Recording Neural Activity of Unfolded Hippocampal Tissue Using Penetrating Microelectrode Array
Abstract
Source: Zhang, M., et al. Neural Activity Propagation in an Unfolded Hippocampal Preparation with a Penetrating Micro-electrode Array. J. Vis. Exp. (2015).
This video demonstrates the procedure to set up a recording chamber with a penetrating microelectrode array (PMEA) to capture neural signals from an unfolded hippocampus. This method allows us to study the speed and direction of propagation of neural activity within the unfolded hippocampus in vitro.
Protocol
All procedures involving animal samples have been reviewed and approved by the appropriate animal ethical review.
1. Solutions for Surgery and Experimental Recording
- Prepare a normal artificial cerebrospinal fluid (aCSF) buffer containing (mM) NaCl (sodium chloride) 124, KCl (potassium chloride) 3.75, KH2PO4 (potassium dihydrogen phosphate) 1.25, MgSO4 (magnesium sulfate) 2, NaHCO3 (sodium bicarbonate) 26, Dextrose 10, and CaCl2 (calcium chloride) 2. Use this normal aCSF for tissue recovery after dissection and for the washing system at the beginning of the experiment.
- Prepare sucrose aCSF that is used during the hippocampus dissection and contains (mM): Sucrose 220, KCl 2.95, NaH2PO4 (sodium dihydrogen phosphate) 1.3, MgSO4 2, NaHCO3 26, Dextrose 10, and CaCl2 2. In order to induce epileptiform activity in the intact hippocampal tissue preparation, add 4-Aminopyridine (4-AP) to normal aCSF at the concentration of 100 µM.
2. Placing the Unfolded Hippocampus onto the penetrating microelectrode array (PMEA) to Record the Neural Activity
- To prepare the experimental setup, fill one bottle with normal aCSF and another bottle with 4-AP aCSF. In both bottles, bubble 95% O2/ 5% CO2 from the very beginning of each experiment. Use a tri-valve connector to control which solution will be selected during an experiment. Connect a vacuum tube at the outlet of the chamber to pump the solution into a dust container. Heat the pipeline before delivering it into the recording chamber and keep the solution at a controlled temperature level (35 °C).
- When the recording chamber's inlet and outlet are closed, use a custom-made glass pipette dropper to transfer and place the unfolded hippocampus into the chamber. Under the microscope, position the unfolded hippocampus using a regular small paint brush while the tissue is floating in the solution. Place the unfolded hippocampus with its alveus side facing down, the CA3 area pointing away, and the CA1 field pointing towards the researcher.
- Carefully suck away the solution in the chamber using a vacuum pipette from the edge of the recording chamber to lower the solution level until the chamber is dried and the tissue is lowered onto the array. Then, carefully place a custom-made tissue anchor (Figure 1) (No. 7 in Specific Materials and Equipment) on top of the tissue to hold the unfolded hippocampus onto the array. Put a few drops of solution into the recording chamber to refill it, and gradually open the inlet and outlet to adjust the flow rate to about two drops per second in the IV drip chamber.
- Incubate the tissue in the recording chamber with normal aCSF for about 1 min to recover, then switch the solution supply to 4-AP dissolved aCSF and adjust the flow rate properly. Incubate the tissue in 4-AP dissolved aCSF for about 5 to 10 min, and then the researcher could start the software to record the signal when spontaneous activity appears.
Representative Results
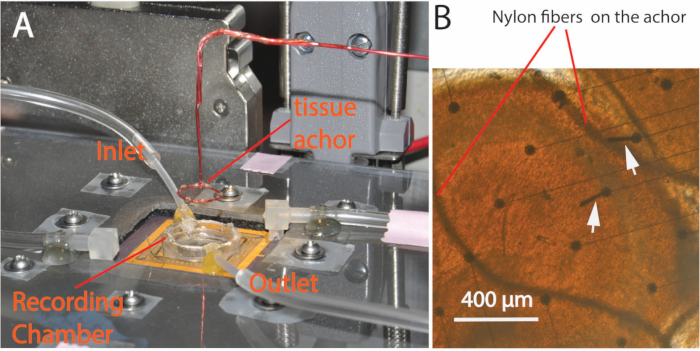
Figure 1. Experimental setup. (A) A plastic cover lid with screws is placed over the circuits to protect it against possible water damage. The recording chamber has both inlet and outlet tubes to carry the solution flow. A custom-made tissue anchor glued with a Nylon fiber mesh is used to secure the tissue during the experiments. (B) Picture taken from the bottom of the glass substrate of the PMEA showing the tissue anchor holding a sample slice during an experiment. The curved wires are the Nylon fibers from the mesh pressing on top of the tissue. The round dots are the bases of microelectrodes. Arrows point to electrodes that were damaged after several experiments.
Disclosures
The authors have nothing to disclose.
Materials
| small paint brush | Lowe's | tem #: 105657 Model #: 90219 | The one with the smallest size in a normal paint brush package |
| Fire polished glass help tool | N/A | N/A | This tool was fire polished and made from the regular Pasteur glass pipettes. |
| Custom made glass needle | N/A | N/A | This tool was fire polished and made from the regular Pasteur glass pipettes. |
| Custom made glass tool with a metal wire loop | N/A | N/A | This tool was fire polished and made from the regular Pasteur glass pipettes with a reshaped metal wire loop. |
| Custom made glass solution dropper | N/A | N/A | This tool was made from the regular Pasteur glass pipettes with its tips cut and a rubber head attached with the cut end. |
| Custom made tissue anchor | N/A | N/A | Nylon fiber mesh was glued on a insulated copper wire ring. The tissue anchor was hold by an micromanipulator. |
| Custom fabricated microelectrode array | N/A | N/A | More detail about the array please refer to Kibler, et al, 2011. |
| Custom made filter and amplifiers circuits for the array | N/A | N/A | More detail about the array please refer to Kibler, et al, 2011. |
| Data acquisition processor 3400a | Microstar Laboratories | N/A | This is a complete data acquisition system with A/D converter. |

