Implanting a Wireless Bidirectional Microelectrode System in a Rat Brain
Abstract
Source: Melo-Thomas, L., et al., A Wireless, Bidirectional Interface for In Vivo Recording and Stimulation of Neural Activity in Freely Behaving Rats. J. Vis. Exp. (2017)
The video demonstrates a procedure for implanting a wireless bidirectional multichannel electrode unit into a rat brain to record and stimulate multichannel neural networks.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Animals
- House male adult Wistar rats (200-250 g) in groups of 3-4 under standard laboratory conditions for at least one week before surgery to allow acclimatization.
- Two days after surgery, house rats in pairs. Cover single cages with high acrylic lids. Avoid conventional lids made of metal grid since implants can get stuck, increasing the risk that they become damaged and/or unstable over time.
2. Stereotactic surgery
- Before starting the surgery, organize and prepare the following equipment and materials:
- Obtain sterile surgical equipment consisting of sterile scissors, blunt-end forceps, spatulas, surgical clippers, dental drill, and cotton buds.
- Obtain drugs and chemicals including isoflurane, xylocaine, tramadol hydrochloride, dexpantenol eye salve, 3% hydrogen peroxide, povidone iodine and 70% ethanol.
- Obtain fixation material including stainless steel screws, acrylic resin, ultraviolet glue, and cap protector.
- Obtain a microelectrode unit, consisting of (i) a recording single electrode (quartz glass insulated platinum tungsten microelectrode, with conical tip shape, outer diameter: 80 µm, conical tip, impedance at 1 kHz: 500 kOhm) or a tetrode (quartz glass insulated platinum/tungsten 4 cores microelectrode, outer diameter: 100 µm, conical tip, impedance at 1 kHz: 500-800 kOhm); (ii) a stimulation electrode (platinum/iridium wire (90% platinum, 10% iridium), core diameter 125µm, outer diameter 150 µm, impedance <10 kOhm) connected to a contact plate and (iii) a platinum wire reference electrode (shaft diameter, 100 µm; Figure 1A).
- Obtain an electrode holder glued with water-soluble glue to the microelectrode unit and test for functionality at least 2 h in advance (Figure 1B).
- Obtain a conventional tethered system consisting of a differential preamplifier, a main amplifier, and a bandpass filter amplifier for recordings.
- Obtain additional material, such as gloves, heating pad, syringes, and physiological saline.
- Obtain home cages (L x W x H: 42 cm x 26 cm x 38 cm).
- Procedure
NOTE: Electrode implantation is performed during a conventional stereotactic surgery under isoflurane anesthesia.- Ensure that the experimenter is wearing gloves, a surgical mask, and a lab coat.
- Initiate anesthesia by placing the animal in an induction chamber (isoflurane 4-5%, oxygen flow 1 L/min, duration ~5 min).
- Test for loss of reflexes (tail and toe reflexes) with forceps to confirm deep anesthesia.
- Place the animal's head in an anesthesia mask fixed around the upper incisor bar of the stereotactic frame and adjust the anesthesia (isoflurane 2-3%, oxygen flow 0.7-0.8 L/min).
- Fix and horizontally align the animal's head in the stereotactic apparatus using ear bars and upper incisor bar.
- Shave the surgical field using surgical clippers or a scissor and sterilize with povidone-iodine.
- Place the animal on a heating pad to prevent hypothermia and treat the eyes with dexpanthenol eye salve to prevent them from drying.
- Inject xylocaine (0.3-0.4 mL, subcutaneously, s.c.) in the center of the surgical field.
- Test for loss of reflexes again.
- Make a small incision (1.5 cm) with a scalpel in the middle of the surgical field to expose the skull. Separate the skin gently and remove residual tissue using forceps, scissors, and a spatula.
- Carefully clean the skull using hydrogen peroxide-coated cotton buds.
- Drill 4-5 small holes (4.7 mm) in the skull for fixation of stainless steel screws.
- Connect the microelectrode unit/electrode holder to the preamplifier and attach it to the stereotactic micromanipulator (Figure 1B and 1C).
- Drill a hole (approximately 7 mm) in the skull above the target area using coordinates from a brain atlas according to the animal used. In the present study, position the electrode tips aimed at the inferior colliculus using the following coordinates, with the bregma serving as the reference: anterior/posterior, −8.8 mm; medial/lateral, 1.5 mm; and dorsal/ventral, 3.5 mm.
- Absorb any blood with cotton buds.
- Vertically introduce the microelectrode unit until the electrode tips reach the target area.
- Position the ground cable along the stainless-steel screws and under the skin.
- Monitor spike activity and carefully adjust electrode position with micromanipulator until reaching a zone of active neurons in the target structure and detect neural activity with a signal-to-noise ratio suited for spike sorting.
- Fix the microelectrode unit to the skull with ultraviolet glue and cover the contact plate and screws with acrylic resin.
- Inject physiological saline (1 mL i.p.) and tramadol (25 mg/kg, s.c.) to prevent dehydration and ensure post-operative analgesia, respectively.
- Disconnect the microelectrode unit from the electrode holder using a brush soaked in water.
- Stop anesthesia, and carefully remove the rat from the stereotactic frame. Disconnect the preamplifier from the microelectrode unit.
- Connect the cap protection on the microelectrode unit implanted and disconnect it only during the experimental procedures.
Representative Results
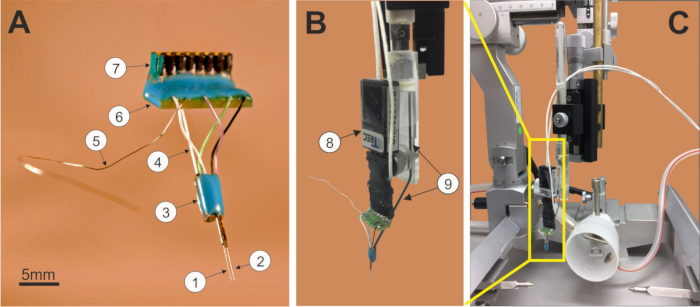
Figure 1: TWS microelectrode unit. (1) recording single electrode/tetrode, (2) stimulation electrode, (3) electrode fiber connection board, (4) flexible connection cables, (5) ground wire, (6) connector board, (7) male or female connector for TWS system (A); TWS microelectrode unit connected to the preamplifier (8) and the holder (9); (B) ready to be attached to a stereotaxic frame (C).
Disclosures
The authors have nothing to disclose.
Materials
| Thomas Wireless System (TWS) version 2.0 | Thomas RECORDING GmbH | AN001165 | The Thomas Wireless System (TWS) version 2.0 is a portable multichannel telemetry system with laptop computer, a preinstalled Microsoft Windows operating system and TWS control software. The TWS includes: low noise 4 channel pre– and programmable main amplifier with fixed bandwidth, single channel constant–current stimulator for application of biphasic current pulses, software programmable micro stimulator, implantable connector system and a basic head stage unit for mounting to an animal. The system is delivered with a transceiver with USB port connection for laptops or desktop personal computers, the control software running under Microsoft operating system Windows. The TWS system can be used for extracellular neural stimulation and recording in freely behaving small animals (e.g. rats, guinea pigs). This system can be adapted to be used in larger animals (e.g. primates) as well. |
| Software for Thomas Wireless System (TWS) | Thomas RECORDING GmbH | inlcuded in AN001165 | The software for the Thomas wireless system is running under Microsoft Windows operating system and provides the graphical user interface (GUI) for the Thomas Wireless System (TWS). The TWS GUI offers complete control of the TWS functions 4 channel recording and 1 channel stimulation. |
| Implantable tetrode for recording (4 channels) and stimulation (single channel) | Thomas RECORDING GmbH | AN001132 | Implantable tetrode for recording (4 channels) and stimulation (single channel) for use with Thomas Wireless Systems (TWS). Recording tetrode specifications: tetrode fiber material: quartz glass insulated platinum tungsten fiber, tetrode fiber outer diameter: 100µm, tip shape D, impedance 0.5-0.8MOHm; Reference electrode: tip shape: D; Impedance: 300-500kOhm; Material: quartzglass insulated platinum/tungsten; Stimulation electrode specification: fiber material: platinum/iridium, diameter: 125µm, lacquer insulated, tip shape : D, impedance: < 10kOhm, dimensions of the electrode can be specified by the end user |
| Implantable microelectrode for recording (single channel) and stimulation (single channel) | Thomas RECORDING GmbH | AN001118 | Implantable microelectrode for recording (single channel) and stimulation (single channel) for use with Thomas Wireless Systems (TWS). Recording electrode specifications: electrode fiber material: quartz glass insulated platinum tungsten fiber, electrode fiber outer diameter: 80µm/250µm (please specify), tip shape D, impedance 0.5-0.8MOHm; Reference electrode: tip shape: D; Impedance. 300-500kOhm; Material: quartzglass insulated platinum/tungsten; Stimulation electrode specification: fiber material: platinum/iridium, diameter: 125µm, lacquer insulated, tip shape : D, impedance: < 10kOhm |
| Holder for electrode implantation | Thomas RECORDING GmbH | AN000838 | Special bent metal rod for microelectrode implantation for standard electrode holders. The rod is used to hold an implantable electrode. The implantable electrode is fixed to the rod with special Thomas RECORDING water soluable glue (AN001080). (Electrode holder is not included) |
| Replacement accumulator power supply for the Thomas Wireless System (3,7V/230mAh) | Thomas RECORDING GmbH | AN001208 | Replacement rechargeable battery (accumulator) for Thomas Wireless System with a capacity of 230mA for approximately 1h operation time. (size: 27mm x 20mm x 6mm, weight app. 6g) |
| Replacement accumulator power supply for the Thomas Wireless System (3,7V/450mAh) | Thomas RECORDING GmbH | AN001209 | Replacement rechargeable battery (accumulator) for Thomas Wireless System with a capacity of 450mA for more than 1h operation time. (size: 48mm x 30mm x 4mm, weight app. 11g) |
| Accumulator charger for Thomas Wireless System (TWS) rechargable accumulator | Thomas RECORDING GmbH | AN001207 | Mains powered charger for the Thomas Wirless System (TWS) rechargable accumulators (AN001209 and AN001209) |
| Water soluble glue | Thomas RECORDING GmbH | AN001080 | Thomas RECORDING water soluble electrode glue is a specially selected product for use with implantable microelectrodes in neuroscientific research. Its unique properties ensure a rigid connection between electrode and mounting device although it is easily removable with warm water. The Thomas RECORDING water soluble electrode glue can be used out-of-the-box, without any time consuming preparation. Thomas RECORDING water soluble electrode glue is not harmful to humans, animals or the environment. Quantity: 1 box of 10 gramms |
| Miniature differential preamplifier | Thomas RECORDING GmbH | AN000329 | The Miniature Differential Pre-Amplifier, Model MDPA-2 is a 2-channel, differential input preamplifier that is designed for low noise recordings from excitable tissue. It is intended for extracellular recording in conjunction with the implantation of implantable microelectrodes for freely moving animal appliactions with the Thomas Wireless System (TWS). The 2-Channel Miniature Differential Preamplifier (MDPA-2) is connected to the implantable microelectrodes for providing the initial tenfold amplification stage. Ideally Thomas RECORDING quartz glass insulated platinum/tungsten electrodes are used to yield optimal recording results with high signal amplitudes and low noise levels. The MDPA-2 has additional common ground and reference electrode inputs. |
| Connection cable | Thomas RECORDING GmbH | AN000330 | Connection cable to connect the Thomas Miniature differential preamplifier (MDPA-2) to a main amplifier and an accumulator power supply. |
| Rechargeable power supply for the miniature preamplifier | Thomas RECORDING GmbH | AN000328 | Rechargeable accumulator power supply for the Miniature differential preamplifier (MDPA-2). |
| Accumulator charger (US) | Thomas RECORDING GmbH | AN000167 | Accumulator charger for the power supply AN000328 (US mains power outlet conenctor) |
| Accumulator charger (EU) | Thomas RECORDING GmbH | AN000168 | Accumulator charger for the power supply AN000328 (EU mains power outlet connector) |
| Differential preamplifier/main amplifier/bandpass filter | Thomas RECORDING GmbH | AN000677 | TREC AC Main Amplifier (LabAmp-03) is a single-channel, differential main amplifier for neurophysiological applications (e.g. extracellular recording with microelectrodes). This Instrument is designed to work with the miniature Differential Pre-Amplifier, Model MDPA-2. The single channel of the LabAmp-03 contains a high-gain, low-noise differential amplifier stage followed by low frequency and high-frequency filters. The amplifier has two different filter amplifiers, a single unit activity (SUA) filter –amplifier and a local field potential (LFP) filter amplifier, both are connected parallel in the signal path. Record Mode offers two levels of signal gain (x10, x100) in a first stage and 4 additional levels (x5, x10, x25 and x50) in a final amplifier stage. Each amplifier has different bandpass characteristics for single unit activity (SUA) 500Hz…20kHz and local field potentials (LFP) 0,1Hz…140Hz. An audio monitor and a window discriminator is integrated in the device. The LabAmp-03 has an integrated audio monitor with loudspeaker. This unit provides audio reproduction of electrophysiological signals. The unit combines an audio amplifier in a compact, rugged package. This is especially suited to monitoring neural firing and muscle contractions. The audio monitor input is internally connected to the SUA-Filter amplifier output. The LabAmp-03 is delivered with external power supply for a mains power operation voltage range of 100-240V AC/50-60Hz. |
| USB Oscilloscope | Thomas RECORDING GmbH | AN001096 | USB PC Oszilloskop, 2 Kanal. This 2-channel PC oscilloscope is perfect suitable for mobile use on a laptop and permanent installation in control cabinets, industrial equipment and many other applications where a small, lightweight and powerful oscilloscope is required. This oscilloscope is connected to the signal output of the main amplifier is for display of recorded extracellular activity during the implanation of the implantable microelectrodes for the Thomas Wireless System (TWS). The user can acquire the measurement data over the several data-interfaces directly on the PC with includes PC software. |
| Stimulus generator | Multichannel Systems | STG3008-FA | Stimulus Generator for Current (STG) and Voltage Driven Stimulation fulfill three functions: current driven stimulation, voltage driven stimulation, controlling and timing. The STG is available with 2, 4 or 8 independet output channels. Featuring integrated isolation units for each output channel, the STG is able to provide any arbitrary waveform. |
| Cap protector for the electrode | Thomas RECORDING GmbH | AN001193 | Protective cap for implantable electrode unit for the Thomas Wireless System |
| Surgical equipment | Scissors, blunt-end forceps, spatulas, surgical clippers, dental drill, and cotton buds | ||
| Drugs and chemicals | Isoflurane, xylocaine, tramadol hydrochloride (Tramadol-CT, AbZ-Pharma GmbH, Ulm, Germany), dexpantenol eye salve (Bepanthen, Bayer AG, Leverkusen, Germany), 3% hydrogen peroxide, povidone-Iodine (Betaisodona, Mundipharma GmbH, Limburg, Germany) and 70% ethanol; | ||
| Fixation material including | Stainless steel screws (BN650 M1.2×5; 4.7 mm ), acrylic resin (Paladur, Heraeus Holding GmbH, Hanau, Germany), ultraviolet glue (Cyberbond U3300, Cyberbond Europe GmbH, Germany) and cap protector (Thomas Recording GmbH, Giessen, Germany); | ||
| Additional material | Gloves, heating pad, syringes, and physiological saline. | ||
| Small Animal Stereotaxic Instrument (SASI) | Thomas RECORDING GmbH | AN000287 | The model should be chosen according to the animal (rat, guinea pig, monkeys, etc) used in the study |
| Video camera | EverFocus | EverFocus, model: EQ150 |

