An Electrophysiological Study of Retinogeniculate and Corticogeniculate Synapses in Mouse Brain Slices
Abstract
Source: Chen, X., et al. Electrophysiological Investigations of Retinogeniculate and Corticogeniculate Synapse Function. J. Vis. Exp. (2019).
The video describes the electrophysiological recording of retinogeniculate and corticogeniculate synapses in mouse brain slices. The slices containing the dorsal lateral geniculate nucleus (dLGN) are placed in a recording chamber. A stimulating pipette is positioned on either the optic tract to stimulate retinal ganglion cells (RGCs) and investigate retinogeniculate synapses or the nucleus reticularis thalami to stimulate cortical neurons and investigate corticogeniculate synapses. Upon stimulation, the RGCs and cortical neurons release excitatory neurotransmitters, which produce synaptic currents in the LGN relay neurons. These currents are recorded using a patch-clamp recording pipette.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
1. Solutions
- Dissection solution
- To reduce excitotoxicity, prepare a choline-based solution to be used during the dissection as presented here (in mM): 87 NaCl, 2.5 KCl, 37.5 choline chloride, 25 NaHCO3, 1.25 NaH2PO4, 0.5 CaCl2, 7 MgCl2, and 25 glucose. Prepare the dissection solution less than 1 week before the experiment.
- Recording solution
- Prepare an artificial cerebral spinal fluid (ACSF) solution containing (in mM): 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 2.5 KCl, 2 CaCl2, 1 MgCl2, and 25 glucose. Add 50 µM D-2-Amino-5-phosphonovaleric acid (D-APV) and 10 µM SR 95531 hydrobromide (gabazine) to block N-methyl-D-aspartate (NMDA) and GABAA receptors, respectively.
- Intracellular solution
- Prepare an intracellular solution containing (in mM): 35 Cs-gluconate, 100 CsCl, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 10 ethylene glycol tetraacetic acid (EGTA), and 0.1 D-600 (methoxyverapamil hydrochloride). Adjust the pH to 7.3 with CsOH. Filter, aliquot, and store the stock solution at -20 °C until use.
2. Dissection
- Prepare two slice chambers, of which one is filled with 100 mL of the dissection solution and the other with 100 mL of recording solution. Place the two beakers into a water bath (37 °C) and bubble the solutions with carbogen for at least 15 min before the dissection.
- Fill a plastic beaker with 250 mL of near ice-cold (but not frozen) dissection solution. Bubble with carbogen at least 15 min before use.
- Fill a plastic Petri dish (100 mm x 20 mm), in which the brain dissection will be performed, with the cold dissection solution. Place a piece of filter paper into the lid of the Petri dish.
- Cool down the dissection chamber of the vibratome.
- Anesthetize a mouse with 2.5% isoflurane, e.g., in the mouse cage. As soon as the mouse does not respond to a tail pinch, decapitate the mouse near the cervical medulla and immerse the head into the ice-cold dissection solution in the base of the Petri dish.
- Cut the skin of the head from caudal to nasal and keep it laterally with fingers to expose the skull. To take out the forebrain, first cut the skull along with the posterior-anterior midline, and then cut two more times through the coronal suture and lambdoid suture (Figure 1A).
- Remove the skull between the coronal suture and lambdoid suture such that the cerebrum is exposed. Cut the olfactory bulb and separate the forebrain from the midbrain with a fine blade (Figure 1B). Remove the brain from the skull with a thin spatula and place it onto the filter paper in the lid of the Petri dish.
NOTE: Steps 2.6 and 2.7 should be performed as fast as possible to reduce cell death. - To preserve the integrity of the sensory and cortical inputs to the dLGN in the brain slice, separate the two hemispheres with a parasagittal cut with an angle of 3−5° (Figure 1C, D). Dry the mediolateral planes of the two hemispheres by placing them on the filter paper and then glue them onto the cutting stage with an angle of 10−25° from the horizontal plane (Figure 1E).
- Place the stage in the center of the metal buffer tray and gently pour the rest of the ice-cold dissection solution into the buffer tray. Perform this step carefully since a strong pour might remove the pasted brain (Figure 1F).
- Place the buffer tray into the ice-filled tray, which helps to maintain the low temperature during dissection. Cut 250 µm slices with a razor blade at a speed of 0.1 mm/s and an amplitude of 1 mm.
- Keep slices in the holding chamber filled with oxygenated dissection solution at 34 °C for around 30 min and then allow the slices to recover in the recording solution at 34 °C for another 30 min.
- After recovery, remove the slice holding chamber from the water bath and keep slices at room temperature (RT) until used in experiments.
3. Electrophysiology
- Pull pipettes using borosilicate glass capillaries and a filament puller. Maintain the resistance of recording pipettes at 3−4 MΩ. Pull stimulating pipettes using the same protocol but break the tip slightly after pulling to increase the diameter. Fill recording pipettes with the intracellular solution and biocytin, while filling stimulating pipettes with the recording solution.
- Place the slices into the recording chamber and continuously perfuse the slices with oxygenated recording solution at RT. Check all the slices and select the ones that display an intact optic tract.
- Visualize the slice and cells with an upright microscope equipped with infrared differential interference contrast (IR-DIC) video microscopy.
NOTE: Relay neurons are differentiated from interneurons by their larger soma size and more primary dendrites (branches emerging from the soma). Interneurons display bipolar morphology with a smaller soma. - Place the stimulating pipette onto the slice before patching the cell with the recording pipette. To investigate retinogeniculate synapses, place the stimulating pipette directly onto the optic tract, where the axon fibers from retinal ganglion cells are bundled (Figure 2A). To analyze corticogeniculate synapses, place the stimulating electrode on the nucleus reticularis thalami, which is rostroventrally adjacent to the dLGN (Figure 2B).
- Once the recording pipette is immersed into the recording solution, apply a 5 mV voltage step to monitor the pipette resistance. Set the holding potential to 0 mV and cancel the offset potential so that the holding current is 0 pA.
- Approach the cell with the recording pipette while applying positive pressure. When the pipette is in direct contact to the cell membrane, release the positive pressure, and set the holding potential to -70 mV. Apply a slight negative pressure to allow the cell membrane to attach to the glass pipette such that a gigaohm seal forms (resistance >1 GΩ). Compensate the pipette capacitance and open the cell by application of negative pressure pulses.
- To investigate the synaptic function, apply 0.1 ms current pulses (ca. 30 µA) via the stimulation pipette. If no synaptic responses are observed, the stimulating pipettes may have to be replaced. Be careful during placing the stimulating pipettes to a different position such that the recorded cell is not lost.
NOTE: In the example recordings shown in Figure 2, synaptic short-term plasticity was investigated by stimulating the optic tract or corticogeniculate fibers twice with 30 ms inter-stimulus intervals. The paired-pulse ratio (PPR) was calculated by dividing the amplitude of the second excitatory postsynaptic current (EPSC) by that of the first EPSC (EPSC2/EPSC1). Each stimulation protocol was repeated 20 times. The EPSC amplitude was measured from average currents of the 20 sweeps. The time interval between each repetition was at least 5 s to avoid short-term plasticity induced by the repetitions. - Monitor the series resistance continuously by applying a 5-mV voltage step. Only use the cells with the series resistance smaller than 20 MΩ for analysis.
Representative Results
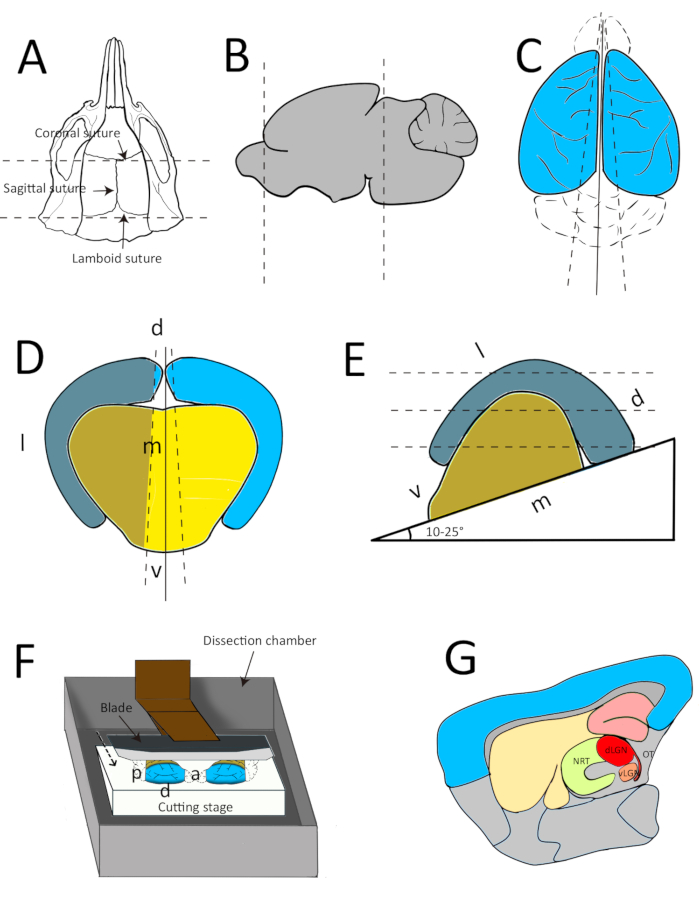
Figure 1: Schemes representing the dissection protocol. (A) Horizontal view of the mouse skull, showing the position of the initial cuts. The first cut was performed along with the sagittal suture, followed by another two cuts along with the coronal suture and lambdoid suture, respectively. Dashed lines represent the incisions. (B) Sagittal view of the whole brain. Dashed lines indicate that the cerebrum is isolated from the olfactory bulb and midbrain. (C-D) These panels show the first cutting angle to separate the two hemispheres from horizontal (C) and coronal (D) view, respectively. (E) Coronal view of one hemisphere on the cutting stage. Dashed lines indicate the slicing direction. l, m, d, and v represent the lateral, medial, dorsal and ventral aspects, respectively, for the hemisphere in panels D and E. (F) Diagram of the dissection chamber with two hemispheres on the cutting stage. The dashed arrow indicates the moving direction of the cutting blade. a, p, and d represent the anterior, posterior, and dorsal aspects of the left hemisphere, respectively.
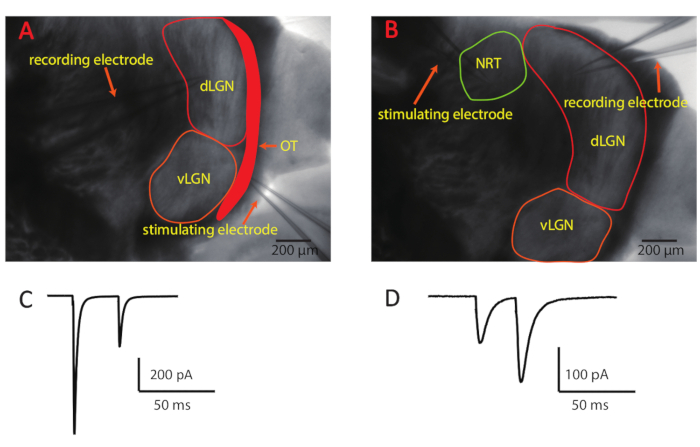
Figure 2: Slice preparation of dLGN containing the retinogeniculate and corticogeniculate pathways and example recordings. (A-B) These panels show a dLGN slice with preservation of retinogeniculate and corticogeniculate inputs. The stimulation electrode was placed on the optic tract to activate retinogeniculate synapses (A) and on the nucleus reticularis thalami to activate corticogeniculate synapses (B).
Disclosures
The authors have nothing to disclose.
Materials
| Amplifier | HEKA Elektronik | EPC 10 USB Double patch clamp amplifier | |
| CaCl2 | EMSURE | 1.02382.1000 | |
| choline chloride | Sigma-Aldrich | C1879-1KG | |
| Confocal Laser Scanning Microscope | Leica Microsystems | TCS SP5 | |
| CsCl | EMSURE | 1.02038.0100 | |
| Cs-gluconate | Self-prepared | Since there was no commercial Cs-gluconate, we prepared it by ourselves | |
| D-600 | Sigma-Aldrich | M5644-50MG | |
| D-APV | Biotrend | BN0085-100 | |
| Digital camera for microscope O | Olympus | XM10 | |
| EGTA | SERVA | 11290.02 | |
| Glucose | Sigma-Aldrich | 49159-1KG | |
| HEPES | ROTH | 9105.2 | |
| High Current Stimulus Isolator | World Precision Instruments | A385 | |
| KCl | EMSURE | 1.04936.1000 | |
| MgCl2 | EMSURE | 1.05833.0250 | |
| Micromanipulators | Luigs & Neumann | SM7 | |
| Miroscope | Olympus | BX51 | |
| mounting medium | ThermoFisher Scientific | P36930 | |
| NaCl | ROTH | 3957.1 | |
| NaH2PO4 | EMSURE | 1.06346.1000 | |
| NaHCO3 | EMSURE | 1.06329.1000 | |
| Pipette | Hilgenberg | 1807502 | |
| Puller | Sutter | P-1000 | |
| razor blade | Personna | 60-0138 | |
| Semiautomatic Vibratome | Leica Biosystems | VT1200S | |
| SR 95531 hydrobromide | Biotrend | AOB5680-10 | GABAA-receptor antagonist |

