Behav Brain Res. 272, 8-15 (2014).
- Bierman, A. M., Tankersley, C. G., Wilson, C. G., Chavez-Valdez, R., Gauda, E. B. Perinatal hyperoxic exposure reconfigures the central respiratory network contributing to intolerance to anoxia in newborn rat pups. J App Physiol. 116, 47-53 (2014).
- Umezawa, N., et al. Orexin-B antagonized respiratory depression induced by sevoflurane, propofol, and remifentanil in isolated brainstem-spinal cords of neonatal rats. Respir Physiol Neurobiol. 205, 61-65 (2015).
- Ruangkittisakul, A., Secchia, L., Bornes, T. D., Palathinkal, D. M., Ballanyi, K. Dependence on extracellular Ca2+/K+ antagonism of inspiratory centre rhythms in slices and en bloc preparations of newborn rat brainstem. J Physiol. 584, 489-508 (2007).
- Cayetanot, F., Bodineau, L., Frugiere, A. 5-HT acting on 5-HT(1/2) receptors does not participate in the in vitro hypoxic respiratory depression. Neurosci Res. 41, 71-78 (2001).
- Onimaru, H., Homma, I. Whole cell recordings from respiratory neurons in the medulla of brainstem-spinal cord preparations isolated from newborn rats. Pflugers Archiv : European journal of physiology. 420, 399-406 (1992).
- Paton, J. F. Rhythmic bursting of pre- and post-inspiratory neurones during central apnoea in mature mice. J Physiol. 502 (Pt 3), 623-639 (1997).
- Morin-Surun, M. P., Boudinot, E., Kato, F., Foutz, A. S., Denavit-Saubie, M. Involvement of NMDA receptors in the respiratory phase transition is different in the adult guinea pig in vivo and in the isolated brain stem preparation. J Neurophysiol. 74, 770-778 (1995).
- Otsuka, H. Effects of volatile anesthetics on respiratory activity and chemosensitivity in the isolated brainstem-spinal cord of the newborn rat. Hokkaido Igaku Zasshi. 73, 117-136 (1998).
- Gestreau, C., et al. Task2 potassium channels set central respiratory CO2 and O2 sensitivity. PNAS. 107, 2325-2330 (2010).
- Caravagna, C., Soliz, J. PI3K and MEK molecular pathways are involved in the erythropoietin-mediated regulation of the central respiratory command. Respir Physiol Neurobiol. 206C, 36-40 (2014).
- Tree, K., Caravagna, C., Hilaire, G., Peyronnet, J., Cayetanot, F. Anandamide centrally depresses the respiratory rhythm generator of neonatal mice. 신경과학. 170, 1098-1109 (2010).
- Arata, A. Respiratory activity of the neonatal dorsolateral pons in vitro. Respir Physiol Neurobiol. 168, 144-152 (2009).
- Onimaru, H., Homma, I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 23, 1478-1486 (2003).
- St-John, W. M., Paton, J. F. Characterizations of eupnea, apneusis and gasping in a perfused rat preparation. Respir Physiol. 123, 201-213 (2000).

