Applying Noise Currents to Medial Vestibular Nucleus Neurons Using Whole-Cell Patch-Clamp Technique
Abstract
Source: Stefani, S. P., et al. Stochastic Noise Application for the Assessment of Medial Vestibular Nucleus Neuron Sensitivity In Vitro. J. Vis. Exp. (2019).
This video demonstrates a protocol involving the application of subthreshold noise currents to medial vestibular nucleus neurons using the whole-cell patch-clamp technique to study the impact of the noise currents on neuronal sensitivity to electrical stimuli.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
1. Animals
NOTE: Mice were obtained from the Australian Rodent Centre (ARC; Perth, Australia) and held at the Medical Foundation Building Animal Facility at the University of Sydney.
- Maintain the mice on a standard 12 h light/dark cycle with environmental enrichment.
- Use male and female C57BL/6 mice (3–5 weeks old) for all experiments.
2. Preparation of Solutions
- Prepare 1 L of artificial cerebrospinal fluid (ACSF) composed of 29 mM NaHCO3, 11 mM glucose, 120 mM NaCl, 3.3 mM KCl, 1.4 mM NaH2PO4, 2.2 mM MgCl2, 2.77 mM CaCl2.
- Prepare 200 mL of sucrose-ACSF (sACSF) containing 29 mM NaHCO3, 11 mM glucose, 241.5 mM sucrose, 3.3 mM KCl, 1.4 mM NaH2PO4, 2.2 mM MgCl2, 2.77 mM CaCl2. Prior to adding CaCl2 to the ACSF and sACSF, gas the solutions with carbogen (95 % O2 and 5 % CO2) to establish a pH of 7.4 and avoid calcium precipitation (cloudiness).
- Prepare K+-based intracellular solution composed of 70 mM potassium gluconate, 70 mM KCl, 2 mM NaCl, 10 mM HEPES, 4 mM EGTA, 4 mM Mg2-ATP, 0.3 mM Na3-GTP, with a final pH of 7.3 (adjusted using KOH).
NOTE: It is recommended that intracellular solutions be filtered with 0.22 μm filters and stored in 0.5 mL aliquots at -20 °C.
3. Preparation of the Brainstem
- Before brainstem extraction, equilibrate the sACSF with carbogen and cool at -80 °C for 25 min to form an ice slurry.
- Anaesthetize the mouse with isoflurane (3–5 %) saturated in oxygen (3 mL/min). Once the hind paw reflexes are absent, decapitate the mouse with sharp stainless-steel scissors.
- Expose the skull by making a sagittal incision in the skin using a razor blade (#22 rounded).
- Using the pointed end of a pair of standard pattern scissors, make a small incision at the lambda and cut along the longitudinal fissure.
- Carefully reflect away the paired parietal and occipital bones using a pair of shallow-bend Pearson rongeurs.
NOTE: The brain is continuously bathed in situ using the previously prepared ice-cold sACSF slurry during this procedure. - Isolate the brainstem from the forebrain and its bony encasing using a razor blade (#11 straight) to cut down the parieto-occipital sulcus and at the caudal medulla.
- Mount the isolated brainstem ventral end on a previously cut trapezoidal polystyrene block. Use a wick of tissue paper to remove excess fluid around the dissected tissue and ensure good tissue adhesion to the cutting stage.
NOTE: The polystyrene block is cut in a trapezoidal shape to ensure the rostral end of the midbrain fits and tapers into the spinal cord. - Use cyanoacrylate glue to fix the polystyrene block with the attached brainstem rostral end down to the cutting stage.
- Using an advance speed of 0.16 mm/s and vibration amplitude of 3.00 mm, prepare 200 μm transverse slices of the MVN.
NOTE: The location of the MVN is determined using the Paxinos and Franklin mouse brain atlas. The MVN (listed as MVe in the atlas) lies immediately ventrolateral to the 4th ventricle and is largest right before the attachment of the cerebellum (between the inferior colliculi and the obex). - Use a plastic-trimmed pipette to transfer slices onto a filter paper disc sitting in carbogenated ACSF at 25 °C for at least 30 min before recording.
4. Instruments
- Use a standard electrophysiological setup to perform the whole-cell patch clamp technique.
- Prepare micropipettes using a two-step protocol (heat step 1: 70; heat step 2: 45) on a micropipette puller (see the Table of Materials).
- When placed in the bath, micropipettes should have a final resistance ranging from 3 to 5 MΩ with an internal solution.
NOTE: Settings used may vary depending on the temperature within the room and can change quite frequently.
5. Whole-cell Patch Clamp Electrophysiology
- To obtain whole-cell patch clamp recordings from individual neurons in the MVN, a K+-based internal solution is used within the recording pipette.
- Transfer a single tissue slice from the incubation chamber to the recording chamber and secure it using nylon thread on a U-shaped weight. Continuously perfuse the recording chamber with carbogenated-ACSF at 25 °C at a 3 mL/min flow rate.
- After filling a micropipette with internal solution, locate the MVN using a low-power (10x) objective lens. Using a high-power (40x) objective, individual neurons within the MVN can be located.
NOTE: Cell quality is essential in ensuring quality recordings and durability of the cell when attempting to achieve the whole-cell configuration. A suitable cell will demonstrate a spherical shape, a reflective cell membrane, and an invisible nucleus. An unsuitable cell will have a large visible nucleus (egg-like) and a swollen/shrunken appearance. - Before breaching the tissue with the pipette, apply a small amount of positive pressure to push debris away from the pipette tip.
- Move the pipette using the micromanipulator towards the chosen neuron, and a small dimple should form on the neuronal membrane.
Release positive pressure and apply a small amount of negative pressure. - Once a 1 GΩ seal is achieved, apply gentle, short, and sharp negative pressure to the pipette holder through the suction port to rupture the membrane and create a whole-cell configuration.
- Make whole-cell current clamp recordings using standard techniques.
6. Applying Sinusoidal and Stochastic Noise to Individual Medial Vestibular Nucleus Neurons
- Apply the stochastic and sinusoidal noise at a range of amplitudes from 3 to 24 pA to determine neuronal threshold and firing rate.
- Determine the sensory threshold by grouping lower and higher stimulus intensities and perform an ANOVA to observe any differences (as shown in Figure 1).
- Calculate the average firing rate over the 10 s period where the depolarizing current step was/will be injected for each individual current level (i.e., 7 total episodes; Figure 2).
- Use the average firing rate values to generate a firing rate versus the current plot and perform a linear regression analysis to determine the gradient of the line of best fit. The gradient of the best-fit line indicates neuronal gain.
Representative Results
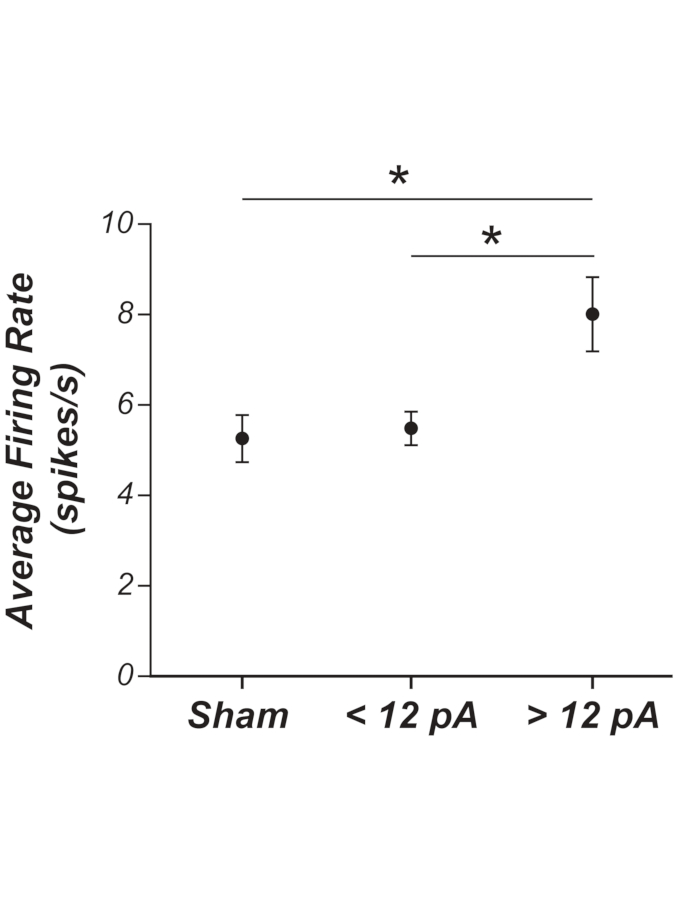
Figure 1: Objective determination of 12 pA threshold. Firing rates for less than 12 pA (3 and 6 pA) and more than 12 pA (18 and 24 pA) were pooled and averaged. These averages were then analyzed using an ANOVA and statistical significance between sham and >12 pA and between <12 pA and >12 pA. *p < 0.05.
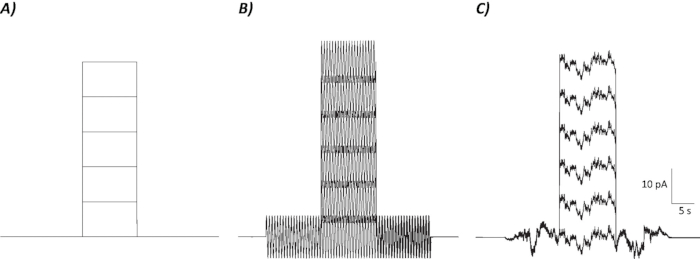
Figure 2: Diagrammatic profiles of control, sinusoidal, and stochastic noise protocols.(A) Control (no noise) protocols applied to MVN neurons. (B) Sinusoidal noise protocol with a frequency of 2 Hz. (C) Stochastic noise protocols where majority of the power spectrum is ≤2 Hz. Each protocol presented here has an amplitude of ±6 pA with a 10 s depolarizing current increasing by 10 pA up to 50 pA. The true stimulus does not have a depolarizing current step and is therefore the first episode of these protocols to determine neuronal gain changes.
Disclosures
The authors have nothing to disclose.
Materials
| CaCl2 | Scharlau | CA01951000 | Used for ACSF and sACSF |
| D-(+)-Glucose | Sigma | G8270 | Used for ACSF and sACSF |
| EGTA | Sigma | E0396-25G | Used for K-based intracellular solution |
| HEPES | Sigma | H3375-25G | Used for K-based intracellular solution |
| KCl | Chem-supply | PA054-500G | Used for ACSF, sACSF and intracellular solution |
| K-gluconate | Sigma | P1847-100G | Used for K-based intracellular solution |
| Mg-ATP | Sigma | A9187-500MG | Used for K-based intracellular solution |
| MgCl | Chem-supply | MA00360500 | Used for ACSF and sACSF |
| Na3-GTP | Sigma | G8877-100MG | Used for K-based intracellular solution |
| NaCl | Chem-supply | SO02270500 | Use for ACSF and intracellular solution |
| NaH2PO4.2H2O | Ajax | AJA471-500G | Used for ACSF and sACSF |
| NaHCO3 | Sigma | S5761-1KG | Used for ACSF and sACSF |
| Sucrose | Chem-supply | SA030-500G | Used for sACSF |
| Isoflurane | Henry Schein | 1169567762 | Used for anaesthetising mice |
| EQUIPMENT | |||
| Borosilicate glass capillaries | Warner instruments | GC150T-7.5 | 1.5mm OD, 1.16mm ID, 7.5cm length |
| Data acquisition software | Axograph | Used for electrophysiology and analysis | |
| Friedmen-Pearson Rongeurs | World precision instruments | 14089 | Used for dissection |
| Micropipette puller | Narishige | PP-830 | Used for micropipette |
| Multiclamp amplifier | Axon instruments | 700B | Used for electrophysiology |
| pH meter | Sper scientific | 860033 | Used for internal solution |
| Standard pattern scissors | FST | 14028-10 | Used for dissection |
| Sutter micromanipulator | Sutter | MP-225/M | Used for electrophysiology |
| Upright microscope | Olympus | BX51WI | Used for electrophysiology |
| Vibratome | Leica | VT1200 | Used for slicing brain tissue |

