An Isolated Semi-intact Preparation of the Mouse Vestibular Sensory Epithelium for Electrophysiology and High-resolution Two-photon Microscopy
Summary
Analysis of vestibular hair cell function is complicated by their location deep within the hardest part of the skull, the petrous temporal bone. Most functional hair cell studies have used acutely isolated hair cells. Here we describe a semi-intact preparation of mouse vestibular epithelium for electrophysiological and two-photon microscopy studies.
Abstract
Understanding vestibular hair cells function under normal conditions, or how trauma, disease, and aging disrupt this function is a vital step in the development of preventative approaches and/or novel therapeutic strategies. However, the majority of studies looking at abnormal vestibular function have not been at the cellular level but focused primarily on behavioral assays of vestibular dysfunction such as gait analyses and vestibulo-ocular reflex performance. While this work has yielded valuable data about what happens when things go wrong, little information is gleaned regarding the underlying causes of dysfunction. Of the studies that focus on the cellular and subcellular processes that underlie vestibular function, most have relied on acutely isolated hair cells, devoid of their synaptic connections and supporting cell environment. Therefore, a major technical challenge has been access to the exquisitely sensitive vestibular hair cells in a preparation that is least disrupted, physiologically. Here we demonstrate a semi-intact preparation of the mouse vestibular sensory epithelium that retains the local micro-environment including hair cell/primary afferent complexes.
Introduction
Despite the significant contribution of the vestibular system to our everyday lives, a clear understanding of the processes responsible for the observed decline in vestibular function with age remain elusive. One reason for this lack of knowledge is that decline in vestibular function has almost exclusively been explored using behavioral assays, including the vestibulo-ocular reflex (VOR), a precise indicator of extrinsic vestibular function, but provides limited insights into the changes of intrinsic components. This is a major impediment to our understanding of vestibular hair cell function in health, disease, or aging.
While there have been many studies of individual vestibular hair cells, a major shortcoming has been the reliance on acute hair cell preparations, where hair cells and even calyx afferent terminals are removed from their normal environment via mechanical and/or enzymatic treatment. Such approaches inevitably disrupt the delicate microarchitecture between hair cell and calyx, and hair cell and supporting cell. With the development of semi-intact preparations 1-5, and an isolated mouse labyrinth preparation 6, there is now an opportunity to study the various forms of synaptic communication under conditions that more closely resemble those in vivo. Indeed, Lim et al. (2011) showed marked differences in whole cell currents recorded from acutely isolated type I vestibular hair cells compared to those that remained embedded within the neuroepithelium. Specifically, potassium is thought to accumulate in the intercellular space, between the hair cell and calyx afferent, and significantly alter hair cell response7. This type of information would be impossible to obtain without the semi-intact preparation of the vestibular sensory epithelium described here. We demonstrate the semi-intact preparation of the mouse crista 3, and show representative results obtained from whole-cell patch electrophysiology, and two-photon calcium imaging.
Protocol
1. Animals
- Mice were obtained from the Australian Rodent Centre (ARC; Perth, Australia) and held at the University of Sydney Bosch Animal Facility on a normal 12-hr light/dark cycle with environmental enrichment. All experiments described were approved by The University of Sydney Animal Ethics Committee.
- Male and female mice (C57/Bl6) were used for all experiments since this strain are commonly used as the background for genetic manipulation, and can be considered equivalent to wildtype 8-9.
2. Tissue Preparation
- Prepare 300 ml of a glycerol-based artificial cerebrospinal fluid (ACSF) consisting of (in mM): 26 NaHCO3, 11 glucose, 250 glycerol, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, and 2.5 CaCl2. Prior to the addition of CaCl2, gas the solution with carbogen (95% O2 and 5% CO2) to establish a pH of 7.4 and avoid calcium precipitation (cloudiness). Freeze the solution in a -80 °C freezer for 45 min so that an ice slurry is formed.
- Deeply anaesthetize mice with ketamine (100 mg/kg) (Parnell, Alexandria, Australia) via an intra-peritoneal injection.
- Once hind-limb pinch reflexes are absent decapitate mice using sharp stainless steel scissors and make a sagittal skin incision using a razor blade (rounded #22) to expose the skull. At this point and throughout steps 2.3-2.8 the cranial vault, brain, and underlying vestibular apparatus should be kept as cool as possible by regular application of ice-cold ACSF over the tissue.
- Using the pointed arm of standard pattern scissors (FST, North Vancouver, Canada) make a small incision in the skull at Lambda and cut along the sagittal suture. Ensure that the brain is not “dragged” by the shear blade during this step.
- Carefully peel the parietal bones away laterally and the occipital bone posteriorly using shallow bend pearson rongeurs (FST).
- A small stainless steel spatula is then used to gently lift the brain from the surface of the middle and posterior cranial fossae, and the exposed vestibulocochlea nerve (CNVIII) cut midway between the inner ear and the brainstem with a pair of fine iris scissors. Cutting this nerve minimizes undue tension on axons of the primary afferents and their connections with hair cells.
- Following transection of CN VIII the brain is removed in toto.
- The vestibular labyrinth is now clearly visible in the middle cranial fossa, with the cochlea pointing anteromedially. Rongeur either side of the vestibular labyrinth before gently excising by gripping the anterior semicircular canal and pulling laterally.
- Immerse the excised labyrinths (Figure 1) in a dissecting dish containing the ice-cold, continuously gassed ACSF described in section 2.1. Under a stereomicroscope, hold the labyrinth to the base of the dish by gripping the cochlea. Use fine forceps to scratch away at the bone overlying the anterior semicircular canal ampulla. Once a small hole in the bone is achieved begin to flick the bone away from the ampulla. Caution should be taken not to push the forceps through the bone as this can cause damage to the underlying membranous labyrinth and sensory epithelium. Continue this procedure until both the anterior and adjacent horizontal semicircular membranous ducts and ampullae are exposed (Figure 2).

Figure 1. The isolated mouse vestibular labyrinth. A. Left panel) Schematic representation of the isolated mouse vestibular labyrinth. Important points of reference for accessing the vestibular sensory epithelium, the cochlea, anterior, and horizontal semicircular canals are labeled. Asterisks indicate the semicircular canal ampulla containing the vestibular sensory epithelium. B. Right panel) Photomicrograph of the isolated vestibular labyrinth from a 1-month-old mouse.

Figure 2. Exposure of the Membranous vestibular labyrinth. The bone overlying the anterior and horizontal semicircular canal ampullae have been scratched away to reveal the black/brown-speckled membranous ampullae and associated ampullary nerves (CNVIII). The schematic in the bottom panel represents the structures in the highlighted region of the photomicrograph and shows the relationship of the semicircular canal ducts to the ampullae and CNVIII.
- Using fine forceps, gently lift the ampullae and associated utricle away from the bony labyrinth, ensuring that the central region of the ampullae (containing the sensory epithelium) is not damaged. In some cases the proximal part of the semicircular membranous duct may need to be cut with iris scissors to release the ampullae from the bone.
- Transfer the triad containing the two ampullae and utricle into a Petri dish filled with Leibovitz’s L-15 culture medium (Sigma-Aldrich, St. Louis, MO). Use a fiber optic to “backlight” the tissue. This allows clear visualization of the crista containing the sensory epithelium within the ampullae.
- Carefully make an incision in the speckled “roof” of the utricle with the fine iris scissors. Continue this incision through the roof of the anterior and then the horizontal ampullae. Make the incision as close to the edge of the sensory epithelium as possible without contacting it (Figure 3A). Ensure that there are no pieces of membrane overlying the sensory epithelium (Figure 3B).
- Transfer the isolated semi intact preparation to a small glass-bottomed recording chamber filled with L-15 media. Weigh down the preparation using a grid of fine nylon fibers secured to a flattened U-shaped platinum wire (Figure 3B). Ideally, the fibers of the grid should not overlie the sensory epithelium, however in some cases where more stability is required, a single fiber transecting the sensory epithelium maybe preferred.

Figure 3. The isolated semi-intact preparation of the vestibular sensory epithelium. A. Schematic representation of the semi-intact preparation and electrode configuration. The ampulla overlying the crista has been “de-roofed” to expose the surface of the sensory epithelium (green). B. Photomicrograph of the semi-intact ‘triad’ preparation showing the anterior (ac) and horizontal (hc) crista (utricle obscured behind ac). Note the nylon fiber used to secure the preparation to the base of the recording chamber. Scale bar: 100 μm. C. A recording electrode positioned on an individual vestibular hair cell. Scale bar: 15 μm.
3. Electrophysiology
- Perfuse the semi-intact preparation with continuously oxygenated L-15 medium. The media contains a pH indicator (phenol red) and color of the medium should be monitored throughout the recordings. The pH with oxygen should be 7.3-7.4 and correspond to a red color.
- Prepare recording pipettes from 1.5 mm (1.19 mm ID) borosilicate glass using a two-step protocol (heat step 1: 72; and heat step 2: 50) on a micropipette puller (Narishige, Japan, model PP-830) to achieve a final impedance of 3-4 MΩ.
- Fill the shank of the pipette with 3-4 mm of Potassium fluoride-based internal solution containing (in mM) 110 KF, 12 KCl, 27 KOH, 1 NaCl, 10 HEPES, 10 EGTA, 1.8 MgCl2, 3 D-glucose, and 2 Na-ATP; pH 7.4 with KOH.
- Wrap the shank of the pipette 2-3 times and as far down to the tip as possible with a thin strip of parafilm to insulate the electrode and reduce pipette capacitance.
- Position the pipette over the sensory epithelium under low power magnification (5X) on an upright microscope (Olympus BX51). Switch to high power (40X) and visualize individual vestibular hair cells with an attached CCD camera.
- Position pipette on the membrane of a visualized hair cell (Figure 3C) using a micromanipulator (Sutter Instruments, California, USA). Once a gigaohm seal is achieved, rupture the cell membrane with a small amount of negative pressure applied through a suction port on the pipette holder.
- Make whole-cell voltage clamp recordings using standard techniques 8-9.
4. Two-photon Microscopy
- Prepare a 0.9% saline solution containing 5 mM Oregon Green 488 BAPTA-1 (OGB-1; hexapotassium salt; Invitrogen, Germany).
- While still submerged, place the “de-roofed” semi-intact triad preparation onto a small piece of filter paper (4X4 mm, 0.8 μm thick; Millipore, Germany) ensuring that the sensory epithelium is unobstructed from above.
- Transfer the filter paper and preparation onto the saline covered base platinum electrode (7 μl) of an electroporator consisting of the combination of a pulse generator and a wide-band amplifier, and cover with a further 5 μl of 5 mM solution of the synthetic calcium indicator dye Oregon Green 488 BAPTA-1 (Figure 4).
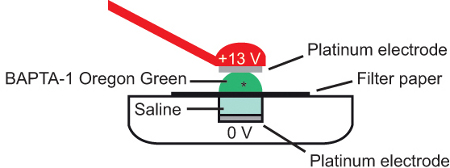
Figure 4. Bulk electroporation. A. schematic showing cross-section of electroporation configuration. The semi-intact preparation (*) is placed on a piece of filter paper immersed in calcium dye (Oregon Green 488 BAPTA-1) and current applied between 2 platinum electrodes. Adapted from Briggman and Euler, 2011 10.
- Bring the top platinum electrode parallel with the base electrode at a distance of approximately 2 mm, being careful not to disrupt the orientation of the preparation when contact with the Oregon Green 488 BAPTA-1 solution is made.
- Pass a brief current pulse across the preparation (Parameters for the current; +13 V, 10 msec pulse width, 1 Hz frequency, 10 square-wave pulses) 10-11.
- On the bottom of a glass bottomed recording chamber filled with L-15 medium, place a small dab of silicon oil and position the semi-intact preparation onto it, again ensuring that the sensory epithelium is unobstructed from above. To ensure stability throughout imaging, replace the nylon grid over the preparation as described above (see point 2.13).
- Using the two-photon microscope make optical recordings of spontaneous calcium activity in the sensory epithelium (Figure 7) using standard imaging protocols 10-11.
Representative Results
The electrophysiological properties of vestibular hair cells are dependent on the complex microarchitecture within which they are embedded 7. Figure 5 shows that the semi-intact vestibular epithelium preparation can be used to differentiate between type I hair cells (Figure 5A), type II hair cells (Figure 5B), and the calyx primary afferent (Figure 5C) based on characteristic whole cell conductances. These characteristics include a pronounced “sag” during depolarization and large “collapsing” tail currents in the type I hair cell, and the presence of transient inward sodium currents representing action potentials in the calyx primary afferent (Arrow in Figure 5C).
The preparation can also be used to quantify the impact of aging on individual vestibular hair cell types. Figure 6 compares the conductance (Figure 6A) and inactivation characteristics (Figure 6B) of type II vestibular hair cells in response to step depolarization from a negative resting potential in young and older mice. For the type II hair cells – the least sensitive subtype- significant differences were not observed in either parameter (see discussion).
In addition to the analysis of whole-cell properties, the semi-intact preparation also lends itself to large-scale analysis of the subcellular mechanisms underlying hair cell function. Figure 7 shows the differential localization of calcium to the type I hair cells and calyx primary afferents, as well as the sub cellular organization of calcium within these cells. Figure 7A shows the change in calcium fluorescence in an individual type I hair cell to the addition of 100 mM KCl. The time course of this change is plotted in Figure 7B. Importantly, the preparation allows for the investigation of hair cell activity at subcellular resolution, in multiple cells simultaneously, and in a “near normal” environment (Figure 7C)- a feature not possible using isolated hair cells and single cell electrophysiology.

Figure 5. Whole cell currents recorded from hair cells in the mouse vestibular sensory epithelium. A. Whole cell currents recorded from a type I vestibular hair cell characterized by large “collapsing” tail currents (Arrow). B. Whole cell currents recorded from a type II vestibular hair cell. Note the absence of tail currents. C. Whole cell recording from a calyx primary afferent characterized by transient sodium-mediated currents (Arrow) following depolarization from a negative resting potential (-120 mV). All cells were held at -60 mV.
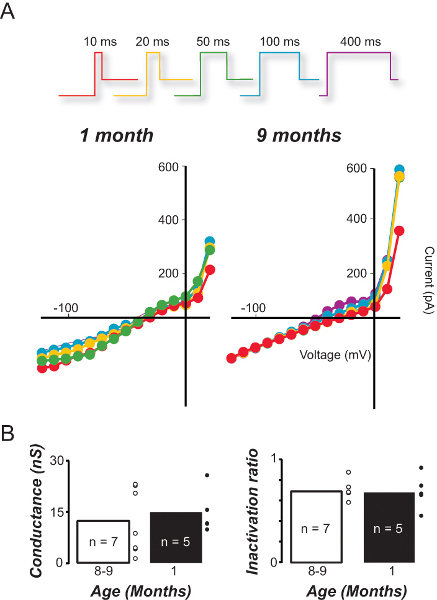
Figure 6. Comparison of sensitivity and inactivation in type II hair cells from young and older mice. A. I/V relationships for type II hair cells recorded from young (1 month) and older (9 month) mice in response to increasing depolarization duration. B. The average conductance and inactivation ratio of type II hair cells in young and old mice based on the 100 msec I/V curves.
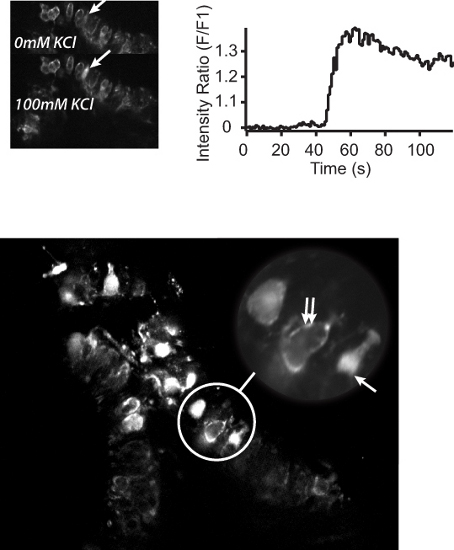
Figure 7. Two-photon calcium imaging of the semi-intact mouse vestibular sensory epithelium preparation. A. Top left panel) Two-photon micrographs showing BAPTA-1 Oregon Green calcium activation in response to normal L-15 perfusate (top) or L-15 containing 100 mM KCl (bottom). Arrows indicate an individual hair cell before and after the addition of KCl. B. Top right panel) Calcium fluorescence profile of the cell highlighted in A. C. Bottom panel) Two-photon micrograph of the sensory epithelium showing Oregon Green 488 BAPTA-1 loading of individual hair cells. Bottom panel Inset: subcellular localization of calcium to the type I hair cell cytoplasm (arrow) or the calyx primary afferent surrounding a type I hair cell (double arrows).
Discussion
The mechanisms underlying our sense of balance have received limited attention in comparison with other sensory systems, e.g. the visual and auditory systems. Of the studies that have investigated changes in vestibular or balance function, most have focused on behavioral measures including the vestibulo-ocular reflex, with incomplete knowledge of the fundamental building blocks of balance- the vestibular hair cells themselves. Those studies that have concentrated on the hair cells have almost invariably done so using acutely isolated hair cells removed from their native environment. In this article we demonstrate a semi-intact preparation of the mouse vestibular sensory epithelium that expands upon these foundation studies.
While vestibular hair cells are relatively robust, the utility of the semi-intact preparation is critically dependent on the speed of excision. Typically, a preparation will remain viable at room temperature for a period of approximately 2-5 hr after excision. Therefore, minimizing the time taken from when the animal is euthanized to recording is paramount. The excision can be relatively easy and quick for a 3 week old mouse (5-7 min total), but gets progressively more difficult in an older animal (up to 25 min in 9 month old mice). In these older animals, dissection temperature is a critical determinant – by ensuring that the preparation is continually bathed in ice-cold oxygenated ACSF normal catabolic processes are inhibited.
As described in section 3.1 the preparation was continually perfused with oxygenated Leibovitz’s medium, L-15, and whole-cell voltage clamp recordings were made using glass microelectrodes filled with KF internal solution 12. This combination of external and internal environments has been shown to provide more stable and longer- duration recordings than other potassium-based internals and/or Ringer’s based extracellular solutions 13-14. This stability is crucial for the long protocols required to study electrophysiological properties and calcium dynamics of hair cells using patch-clamp and two-photon techniques respectively.
The fundamental objective of the semi-intact preparation is to provide a model for the study of vestibular hair cells that is disturbed as little as possible. While the preparation provides clear advantages over acutely isolated hair cells – for example, the full dynamics of whole cell currents are only revealed using the semi-intact preparation 3, 7– disruptions cannot be avoided. First, it is difficult to assess the impact of removing the “roof” of the ampulla (and presumably the attached cupula) on spontaneous hair cell signaling. Under normal physiological conditions, hair cells respond to bending of the cupula/hair bundle complex. Without this complex it is conceivable that spontaneous hair cell signaling may not be representative of in vivo conditions. Second, electrophysiological recordings in the semi-intact preparation are mostly limited to measurements of spontaneous activity or responses to simulated natural activity (i.e. direct current injections into the hair cell or mechanical bending of hair bundles). Therefore it is not possible to study the underlying hair cell properties in response to real-life activation (i.e. head movements). Nevertheless, the semi-intact preparation represents a new tool to help us understand hair cell function, and opens a number of avenues for future research.
One major advantage that the semi-intact preparation provides over acutely isolated hair cell preparations is that it allows simultaneous recordings from multiple hair cells and/or their associated primary afferents. Using dual, or multi-cell patch clamp recording techniques this feature provides a means of assessing directly hair cell/primary afferent signaling, something that cannot be achieved with traditional isolated preparations. In addition, using two-photon microscopy, the semi-intact preparation allows for simultaneous measurement of sub-cellular function across the full extent of the sensory epithelium. This means that information regarding calcium signaling and cellular metabolism can be accrued rapidly and in context. Finally, using mice allows for targeted genetic manipulation (e.g. green fluorescent protein tagged to calretinin expression as a marker for type I hair cells and calyx primary afferents). The semi-intact preparation provides a method to study the activity of hair cells and primary afferents differentially, and simultaneously. This alone, would provide a wealth of new information regarding how vestibular hair cells and primary afferents interact to detect and then encode information to be transmitted to the brain regarding head and body position.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Funding for this work was provided by a Garnett Passe and Rodney Williams Memorial Foundation Project grant to R. Lim and A.J. Camp.
Materials
| REAGENTS | |||
| Leibovitz medium L-15 | Sigma Aldrich | L4386-10X1L | |
| BAPTA-1-oregon green | Invitrogen | O6806 | |
| EQUIPMENT | |||
| Stereo microscope | Leica Microsystems | A60S | |
| Upright microscope | Olympus | BX51WI | |
| Two-photon microscope | Olympus/La Vision | BX51WI/ TriMScope II | |
| Dumont #5 SF Forceps | FST | 11252-00 | |
| Friedman-Pearson Rongeurs | FST | 16221-14 | |
| Standard Pattern Scissors | FST | 14001-12 | |
| InstraTECH A-D converter | HEKA | ITC-18 | |
| Sutter Micromanipulator | Sutter | MP-225/M | |
| multiclamp amplifier | Axon Instruments | 700B | |
| Data acquisition software (electrophysiology) | Axograph | N/A | |
| Imspector Data acquisition software (two-photon) | Max Planck innovation | N/A |
References
- Dulon, D., Safieddine, S., Jones, S. M., Petit, C. Otoferlin is critical for a highly sensitive and linear calcium-dependent exocytosis at vestibular hair cell ribbon synapses. J. Neurosci. 29, 10474-10487 (2009).
- Highstein, S., Art, J., Holstein, G., Rabbitt, R. Simultaneous pre- and post-synaptic recording from the peripheral vestibular calyx and its included type I hair cell. , (2009).
- Kindig, A. E., Lim, R., Callister, R. J., Brichta, A. M. Voltage dependent currents in type I and II hair cell and calyx terminals of primary afferents in an intact in vitro mouse vestibular crista preparation. , (2009).
- Chatlani, S., Goldberg, J. M. Whole-cell recordings from calyx endings in the turtle posterior crista. , (2010).
- Songer, J. E., Eatock, R. A. Transduction in the mammalian saccule. , (2010).
- Lee, H. Y., Camp, A. J., Callister, R. J., Brichta, A. M. Vestibular primary afferent activity in an in vitro preparation of the mouse inner ear. J. Neurosci. Methods. 145, 73-87 (2005).
- Lim, R., Kindig, A. E., Donne, S. W., Callister, R. J., Brichta, A. M. Potassium accumulation between type I hair cells and calyx terminals in mouse crista. Exp. Brain Res. 210, 607-621 (2011).
- Camp, A. J., Callister, R. J., Brichta, A. M. Inhibitory synaptic transmission differs in mouse type A and B medial vestibular nucleus neurons in vitro. J. Neurophysiol. 95, 3208-3218 (2006).
- Camp, A. J., et al. Attenuated glycine receptor function reduces excitability of mouse medial vestibular nucleus neurons. 신경과학. 170, 348-360 (2010).
- Briggman, K. L., Euler, T. Bulk electroporation and population calcium imaging in the adult mammalian retina. J. Neurophysiol. 105, 2601-2609 (2011).
- Briggman, K. L., Helmstaedter, M., Denk, W. Wiring specificity in the direction-selectivity circuit of the retina. Nature. 471, 183-188 (2011).
- Rennie, K. J., Streeter, M. A. Voltage-dependent currents in isolated vestibular afferent calyx terminals. J. Neurophysiol. 95, 26-32 (2006).
- Hudspeth, A. J., Lewis, R. S. Kinetic analysis of voltage- and ion-dependent conductances in saccular hair cells of the bull-frog, Rana catesbeiana. J. Physiol. 400, 237-274 (1988).
- Rennie, K. J., Ashmore, J. F. Ionic currents in isolated vestibular hair cells from the guinea-pig crista ampullaris. Hear. Res. 51, 279-291 (1991).

