Preparation of Mechanically Stable Self-Assembled Peptides Hydrogels
Summary
This protocol presents three fast and simple preparation methods that use environmental conditions to trigger the self-assembly of peptides into hydrogels. Additionally, the characterization of peptide hydrogels is described, demonstrating that mechanically stable peptide hydrogels can be formed under these straightforward conditions.
Abstract
Peptide hydrogels are highly hydrophilic, three-dimensional network gels formed by the self-assembly of nanofibers or polymers, creating water-locking networks. Their morphology closely resembles that of the extracellular matrix, allowing them to exhibit both the biological functions of peptides and responsive gelation properties. These unique characteristics have led to their extensive application in tissue engineering, three-dimensional cell culture, cancer therapy, regenerative medicine, and other biomedical fields. This article describes three methods for preparing ECF-5 peptide hydrogels using self-assembling peptides with environmentally responsive gelation processes: (1) pH-responsive gelation: varying pH levels induce the protonation or deprotonation of amino acid residues, altering electrostatic interactions between peptide molecules and promoting their self-assembly into hydrogels; (2) Metal ion addition: polyvalent metal ions chelate with negatively charged amino acid residues, acting as bridges between peptides to form a network hydrogel; (3) Solvent exchange: hydrophobic peptides are initially dissolved in non-polar organic solvents and subsequently induce self-assembly into hydrogels upon transitioning to a polar aqueous environment. These methods utilize conventional experimental procedures to facilitate peptide self-assembly into hydrogels. By designing peptide sequences to align with specific gelation-inducing conditions, it is possible to achieve finely tuned micro/nanostructures and biological functions, highlighting the significant potential of peptide hydrogels in the biomedical domain.
Introduction
Through the design of peptide sequences, non-covalent interactions between peptides induce self-assembly, leading to the formation of ordered micro- and nanometer structures, including nanotubes, nanoribbons, nanofibers, and spherical structures1. When self-assembled into micro- and nanometer fibers/ribbons, these structures macroscopically exhibit hydrogel properties. Peptide self-assembling hydrogels differ from polymer hydrogels in that they self-assemble through non-covalent interactions, their gel form is reversible, and they readily respond to specific conditions to transition between solution and gel phases2. For instance, aromatic amino acid peptides can be induced to gelatinize based on solvent switching3,4,5, RADA16 peptides form gels through cationic and anionic electrostatic interactions6, and E1Y9 peptide is induced to form a hydrogel via Ca2+ ions7. Natural amino acids can be metabolized by the human body and offer excellent biocompatibility, a feature that polymer hydrogels cannot achieve8. Proteins are the molecules that execute biological functions, and differences in peptide sequences create their specific biological functions. Therefore, embedding specific biofunctional peptide sequences and endowing them with self-assembling properties can design peptide self-assembling hydrogels with unique biological functions and morphologies9,10,11. This article introduces three methods for preparing peptide hydrogels, where the gelation process is triggered by environmental responsiveness. It also briefly discusses methods for characterizing the mechanical properties and morphology of peptide hydrogels.
The pH regulates the charge of amino acids, triggering the gelation of some peptides. For instance, positively charged amino acids (e.g., arginine, lysine, histidine) are regulated by pH to attain positive or neutral states. Negatively charged amino acids are regulated by pH to achieve negative or neutral states, moving away from their isoelectric point and thereby altering their hydrophilicity in aqueous solutions. Therefore, controlling electrostatic and hydrophobic interactions between peptides facilitates their ordered self-assembly. Zhang et al. designed an amphiphilic pH-responsive self-assembling peptide, methotrexate-coupled KKFKFEFEF, which responds to slightly acidic environments both in vitro and in vivo, enabling a sol-to-gel phase transition. This leads to efficient cellular uptake and endocytosis, thereby delivering anti-cancer drugs and improving chemotherapy effectiveness12. Shen et al.13 designed the FF8 (KRRFFRRK) peptide, which easily self-assembles into fibers at a pH greater than 9.4. Under neutral conditions, microorganisms neutralize their positive charges due to electrostatic interactions with their negatively charged phospholipid membranes, coordinating with phospholipid molecules to self-assemble, causing membrane rupture and enhancing bactericidal effects13.
Triggering peptide supramolecular self-assembly into hydrogels using coordination metals is a relatively rare method14. When metal ions interact electrostatically with peptides, they form salt bridges that connect peptide molecules, leading to non-covalent interactions and self-assembly, which results in gelation properties. For example, Abul-Haija et al.15 designed the tripeptide FFD, which transitions from a liquid to a hydrogel upon the addition of copper ions. Tao et al.16 developed the glutamic acid and phenylalanine-rich peptide E3F3, which self-assembles into fibrous hydrogels in the presence of zinc ions, and is used for prostate drug delivery.
Solvent exchange formation of peptide hydrogels is the most common supramolecular self-assembly triggering condition. After hydrophobic peptides dissolve in organic solvents, their hydrophobic groups are fully exposed. When transferred to an aqueous phase, the hydrophobic groups approach each other, and water molecules facilitate the formation of peptide hydrogen bonds, leading to rapid self-assembly and easy formation of hydrogels. For instance, Zhang et al.17 designed a peptide that could dissolve stably at high concentrations in polar organic solvents and, upon dilution with water, self-assembled into β-sheet structures to form peptide fiber hydrogels. Shen et al.13 designed a reductive peptide ECF-5 (ECAFF), pre-dissolved in dimethyl sulfoxide (DMSO) and then injected into an aqueous phase to form a reductive hydrogel, used for the targeted removal of reactive oxygen species produced by ischemia-reperfusion, which subsequently degraded into a solution after scavenging.
This study selected three simple, rapid, and highly generalizable peptide hydrogel preparation strategies based on previous experiences: (1) pH response method: peptides are dissolved in a solution with a pH far from their isoelectric point, and then the pH is adjusted to near the isoelectric point. This change allows certain self-assembling peptides to form fibers and create peptide hydrogels; (2) Metal ion addition method: coordination cations are added to water-soluble, negatively charged self-assembling peptides. The metal coordination chelation between peptides leads to their self-assembly into hydrogels; (3) Solvent exchange method: high-concentration peptides are dissolved in organic solvents and then diluted into an aqueous phase, inducing gelation behavior.
Protocol
The details of the plasmids, reagents, and equipment used in this study are listed in the Table of Materials.
1. pH response method
- Add 5 mg of ECF-5 peptides to 400 µL of deionized water. Sonicate at 40 kHz for 30 min and mix thoroughly.
- Add 40 µL of sodium hydroxide (1 M, filtered through a 0.22 µm filter) to the peptide solution. Vortex and mix thoroughly. Continue sonication for 15 min until the solution is completely clarified.
- Add 60 µL of hydrochloric acid. Quickly vortex and ensure thorough mixing. Allow the mixture to stand at room temperature for over 30 min to facilitate hydrogel formation.
NOTE: Replace deionized water with the desired buffer solution if needed. If solid particles are large, pulverize them in advance. Sonicate to achieve a homogeneous particle mixture in the liquid. If the peptide is intolerant to alkaline conditions, add 1 M hydrochloric acid solution first and repeat the above steps. Adjust the amounts of acid and base as necessary to reach near the isoelectric point or desired pH, ensuring the gel-forming properties are retained.
2. Metal ion addition method
- Preparation of the material
- Prepare 0.15 M of Tris and 0.1 M of NaCl buffer at pH 7.4. Filter through a 0.22 µm filter and store at 4 °C for up to one week.
- Prepare an ECF-5 self-assembling peptide solution at a concentration of 10 mg/mL using the buffer prepared in step 1.1.1. Dissolve by sonication and store at 4 °C.
- Prepare a 50 mg/mL calcium chloride solution using deionized water. Store at 4 °C.
- Metal ion addition induced peptide hydrogel formation
- Add 40 µL of calcium chloride solution to 460 µL of the ECF-5 peptide solution. Vortex and mix thoroughly. Allow the mixture to stand at room temperature for over 2 h.
NOTE: Avoid using phosphate buffer, as it may lead to calcium ion precipitation.
- Add 40 µL of calcium chloride solution to 460 µL of the ECF-5 peptide solution. Vortex and mix thoroughly. Allow the mixture to stand at room temperature for over 2 h.
3. Solvent exchange method
- Preparation of materials
- Weigh 10 mg of ECF-5 peptide lyophilized powder. Add it to 100 µL of DMSO. Mix thoroughly using ultrasonication and store at 4 °C.
- Prepare a 10 mM of PBS buffer solution. Filter and sterilize. Store at 4 °C and use within 1 week.
- Solvent-induced peptide hydrogel formation
- Rapidly introduce 1 mL of PBS into the DMSO-solubilized peptide. Vortex and mix thoroughly. Let it stand at room temperature for 5 min.
- Add 500 µL of PBS, let stand for 15 min, discard the supernatant, and retain the bottom gel. Repeat this process three times to remove DMSO.
NOTE: If DMSO affects subsequent experiments, consider dissolving the peptide in a higher concentration of DMSO to minimize its impact.
4. Rheomechanical characterization of hydrogel
- Rheological analysis
- Spoon the hydrogel onto a 25 mm aluminum parallel plate. Perform dynamic time sweep rheological analysis of the peptide hydrogels using a rheometer18.
- Frequency and strain sweep analyses
- Conduct frequency and strain sweep analyses18 systematically using a controlled strain of 0.3% and a frequency of 10 rad/s. Ensure temperature control is set to 25 °C.
5. Atomic force microscopy (AFM) characterization of fiber morphology
- Preparation of materials
- Prepare a solution of 95% ethanol and add 5% APTES. Store at -20 °C and use within 1 month.
- Apply double-sided adhesive to remove a layer of mica. Adhere the mica surfaces using the APTES solution and let it stand for 5 min. Rinse thoroughly with pure water before use.
- Dilute and thoroughly mix the hydrogel. Drop it onto the modified mica surface and allow static adsorption for 5 min. Rinse the surface with pure water and dry it before use.
- Detection method
- Utilize a multimode AFM equipped with a scanner to examine peptide fiber or hydrogel morphologies. Use silicon cantilevers with a nominal spring constant of 48 N/m.
- Initialize the instrument to position the needle. Select the tapping mode to scan the sample to acquire images.
Representative Results
The three methods described in this article for preparing peptide hydrogels enable rapid, affordable, and straightforward production. The function of the hydrogel is related to its peptide sequence. Here, the ECF-5 peptide is used as a representative example to demonstrate its physical characteristics, including microscopic morphology and mechanical properties.
As shown in Figure 1A and Supplementary Figure 1, the ECF-5 peptide contains a glutathione-like sequence and a phenylalanyl dipeptide sequence, which endow it with the reducing properties of glutathione and the ability to self-assemble. The ECF-5 peptide was pre-dissolved in DMSO and then rapidly mixed with an aqueous solution. At this stage, the peptide underwent rapid self-assembly, forming a solid gel within minutes. When the final peptide concentration exceeds 1%, the gel can be picked up with tweezers.
Atomic force microscopy (AFM) imaging of the gel surface revealed a network of numerous peptide fibers with dense pores that trap water. Upon dilution, the morphology of individual fibers, as shown in Figure 1D, displays a height of approximately 3.5 nm, forming highly consistent wider fiber bands. This is consistent with the fiber morphology observed in the gel network. Additionally, under neutral conditions, calcium metal ions can also induce the peptide to form a hydrogel, although this process is relatively slower and results in a softer gel.
The macroscopic behavior of hydrogels prepared using the three methods – pH response, metal addition, and solvent exchange – is illustrated in Figure 2A. Compared to deionized water, all hydrogels exhibited semi-solid properties. Rheology is commonly used to differentiate hydrogels from sols.
The macroscopic viscoelasticity of ECF-5 hydrogels obtained through these three gelation methods was compared using rheological techniques. As shown in Figure 2, the ECF-5 hydrogel exhibited the viscoelasticity characteristic of typical hydrogels. At a strain of 0.3%, the relationship between the storage modulus (G') and loss modulus (G'') at different frequencies is depicted. The ECF-5 hydrogel showed a weak correlation with frequency. The storage modulus (G') of hydrogels obtained by the three methods was approximately 10 kPa, which is about 10 times larger than the loss modulus (G'') and demonstrated typical semi-solid hydrogel properties (Figure 2B). This indicates that ECF-5 hydrogels, irrespective of the preparation method, exhibit typical hydrogel viscoelasticity.
Plots of storage modulus (G') and loss modulus (G'') versus strain level at 10 rad/s (Figure 2C) showed that both G' and G'' remained nearly constant over the strain range of 0.1% to 10%, with G' being about 10 times higher than G''. This suggests that the ECF-5 hydrogels are typical viscoelastic hydrogels capable of withstanding stress.
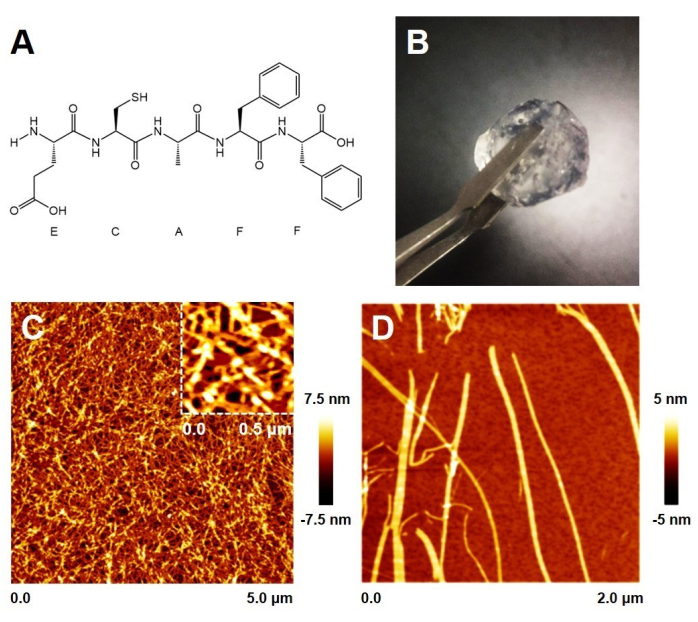
Figure 1: ECF-5 peptide hydrogels. (A) Chemical structure of ECF-5. (B) Macroscopic morphology of ECF-5 peptide hydrogel at 1% concentration. (C) Microstructure of the hydrogel surface. (D) Microscopic morphology of ECF-5 peptide fibers. Please click here to view a larger version of this figure.
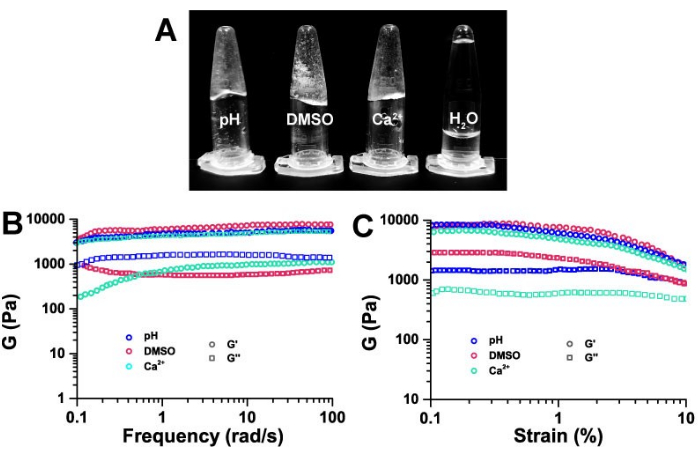
Figure 2: Macroscopic morphology and rheological characterization of hydrogels. (A) Macroscopic morphology of hydrogels under three different induced hydrogel conditions, all with a final concentration of ECF-5 peptide of 3 mg/mL. Frequency (B) and strain (B) dependence of the storage modulus (G') and loss modulus (G'') of ECF-5 hydrogel under three induction conditions at 1% concentration. Please click here to view a larger version of this figure.
Supplementary Figure 1: LC-MS analysis of the peptide ECAFF. (A) Peak at the retention time of 13.238 min is the product peak. The column eluate was monitored at 220 nm and a flow rate of 1 mL/min with 0.1% trifluoroacetic acid in 100% Acetonitrile. ECF-5 yield is 97.33%. (B) ESI/Mass spectrometry confirms the molecular weight of ECF-5 is 616.1. Please click here to download this File.
Discussion
In the past few decades, following the discovery of self-assembling peptide sequences derived from amyloid proteins, numerous self-assembling peptides have been designed based on their properties, demonstrating significant potential for applications in biomedicine and materials science19. Peptide hydrogels have exhibited unique bio-functionalization capabilities in tissue culture, drug delivery, and tumor treatment20.
This article describes simple and rapid preparation methods for three types of peptide hydrogels: pH response, metal ion addition, and solvent exchange. Self-assembling peptides generate different non-covalent interactions based on their sequence characteristics, with hydrophobic interactions being the predominant force, followed by electrostatic interactions. These interactions result in varying self-assembly properties and promote the transition from a soluble to an insoluble state. For example, both amino acids and peptides have specific isoelectric points at which their solubility is at a minimum. When the pH deviates significantly from their isoelectric point, their solubility increases, enabling self-assembly. Thus, adjusting the solution pH can trigger the self-assembly of certain peptides13. However, peptides with highly polar isoelectric points may exhibit unstable structures and are prone to deamidation or hydrolysis.
In the formation of peptide hydrogels induced by metal ions, peptides typically contain negatively charged amino acids such as glutamic acid and aspartic acid. These peptides can connect using metal ions as bridges under the influence of divalent metal ions, thereby triggering self-assembly. Alternatively, peptides with histidine residues can form coordination bonds with certain divalent metal cations, inducing self-assembly. Metal ions facilitate peptide self-assembly by reducing electrostatic repulsion between peptides and enhancing their mutual attraction, leading to the formation of peptide hydrogels14. While this method is suitable for applications that require specific metal ions, it may not be necessary under most physiological conditions, and the metal ions cannot be easily removed.
Some highly hydrophobic peptides are first dissolved in water-soluble organic solvents and then added to an originally insoluble aqueous phase to rapidly form peptide hydrogels. However, many organic solvents can have detrimental effects on biological applications, especially at higher concentrations. The methods described herein are applied specifically to self-assembled peptides. Peptides are designed based on the aforementioned triggering mechanisms to achieve desired biological functions while minimizing adverse effects.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 11674344 and 22201026) and the Key Research Program of Frontier Sciences, CAS (Grant NO. QYZDJ-SSW-SLH019).
Materials
| 3-Aminopropyl)triethoxysilane | Aladdin | A107147 | / |
| Atomic Force Microscopy | Bruker | Multimode Nanoscope VIII | / |
| CaCl2 | Aladdin | C290953 | / |
| Diphenylalanine (FF) | Chinesepeptide | customizable | Purity > 95% |
| DMSO | Sigma-aldrich | 34869 | / |
| ECF-5 Peptides | Chinesepeptide | sequence: ECAFF | Purity > 95% |
| Hydrochloric Acid | Aladdin | H399657 | / |
| Mica | Sigma-aldrich | AFM-71856-02 | / |
| Phosphate Buffered Saline | Aladdin | P492453 | / |
| Rheometer | Anton Paar GmbH | MCR302 | / |
| Silicon Cantilevers | MikroMasch | XSC11 | / |
| Sodium Chloride | Aladdin | C111549 | / |
| Sodium Hydroxide | Aladdin | S140903 | / |
| TRIS Hydrochloride | Aladdin | T431531 | / |
Referencias
- Whitesides, G. M., Mathias, J. P., Seto, C. T. Molecular self-assembly and nanochemistry: a chemical strategy for the synthesis of nanostructures. Science. 254 (5036), 1312-1319 (1991).
- Matson, J. B., Zha, R. H., Stupp, S. I. Peptide self-assembly for crafting functional biological materials. Curr Opin Solid State Mater Sci. 15 (6), 225-235 (2011).
- Itzha, G., et al. Peptide self-assembly as a strategy for facile immobilization of redox enzymes on carbon electrodes. Carbon Energy. 5 (11), e411 (2023).
- Rosa, E., et al. Incorporation of PEG diacrylates (PEGDA) generates hybrid Fmoc-FF hydrogel matrices. Gels. 8 (12), 831 (2022).
- Balasco, N., et al. Self-assembled materials based on fully aromatic peptides: The impact of tryptophan, tyrosine, and dopa residues. Langmuir. 40 (2), 1470-1486 (2024).
- Bolan, F., et al. Intracerebral administration of a novel self-assembling peptide hydrogel is safe and supports cell proliferation in experimental intracerebral haemorrhage. Transl Stroke Res. , (2023).
- Tsutsumi, H., et al. Osteoblastic differentiation on hydrogels fabricated from Ca2+ responsive self-assembling peptides functionalized with bioactive peptides. Bioorg Med Chem. 26 (12), 3126-3132 (2018).
- Li, S., et al. Self-assembled peptide hydrogels in regenerative medicine. Gels. 9 (8), 653 (2023).
- Guan, T., Li, J., Chen, C., Liu, Y. Self-assembling peptide-based hydrogels for wound tissue repair. Adv Sci. 9 (10), e2104165 (2022).
- La, M. S., Di, N. C., Onesto, V., Marasco, D. Self-assembling peptides: From design to biomedical applications. Int J Mol Sci. 22 (23), 12662 (2021).
- Gao, Y., et al. Advances in self-assembled peptides as drug carriers. Pharmaceutics. 15 (2), 482 (2023).
- Zhang, J., et al. Injectable and pH-responsive self-assembled peptide hydrogel for promoted tumor cell uptake and enhanced cancer chemotherapy. Biomater Sci. 10 (3), 854-862 (2022).
- Shen, Z., et al. Biomembrane induced in situ self-assembly of peptide with enhanced antimicrobial activity. Biomater Sci. 8 (7), 2031-2039 (2020).
- Shao, T., Falcone, N., Kraatz, H. B. Supramolecular peptide gels: Influencing properties by metal ion coordination and their wide-ranging applications. ACS Omega. 5 (3), 1312-1317 (2020).
- Abul-Haija, Y. M., et al. Cooperative, ion-sensitive co-assembly of tripeptide hydrogels. Chem Commun. 53 (69), 9562-9565 (2017).
- Tao, M., et al. Zinc-ion-mediated self-assembly of forky peptides for prostate cancer-specific drug delivery. Chem Commun. 54 (37), 4673-4676 (2018).
- Apostolopoulos, V., et al. A Global review on short peptides: Frontiers and perspectives. Molecules. 26 (2), 430 (2021).
- Zhang, R. S. T., et al. Rheological characterization and mechanical properties of self-assembled peptide hydrogels. Soft Matter. 15 (11), 2370-2380 (2019).
- Li, T., et al. Peptide-based nanomaterials: Self-assembly, properties and applications. Bioact Mater. 11, 268-282 (2021).
- Sedighi, M., et al. Multifunctional self-assembled peptide hydrogels for biomedical applications. Polymers (Basel). 15 (5), 1160 (2023).

.
