X-Ray Visualization of Intraductal Ethanol-Based Ablative Treatment for Prevention of Breast Cancer in Rat Models
Özet
A procedure for the delivery of a chemical ablative solution to the rat mammary ductal tree for image-guided preventive treatment of breast cancer is described. Mammary epithelial cells can be targeted with minimal collateral tissue damage through cannulation directly into the nipple opening and intraductal infusion of a 70% ethanol-based ablative solution.
Abstract
There are still a limited number of primary interventions for prevention of breast cancer. For women at a high risk of developing breast cancer, the most effective intervention is prophylactic mastectomy. This is a drastic surgical procedure in which the mammary epithelial cells that can give rise to breast cancer are completely removed along with the surrounding tissue. The goal of this protocol is to demonstrate the feasibility of a minimally invasive intraductal procedure that could become a new primary intervention for breast cancer prevention. This local procedure would preferentially ablate mammary epithelial cells before they can become malignant. Intraductal methods to deliver solutions directly to these epithelial cells in rodent models of breast cancer have been developed at Michigan State University and elsewhere. The rat mammary gland consists of a single ductal tree that has a simpler and more linear architecture compared to the human breast. However, chemically induced rat models of breast cancer offer valuable tools for proof-of-concept studies of new preventive interventions and scalability from mouse models to humans. Here, a procedure for intraductal delivery of an ethanol-based ablative solution containing tantalum oxide nanoparticles as X-ray contrast agent and ethyl cellulose as gelling agent into the rat mammary ductal tree is described. Delivery of aqueous reagents (e.g., cytotoxic compounds, siRNAs, AdCre) by intraductal injection has been described previously in mouse and rat models. This protocol description emphasizes methodological changes and steps that pertain uniquely to delivering an ablative solution, formulation consideration to minimize local and systemic side effects of the ablative solution, and X-ray imaging for in vivo assessment of ductal tree filling. Fluoroscopy and micro-CT techniques enable to determine the success of ablative solution delivery and the extent of ductal tree filling thanks to compatibility with the tantalum-containing contrast agent.
Introduction
For women in the USA1, breast cancer (BC) continues to be the most diagnosed cancer type and causes more deaths than any other cancer type except lung cancer. Projections for 2022 estimate that 51,400 women will be diagnosed with carcinoma in situ and 287,850 women will be diagnosed with invasive carcinoma, and that 43,600 women will die from BC1. Despite the prevalence and mortality associated with BC, there are few options available for primary prevention and translational research on novel interventions as primary prevention is not prioritized by federal agencies2. Prophylactic mastectomy is the most effective intervention for primary prevention. However, this procedure is only recommended for high-risk individuals because it is a major surgery with life-changing consequences3. This surgery involves complete removal of the mammary epithelial cells from which carcinogenesis develops as well as the normal surrounding tissue. Individuals are often dissuaded from using this procedure as their first option of primary intervention due to the negative impact of physical, psychological, and social stress. For these reasons, even some high-risk individuals opt to not undergo this procedure and choose instead watchful waiting or similar surveillance strategies3. In previous publication, delivery of 70% ethanol (EtOH) directly into the ductal tree of mouse models was effective at chemically ablating mammary epithelial cells with limited damage to adjacent normal tissue and at preventing breast tumor formation4. EtOH is used in multiple clinical applications as either an ablative agent for local treatment of some cancers or sclerosing agent for local treatment of arteriovenous swelling and malformations5,6,7,8,9,10,11,12,13,14. The low toxicity and safety profile of EtOH is well established, as in some procedures up to 50 mL of 95% EtOH can be administered per session5,10.
Complete removal of mammary epithelial cells from which BC develops is the most crucial component of both prophylactic mastectomy and local delivery of an ablative solution.Therefore, confirmation of complete ductal tree filling is necessary to guarantee that the ablative solution has come in direct contact with all the mammary epithelial cells. Delivering a solution within the ductal tree(s) and its visualization by image-guided fluoroscopy or ductography are possible through clinical procedures that already exist15,16,17. Thus, it will be feasible to readily implement and evaluate this procedure in clinical trials. A key step in establishing the efficacy and translational feasibility of intraductal (ID) ablation as a new intervention for primary prevention will be to demonstrate the feasibility of this X-ray visualization approach in animal models of increasing size and complexity of their ductal tree architecture4,18,19. A protocol that scales up this ablative procedure from mouse20 to rat models is described here. While mouse and rat ductal trees have a similar linear structure and branching pattern, the rat ductal tree is proportionally larger and is surrounded by a much denser stroma. We have implemented a method in the laboratory to successfully inject every mammary gland in a rat over a series of weekly sessions with an ablative solution containing a contrast agent. Session spacing is necessary to ensure the animals have minimal side effects of EtOH (Figure 1 and Figure 2). The procedure involves injection of the ablative solution directly into the nipple opening of an isoflurane-anesthetized rat with a 33 G needle. Some key improvements of the procedure include the use of extended anti-inflammatory treatment, injection of higher volumes per ductal tree than suggested21, and gastight syringes for liquid and gases. The duration of treatment with 5 mg/kg of carprofen (an NSAID) from 48 h before to 1 week after ID injections is comparable to the anti-inflammatory protocol used for the sclerosing therapy of venous malformation in the clinic. The treatment is performed on patients under systemic anesthesia followed by 2 days of anti-inflammatory medications such as NSAIDs. The anti-inflammatory treatment may be extended for a few more days to reduce local inflammation and any potential pain13. As in mice20, intraperitoneal injection of a 5% sucrose solution mitigates the short-term effect of alcohol intoxication in rats. Rats can be injected with up to 1 mL of 70% EtOH (up to 4 ducts; 0.2 g/dL of EtOH content in blood) in a single session when administered with this sucrose solution; animals fully recover within 4 h after ID injections. We perform sequential sessions to allow enough recovery time when injecting more than 4 glands and/or higher EtOH concentrations. Alcohol intoxication in women will be much less likely as ID injection of all ductal trees in both breasts, assuming 16 main ducts16,17and 2 mL per duct22,23, with 70% EtOH would result in less than 0.1 g/dL of EtOH content in blood and may cause mild impairment.
X-ray imaging enables the determination of how successful intraductal delivery is in each individual gland and whether the entire ductal tree is filled (Figure 1, Figure 2, Figure 3). Real-time fluoroscopy imaging in preparation for micro-CT scan and/or 3D reconstruction of DICOM file data can be used to assess the extent of solution delivery into the ductal tree and any leakage into the stroma. Use of fluoroscopy can help to limit the overall radiation dose imposed on the animal. The fluoroscopy technique approximates more closely to the intended clinical application for image-guidance of this ablative treatment. Comparison of FDA-approved iodine-containing Isovue to tantalum oxide (TaOx) nanoparticles has been performed in order to further refine the utility of the ablative solution4,19. It has been found that TaOx is a superior micro-CT contrast agent than Isovue for visualization of the initial filling of the ductal tree in mice4,19. Here, we demonstrate that TaOx is a suitable contrast agent to visualize the initial filling of the rat ductal tree (Figure 2 and Figure 3). Both in translational research and clinical practice applications, the gelling agent ethyl cellulose (EC) has been added to the EtOH solution to minimize diffusion from the intended targeted regions13,14,24,25,26,27,28,29. Studies have shown that addition of up to 1.5% EC to EtOH-containing ablative solutions is compatible with TaOx-based imaging (Figure 3). These as well as further refinements to the ablative solution may assist in ready translation of this image-guided procedure to the clinic.
Protocol
All the experiments that are described were conducted under protocols approved by the Institutional Animal Care and Use Committee at Michigan State University.
1. Extended anti-inflammatory treatment
- Prepare sucralose gel cups as oral dosing of carprofen. Provide rats with this anti-inflammatory treatment from 2 days before receiving ID injection of 70% EtOH to 7 days after the procedure.
- Dilute a working solution of carprofen in sterile PBS for injection into the cup. From 50 mg/mL stock solution, prepare a diluted 2 mg/mL solution colored with 1% v/v sterile blue food dye and inject 500 µL into each cup. Addition of the dye aids in visualization of complete mixing of the drug within the sucralose of the cup.
- Follow the manufacturer's recommendation to prepare the cup for the addition of carprofen. Unless otherwise indicated by the vendor, warm the cup in a water bath at 60 °C for 15 min and dry off upon removal to reduce the risk of contamination.
- Wipe-clean the lid of the sucralose cup with 70% EtOH in the area and introduce the needle of the syringe containing the carprofen working solution. Dispense the appropriate volume (500 µL).
- Cover the puncture with a sticker. Shake the cup energetically for 15 s, and then place that cup in a vortex for an additional 15 s. Visually assess the homogenous and complete mixing before storing for later use. Look for the presence of a dark blue color.
NOTE: Allow the cups to come to room temperature. Store the cups at room temperature if desired but pay attention to drug efficacy guidance from the manufacturer. Alternatively, store the cups at 4 °C and use within a month. Dating the sticker is a good practice for keeping track of the injection date without the risk of a pen or sharp marker puncturing the lid.
- Just before use, wipe down the exterior of the cup with 70% EtOH. Remove the lid before placing the cup into the animal cage. Replace cups every other day or when empty. Check the level of gel daily to ensure adequate dosing. One cup can supply carprofen for up to two rats for up to 2 days; however, rats may consume entire cup sooner.
2. Preoperative preparation
NOTE: Ensure that the animal preparation step precedes the ID injection procedure by 2-3 days.
- Turn on the isoflurane vaporizer (2%-3% isoflurane, 1.5 L/min of oxygen) to anesthetize the rat. Move the animal to a nose cone on a warming pad. Apply eye lubricant to the rat, and then position the animal to its back. Carefully monitor the animal's respiration to ensure that the anesthetic plane is maintained at 1%-3% of isoflurane.
NOTE: An electric razor may be used to remove excess fur before depilation. Extreme care must be taken not to damage any nipples with the razor. For this reason, this step can be skipped. Rats are more sensitive to depilatory cream than mice, so the removal of excess cream is very important. Avoid injecting an ethanol-containing solution into an area that already has an abrasion present from depilation. Some creams have added compounds such as aloe and lanolin that can help minimize the likelihood of abrasions. - Use a cotton-tip applicator to spread the over-the-counter depilatory cream onto the nipple area. Use the applicator to rub the cream into the area for 10-30 s. Check whether the fur has quickly loosened.
- Leave the cream on the rat for the shortest possible interval and remove completely to avoid burning of the skin. Rats are even more sensitive to this procedure than mice.
- After 10-30 s of application, wet gauze with warm water and use it to rinse the cream and the loosened fur from the animal. Perform at least three rinses of the area with fresh moistened gauze and dry with dry gauze after the final rinse. Confirm good visibility and access to the area of the nipple from where the fur is removed. Repeat the depilatory procedure if necessary.
- Place the rat in a clean cage on a heating pad and allow it to recover. Check on the rat to make sure that it is fully recovered from anesthesia before bringing it back to its permanent cage.
- Place one carprofen-dosed (1 mg/cup) sucralose gel cup in the cage for anti-inflammatory treatment. Check the gel consumption daily and replace with a fresh cup as appropriate. Do not leave the cup for more than 2 days. Typically, cups will need to be replaced after 1 day.
3. Intraductal injection
- Prepare the TaOx stock solution at 333.3 mM as described19 using sterile phosphate buffered saline (PBS). Warm the solution if the powder does not fully dissolve. Stir gently. Do not vortex or shake vigorously to avoid bubble formation.
- Mix three parts of 333.3 mM TaOx with seven parts of 100% EtOH for a final 70% EtOH 100 mM/TaOx solution. Optionally, add an appropriate amount of 0.5%-1.5% ethyl cellulose (EC) as a gelling agent to maximize local retention of the ablative solution. Add 1% v/v blue food dye to the ablative solution for visual examination of the delivery into the ductal tree during infusion.
- Prepare a volume appropriate for experimental needs. Gland pairs 1 (cervical) and 6 (inguinal) can be filled with up to 100 μL of the solution while all other pairs can be filled with up to 300 μL.
- Anesthetize the rat as in step 2.1 and move the rat to the nose cone once fully anesthetized. Apply eye lubricant to both the eyes and then place the animal on its back. Secure the rat beneath the stereoscope using tape near the nipples that will be injected, if desired. The weight of the rat is generally enough to keep it from moving substantially without taping.
- To prepare the nipples for injection, remove any dead skin that covers the nipple opening with fine pointed forceps, if possible. Rats often have a plug protruding from the nipple opening that can prevent successful cannulation of the nipple if not removed.
NOTE: It is important to note that larger injection volumes of ablative solutions being used in rats may be more likely to result in superficial skin wounds near the injection site(s). For this reason, injecting every other nipple in a single session is less damaging and irritating to the animal than injecting adjacent nipples. Monitoring the rats for any abrasions for 7 days post-injection helps to ensure no serious health effects from the animal scratching and introducing the possibility of infection from contamination with cage floor debris. Triple antibiotic ointment or washes with chlorhexidine solution can be used to treat any signs of injury infection that may occur (Table 1). - Use a 500 µL syringe with a 33 G needle to aspirate 101-301 µL of ablative solution. Aspirate an extra 1 µL of the solution for possible minor leakage when removing the cannulated needle.
NOTE: These are recommendations for volumes aimed at fully filling the ductal tree(s): up to 100 µL in cervical and inguinal glands, and up to 300 µL in the other glands. For other applications, it may be appropriate to use smaller or larger volumes. - Use a tweezer to gently hold the nipple and cannulate the needle into the nipple opening. Gently continue inserting the needle until the bevel is fully inside the nipple. To accommodate the needle in the nipple, bring the nipple up toward the needle instead of pushing the needle down into the nipple. (Table 1). Take care to follow the path of the nipple opening.
NOTE: Rat nipples are generally much easier to manipulate and cannulate successfully than those in mice due to larger size. However, the increased amount of fat surrounding the nipple opening also makes it more likely to mistakenly inject the fat pad if the needle deviates from the main duct. - Once the needle bevel is completely inserted, slowly infuse the solution at a constant rate of approximately 100 µL/min in rats. Abrupt changes in infusion rate can burst or damage the ductal tree. Wait for 30 s after the end of infusion before removing the needle from the cannulated tree with assistance using forceps; this ensures that the injected volume remains within the ductal tree (Figure 2) and reduces the likelihood of leakage.
- Clean off any spilled solution with moistened gauze or an EtOH wipe to avoid extraneous contrast solution in the images.
- Inject PBS containing 5% sucrose (10 mL/kg) intraperitoneally to mitigate the effects of alcohol intoxication if ethanol is contained within the ID injection solutions. This dose may be given at the beginning and at the end of the procedure.
4. Micro-CT imaging
- After all the desired glands have been injected, move the animal swiftly to the micro-CT system and continue maintaining anesthesia using the incorporated isoflurane vaporizer.
- Straighten the spine of the animal and tape each hind leg in an extended position, so that the leg bones of the animal will be further away from the lower glands of interest and not overlap with the region of interest in the scanned image.
- Tape across the abdomen to minimize breathing artifacts if scanning the lower glands.
NOTE: Animals can be imaged with different scanning parameters (e.g., high resolution, longitudinal scans) if care is taken to determine an appropriate acceptable lifetime dose of radiation for rats and ensuring that the cumulative dose does not exceed this level. Radiation exposure may be further reduced by acquiring fluoroscopy stills and videos without performing scans (Figure 2). - Perform TaOx imaging of the rat ductal tree with good resolution and opportunity for repeated standard (2 min) acquisition scans using the following scan parameters: 90 kVp/88 µA; field of view (FOV), 72 mm; number of slices, 512; slice thickness, 72 µm; voxel resolution, 72 µm3. High-resolution scans for longer time periods (4-14 min) can also be acquired in animals that will not be scanned longitudinally using the same parameters.
- After data acquisition, carefully take the rat away from the anesthesia cone and place in a new clean cage on a heating pad. Check on the rat to make sure it has fully recovered from anesthesia before bringing it back to its permanent cage. Place the carprofen-containing sucralose cup and appropriately replace as described in step 2.5 to ensure that the animals continue to receive anti-inflammatory treatment for the next 7 days.
- Process the scanned images into quick renditions within the micro-CT software to better appreciate any contrast leaks, only partial filling, or overfilling (Figure 2).
- Proceed to the next section to perform formal image processing for publication or detailed analysis of scans if desired (Figure 3).
5. Image analysis
- Use specialized software packages to produce renderings of the filled ductal tree.
NOTE: It is best to segment out the mammary fat pad in order to get best rendition of the injected ductal tree. Spline trace the dark boundaries of the fat pad throughout the complete thickness of the animal in order to achieve this segmentation. - To segment the fat pad (unlike mice, the boundaries of this compartment are not as easily distinguishable from the peritoneal cavity, femoral muscles, and skin due to similar Hounsfield units) within which the ductal tree of interest is contained, selecting the "spline trace" option from the manual menu is the first step in creating a rendering.
- Spline trace the fat pad outline in every third slice. Click on the Propagate Objects option from the semi-automatic menu. This will propagate and connect all the slices into a single segmented object of interest.
NOTE: Changing the threshold within the segmented region allows visualization of the signal only within a certain range of Hounsfield units (HU); for other contrast agents or imaging parameters, this range may need to be adjusted. A software package or Artificial Intelligence analysis may be used to make other measurements and images to show how much the ductal tree was filled. - Set HU values to a low point of 300 and a high point of 3,000 on the semi-automatic menu under the threshold volume tab. This allows for creation of a rendition only displaying the contrast (TaOx) within the ductal tree.
- Set the rendition as primary using the "view" button. This will change the display to only show the 3D rendition of the ductal tree.
NOTE: Perform reconstruction of the ductal tree for further analysis.
Representative Results
Each of the 12 mammary glands of a female rat contains a single ductal tree that opens at the nipple orifice. Despite the differences in size between the mouse and the rat, the developmental timing of the mammary glands and the time that these animals reach adulthood is very similar30,31. A brief description of the key stages of mammary gland development in rats as representative of both rodent species is provided. Terminal end buds (TEBs) are the highly proliferative structures at the tips of the elongating ductal tree that direct ductal branching30,31. The peak of proliferation and density of the TEBs occurs at 3-4 weeks of age during the elongating phase of the ductal tree in pubertal development30. By 9-10 weeks of age, there are few TEBs remaining as the ductal tree has grown to occupy the entire length of the fat pad30. After that, growth and expansion of the ductal tree is proportional to that of the fat pad and of the animal32. Terminal ductal lobular units (TDLUs) in the human breast carry out a similar role to the TEBs in rodents. TDLUs are the main source for initiation of carcinogenesis and progression to BC33,34. We can inject up to 300 µL of 70% EtOH solution to fill the entire ductal tree of the thoracic and abdominal mammary glands of the 9-week-old Sprague-Dawley rat (Figure 1, Figure 2, Figure 3). Unlike mice20, the nipples of the cervical and inguinal glands of the Sprague-Dawley rats are typically suitable for injection in more than 80% of animals, and up to 100 μL of 70% EtOH solution is required to fill the entire ductal tree (Figure 2). We routinely inject up to 10 mammary glands with the ablative solution under study. A typical experimental design consists of two independent weekly ID injection sessions, in which five alternating glands are infused with the ablative solution containing X-ray contrast agent and/or EC as the gelling agent (Figure 2). For TaOx-containing (50-200 mM) ablative solution, fluoroscopy and/or micro-CT scanning is performed after the end of each session to determine and record the individual success of infusing each ductal tree with partial or full amount of infused solution (Figure 2). Immediate and longitudinal imaging after injection enables assessment of how changes in formulation, especially concentration of EC gelling agent, affects and limits outward diffusion of the ablative solution as a function of the injected volume (Figure 3). This imaging analysis provides information to understand the optimal parameters to achieve maximal ablation with minimal collateral tissue damage.
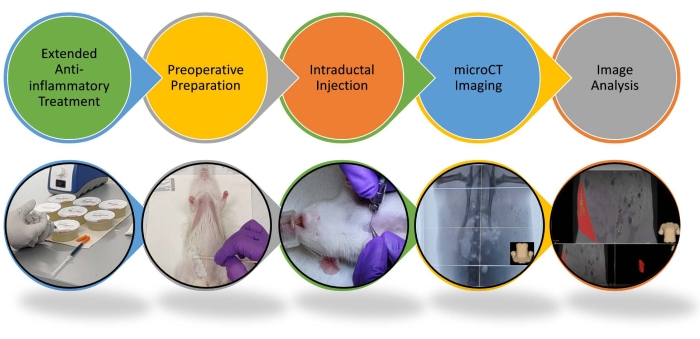
Figure 1: Schematics of the procedure for intraductal injection and image analysis in rats. The step-by-step procedure for intraductal injection and image analysis are highlighted. Please see video for more details. Please click here to view a larger version of this figure.
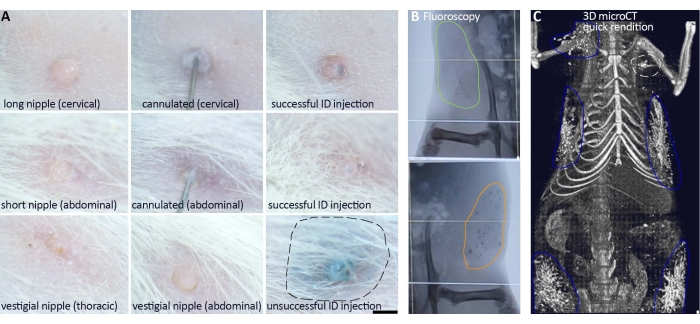
Figure 2: Examples of nipple cannulation and delivery outcome of the ablative solution into multiple mammary glands. (A) Typical presentation of nipple shapes in the Sprague-Dawley rat strain. Nipple length correlates with likelihood of successful cannulation. Longer nipples are easier to cannulate than short nipples, whereas excessively short or vestigial nipples cannot be cannulated. Once cannulated, both long and short nipples can be infused with the solution and achieve similar success rates of delivery. Blue food dye in the injected solution may be used as in vivo evidence of ductal tree filling and delivery success (most apparent, dome formation, for an unsuccessful fat pad injection). Real-time fluoroscopy (B) and 3D micro-CT renditions generated after image acquisition (C) provide in vivo evidence of delivery success and more quantitative assessment of the solution reaching the TEBs. (B) Each abdominal mammary gland of the first pair (#4, #10) received ablative solution with 1% EC (orange outline) or without it (green outline) (C) Successful delivery (blue outline) of the ablative solution in the right cervical (#7), second pair of thoracic (#3, #9) and first pair of abdominal (#4, #10) mammary glands, and unsuccessful injection (dashed white outline) in the left thoracic (#1) gland. Scale bars correspond to 1 mm in the images at different magnification. Please click here to view a larger version of this figure.
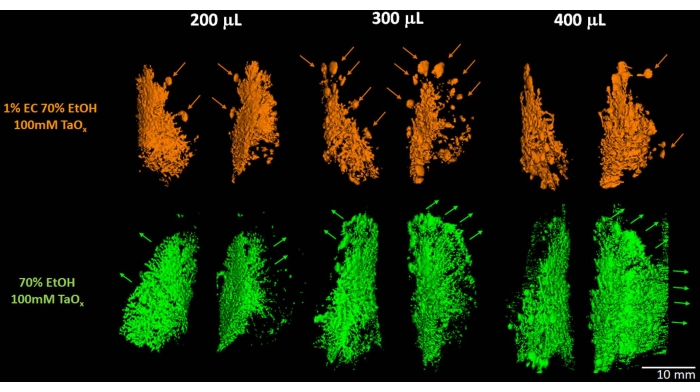
Figure 3: 3D reconstruction and assessment of ablative solution filling and diffusion. 70% EtOH/100 mM TaOx nanoparticles with 1% EC (top) or without EC (bottom) were intraductally injected into the second abdominal mammary gland pair (#4 and #10) and immediately imaged by micro-CT. Each Sprague-Dawley rat received an increasing volume of either solution. Individual ductal trees were reconstructed using an image analysis software package (spline trace + propagate object + threshold rendition). With 1% EC, the solution can be seen reaching the terminal ends. As the delivered volume is increased, the number of TEBs filled is more apparent. Scale bar corresponds to 10 mm in all renditions. Please click here to view a larger version of this figure.
| Issue | Appearance | Solution | |||
| Short nipple (Fig. 2) | Nipple has low profile – hard to grab | It is sometimes easier to hold the skin near the nipple and target the center of the nipple with the needle. The needle will likely dive under the skin. Pulling up slowly may reveal the nipple to be slightly over the tip of the needle and give room to grab and pull it the rest of the way onto the needle. Be very careful when diving below the skin about the angle of the needle. It is easy to inadvertently get a fat pad injection by stabbing at the wrong angle. | |||
| Fat pad injection (Fig. 2) | Swollen around nipple and possibly in nipple itself – easiest to see if color is added to injection solution | If nipple is swelling with first few ul injected, remove needle, and attempt to insert again with more care taken of angle. Begin injection again and watch for further swelling. If swelling continues, abandon attempt. It is very rare to successfully inject a nipple that has started out as a fat pad injection. | |||
| Wounds/scabbing | Open wound or scabbing near injection site of EtOH solution | Rats are more likely than mice to develop wounds or scabbing near the injection area. If wounds are found, apply triple antibiotic ointment to open wounds but leave scabbed wounds alone. Applying ointment to scabs can increase likelihood animal will bother the scab and remove it. Check every 1-2 days until healed depending on severity of wound. Carprofen should be given until healed even if beyond normal window. | |||
| Inject alternating glands | N/A | Larger injection volumes in rats make it more likely to cause skin abrasions if injecting consecutive glands. For least likelihood of trauma to injection area, alternate glands injected within a single session (i.e. inject #1, 3, 4 and 6 rather than #1-4). Spacing between third (#3 and #9) and fourth (#4 and #10) gland pairs allows injection of both of these glands in one session. | |||
Table 1: Helpful tips and troubleshooting
Discussion
As shown here, ID delivery of 70% EtOH preferentially ablates the mammary epithelial cells with limited collateral damage to the surrounding stroma and vasculature in mice4. Local ablation of the ductal tree is effective at preventing tumor formation in mouse models4. Here, we demonstrate that this ablative procedure can be scaled up to rats.
This is the next step in the path to translation of this ablative procedure as an alternative intervention to prophylactic mastectomy for primary prevention of breast cancer in high-risk individuals. Addition of TaOx nanoparticles as an X-ray contrast agent to the ablative solution allows to assess effectiveness of the solution at preventing tumor formation, as it can be determined whether the procedure was successful or not at completely filling the ductal tree. Using fluoroscopy to visualize the injected mammary gland mirrors what will likely be done in the clinic to guide this ID procedure. Image guidance of how much the solution has filled the ductal tree and when to stop infusion will be a key aspect of the clinical implementation to ensure maximal filling of each ductal tree. Troubleshootings and helpful tips are listed in Table 1. Effectiveness of this ablative procedure requires that the infused solution makes direct contact with all the epithelial cells to maximize the rate of cell killing. Spare epithelial cells within one or more trees could eventually serve as a source for BC development. The other groups reported ID delivery of viral particles (e.g., components of Cre/LoxP and/or Cas9/CRISPR systems), hormones and hormone antagonists (e.g., prolactin, fulvestrant), chemotherapeutic agents (e.g., cisplatin), siRNAs and/or antibodies or other targeting agents in mice4,19,21,35,36,37,38,39,40,41,42,43,44,45, rats21,33,46,47,48 and/or rabbits18,49,50,51,52,53. Successful cannulation of up to eight ductal trees per human breast for local delivery of chemotherapy has been reported in independent clinical studies47,54,55. Image guidance for infusion of these other solutions aimed at tumor prevention or geared toward local treatment would similarly maximize their effectiveness.
The scalability and refinement of this procedure from mouse to rat ductal tree is demonstrated here. TaOx nanoparticle in the murine4,19 and rat (unpublished data) ductal tree provide high-resolution imaging that surpasses the FDA-approved iodine-containing X-ray contrast agents. Similarly, we are unaware of other ductal tree imaging approaches in mice40,41 or other animal models18 that can provide comparable resolution to TaOx. Relevant for clinical translation is the fact that the gelling effect of EC in this intermediate size rat models is an enabling formulation refinement to minimize collateral tissue damage. As we continue to assess this ablative ID procedure for its ability to prevent BC, we will be able to determine, more precisely, from which glands BC develops through the added information provided by imaging after ID delivery in chemically induced and other rat models of BC. These data will determine the safety of this procedure and pinpoint any concerns or shortcomings of whether partial or unsuccessful treated ductal trees might be more prone to develop BC in a high-risk woman.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
This work was supported, in part, by the National Cancer Institute R21 CA226579 and R01 CA258314 grants to LFS and by the National Institute of Biomedical Imaging and Bioengineering R01 EB029418 grant to EMS. We are grateful to the MSU Institute for Quantitative (IQ) Health Science and Engineering Imaging Core facility for use of their imaging systems and technical expertise. We thank Dr. Danielle Ferguson for reviewing contents of the video and the figures for adherence to animal welfare guidelines.
Materials
| AnalyzeDirect v12.0 | Caliper | n/a | For micro-CT image processing |
| Carprieve, Carprofen 50 mg/mL | Allivet | 50647 | For anti-inflammatory treatment |
| Ethyl cellulose | Acros Organics | 9004-57-3 | For intraductal injection |
| Evans blue | Sigma | E2129-50G | For injection visualization |
| Hot water bath | Toolots | Yidu_HH-S2 | For preparing carprofen cups |
| MediGel Sucralose Cups | ClearH2O | 74-02-5022 | For delivery of carprofen |
| Model 1750 TTL, PTFE Luer Lock Syringe, 500μL | Hamilton | 81220 | For intraductal injection |
| Photoshop 2021 | Adobe | n/a | For image processing |
| Quantum GX2 microCT Imaging System | Perkin Elmer | CLS149276 | For micro-CT image acquisition |
| Metal Hub Needle, 33 gauge, custom (30° bevel angle, 0.4 in, point style 4) | Hamilton | 7747-01 | For intraductal injection |
| Stereo Microscope SZM Series | AmScope | SM-4TPZ-144 | For intraductal injection |
| Sterile blue food dye | McCormick | 930641 | For injection visualization |
| Sterile phosphate buffered saline (PBS) | ThermoFisher | 14190250 | For solution preparation |
| Stickers | DOT Scientific | DOTSCI-C50 | For preparing carprofen cups |
| Sucrose | Calbiochem | 8550-5KG | For intraductal injection |
| Syringes | Fisher | 14-826-79 | For preparing carprofen cups |
| Vortex | VWR | 10153-834 | For preparing carprofen cups |
| Warming pump/pad(s) | Braintree Scientific | HTP-1500 120V; AP-R 26E | For intraductal injection/preoperative preparation |
Referanslar
- Siegel, R. L., Miller, K. D., Fuchs, H. E., Jemal, A. Cancer statistics, 2022. A Cancer Journal for Clinicians. 72 (1), 7-33 (2022).
- Wild, C. P. The global cancer burden: necessity is the mother of prevention. Nature Reviews. Cancer. 19 (3), 123-124 (2019).
- Padamsee, T. J., Wills, C. E., Yee, L. D., Paskett, E. D. Decision making for breast cancer prevention among women at elevated risk. Breast Cancer Research. 19 (1), 34 (2017).
- Kenyon, E., et al. Ductal tree ablation by local delivery of ethanol prevents tumor formation in an aggressive mouse model of breast cancer. Breast Cancer Research. 21 (1), 129 (2019).
- Kuang, M., et al. Ethanol ablation of hepatocellular carcinoma Up to 5.0 cm by using a multipronged injection needle with high-dose strategy. Radiology. 253 (2), 552-561 (2009).
- Ansari, D., Andersson, R. Radiofrequency ablation or percutaneous ethanol injection for the treatment of liver tumors. World Journal of Gastroenterology. 18 (10), 1003-1008 (2012).
- Zhang, W. Y., Li, Z. S., Jin, Z. D. Endoscopic ultrasound-guided ethanol ablation therapy for tumors. World Journal of Gastroenterology. 19 (22), 3397-3403 (2013).
- Chin, M., Chen, C. L., Chang, K., Lee, J., Samarasena, J. Ethanol ablation of a peripheral nerve sheath tumor presenting as a small bowel obstruction. ACG Case Reports Journal. 3 (1), 31-32 (2015).
- Gueng, M. -. K., Chou, Y. -. H., Tiu, C. -. M., Chiou, S. -. Y., Cheng, Y. -. F. Pseudoaneurysm of the breast treated with percutaneous ethanol injection. Journal of Medical Ultrasound. 22 (2), 114-116 (2014).
- Zhang, J., et al. Comparison between absolute ethanol and bleomycin for the treatment of venous malformation in children. Experimental and Therapeutic Medicine. 6 (2), 305-309 (2013).
- Wohlgemuth, W. A., et al. Ethanolgel sclerotherapy of venous malformations improves health-related quality-of-life in adults and children – results of a prospective study. European Radiology. 27 (6), 2482-2488 (2017).
- Steiner, F., FitzJohn, T., Tan, S. T. Ethanol sclerotherapy for venous malformation. ANZ Journal of Surgery. 86 (10), 790-795 (2016).
- Sannier, K., et al. A new sclerosing agent in the treatment of venous malformations. Study on 23 cases. Interventional Neuroradiology. 10 (2), 113-127 (2004).
- Dompmartin, A., et al. Radio-opaque ethylcellulose-ethanol is a safe and efficient sclerosing agent for venous malformations. European Radiology. 21 (12), 2647-2656 (2011).
- Faguy, K. Ductography: When, how, and why. Radiologic Technology. 92 (5), 487-503 (2021).
- Slawson, S. H., Johnson, B. A. Ductography: how to and what if. Radiographics. 21 (1), 133-150 (2001).
- Sheiman, L. S., Levesque, P. H. The in’s and out’s of ductography: A comprehensive review. Current Problems in Diagnostic Radiology. 45 (1), 61-70 (2016).
- Clark, A., Bird, N. K., Brock, A. Intraductal delivery to the rabbit mammary gland. Journal of Visualized Experiments. (121), e55209 (2017).
- Chakravarty, S., et al. Tantalum oxide nanoparticles as versatile contrast agents for X-ray computed tomography. Nanoscale. 12 (14), 7720-7734 (2020).
- Kenyon, E., et al. Intraductal delivery and x-ray visualization of ethanol-based ablative solution for prevention and local treatment of breast cancer in mouse models. Journal of Visualized Experiments. (182), e63457 (2022).
- Murata, S., et al. Ductal access for prevention and therapy of mammary tumors. Kanser Araştırmaları. 66 (2), 638-645 (2006).
- King, B. L., Love, S. M. The intraductal approach to the breast: raison d’etre. Breast Cancer Research. 8 (2), 206 (2006).
- Love, S. M., Barsky, S. H. Anatomy of the nipple and breast ducts revisited. Cancer. 101 (9), 1947-1957 (2004).
- Lai, Y. E., Morhard, R., Ramanujam, N., Nolan, M. W. Minimally invasive ethyl cellulose ethanol ablation in domesticated cats with naturally occurring head and neck cancers: Six cats. Veterinary and Comparative Oncology. 19 (3), 492-500 (2021).
- Mueller, J. L., et al. Optimizing ethyl cellulose-ethanol delivery towards enabling ablation of cervical dysplasia. Scientific Reports. 11 (1), 16869 (2021).
- Nief, C., et al. Polymer-assisted intratumoral delivery of ethanol: Preclinical investigation of safety and efficacy in a murine breast cancer model. PLoS One. 16 (1), 0234535 (2021).
- Chelales, E., et al. Radiologic-pathologic analysis of increased ethanol localization and ablative extent achieved by ethyl cellulose. Scientific Reports. 11 (1), 20700 (2021).
- Morhard, R., et al. Understanding factors governing distribution volume of ethyl cellulose-ethanol to optimize ablative therapy in the liver. IEEE Trans Biomedical Engineering. 67 (8), 2337-2348 (2020).
- Morhard, R., et al. Development of enhanced ethanol ablation as an alternative to surgery in treatment of superficial solid tumors. Scientific Reports. 7 (1), 8750 (2017).
- Russo, I. H., Russo, J. Developmental stage of the rat mammary gland as determinant of its susceptibility to 7,12-dimethylbenz[a]anthracene. Journal of the National Cancer Institute. 61 (6), 1439-1449 (1978).
- Paine, I. S., Lewis, M. T. The terminal end bud: The little engine that could. Journal of Mammary Gland Biology Neoplasia. 22 (2), 93-108 (2017).
- Hinck, L., Silberstein, G. B. Key stages in mammary gland development: the mammary end bud as a motile organ. Breast Cancer Research. 7 (6), 245-251 (2005).
- Sivaraman, L., et al. Effect of selective ablation of proliferating mammary epithelial cells on MNU induced rat mammary tumorigenesis. Breast Cancer Research Treatment. 73 (1), 75-83 (2002).
- Cardiff, R. D., Wellings, S. R. The comparative pathology of human and mouse mammary glands. Journal of Mammary Gland Biology Neoplasia. 4 (1), 105-122 (1999).
- Brock, A., et al. Silencing HoxA1 by intraductal injection of siRNA lipidoid nanoparticles prevents mammary tumor progression in mice. Scientific Translational Medicine. 6 (217), (2014).
- de Groot, J. S., et al. Intraductal cisplatin treatment in a BRCA-associated breast cancer mouse model attenuates tumor development but leads to systemic tumors in aged female mice. Oncotarget. 8 (37), 60750-60763 (2017).
- Wang, G., et al. Intraductal fulvestrant for therapy of ERalpha-positive Ductal Carcinoma in Situ (DCIS) of the breast – A preclinical study. Carcinogenesis. 40 (7), 907-913 (2019).
- Yoshida, T., et al. Effective treatment of ductal carcinoma in situ with a HER-2- targeted alpha-particle emitting radionuclide in a preclinical model of human breast cancer. Oncotarget. 7 (22), 33306-33315 (2016).
- Chun, Y. S., et al. Intraductally administered pegylated liposomal doxorubicin reduces mammary stem cell function in the mammary gland but in the long term, induces malignant tumors. Breast Cancer Research Treatment. 135 (1), 201-208 (2012).
- Markiewicz, E., et al. High resolution 3D MRI of mouse mammary glands with intra-ductal injection of contrast media. Magnetic Resonance Imaging. 33 (1), 161-165 (2015).
- Markiewicz, E., et al. MRI ductography of contrast agent distribution and leakage in normal mouse mammary ducts and ducts with in situ cancer. Magnetic Resonance Imaging. 40, 48-52 (2017).
- Annunziato, S., et al. Comparative oncogenomics identifies combinations of driver genes and drug targets in BRCA1-mutated breast cancer. Nature Communications. 10 (1), 397 (2019).
- Rutkowski, M. R., et al. Initiation of metastatic breast carcinoma by targeting of the ductal epithelium with adenovirus-cre: a novel transgenic mouse model of breast cancer. Journal of Visualized Experiments. (85), e51171 (2014).
- Xiang, D., Tao, L., Li, Z. Modeling breast cancer via an intraductal injection of cre-expressing adenovirus into the mouse mammary gland. Journal of Visualized Experiments. (148), e59502 (2019).
- Barham, W., Sherrill, T., Connelly, L., Blackwell, T. S., Yull, F. E. Intraductal injection of LPS as a mouse model of mastitis: signaling visualized via an NF-kappaB reporter transgenic. Journal of Visualized Experiments. (67), e4030 (2012).
- Chun, Y. S., et al. Intraductal administration of a polymeric nanoparticle formulation of curcumin (NanoCurc) significantly attenuates incidence of mammary tumors in a rodent chemical carcinogenesis model: Implications for breast cancer chemoprevention in at-risk populations. Carcinogenesis. 33 (11), 2242-2249 (2012).
- Stearns, V., et al. Preclinical and clinical evaluation of intraductally administered agents in early breast cancer. Science Translational Medicine. 3 (106), (2011).
- Okugawa, H., et al. Effect of perductal paclitaxel exposure on the development of MNU-induced mammary carcinoma in female S-D rats. Breast Cancer Research Treatment. 91 (1), 29-34 (2005).
- Falconer, I. R. The distribution of 131 I- or 125 I-labelled prolactin in rabbit mammary tissue after intravenous or intraductal injection. Journal of Endocrinology. 53 (3), 58-59 (1972).
- Fiddler, T. J., Birkinshaw, M., Falconer, I. R. Effects of intraductal prolactin on some aspects of the ultrastructure and biochemistry of mammary tissue in the pseudopregnant rabbit. Journal of Endocrinology. 49 (3), 459-469 (1971).
- Fiddler, T. J., Falconer, I. R. The effect of intraductal prolactin on protein and nucleic acid biosynthesis in the rabbit mammary gland. The Biochemical Journal. 115 (5), 58 (1969).
- Bourne, R. A., Bryant, J. A., Falconer, I. R. Stimulation of DNA synthesis by prolactin in rabbit mammary tissue. Journal of Cell Science. 14 (1), 105-111 (1974).
- Chadwick, A. Detection and assay of prolactin by the local lactogenic response in the rabbit. The Journal of Endocrinology. 27, 253-263 (1963).
- Mahoney, M. E., et al. Intraductal therapy of ductal carcinoma in situ: a presurgery study. Clinical Breast Cancer. 13 (4), 280-286 (2013).
- Love, S. M., et al. A feasibility study of the intraductal administration of chemotherapy. Cancer Preview Research (Phila). 6 (1), 51-58 (2013).

