Separation of Bioactive Small Molecules, Peptides from Natural Sources and Proteins from Microbes by Preparative Isoelectric Focusing (IEF) Method
Özet
The objective is to fractionate and isolate bioactive small molecules, peptides from a complex plant extract, and proteins from pathogenic microbes by employing liquid-phase isoelectric focusing (IEF) method. Further, the separated molecules were identified and their bioactivity confirmed.
Abstract
Natural products derived from plants and microbes are a rich source of bioactive molecules. Prior to their use, the active molecules from complex extracts must be purified for downstream applications. There are various chromatographic methods available for this purpose yet not all labs can afford high performance methods and isolation from complex biological samples can be difficult. Here we demonstrate that preparative liquid-phase isoelectric focusing (IEF) can separate molecules, including small molecules and peptides from complex plant extracts, based on their isoelectric points (pI). We have used the method for complex biological sample fractionation and characterization. As a proof of concept, we fractionated a Gymnema sylvestre plant extract, isolating a family of terpenoid saponin small molecules and a peptide. We also demonstrated effective microbial protein separation using the Candida albicans fungus as a model system.
Introduction
The purification of biomolecules from complex biological samples is an essential and often difficult step in biological experiments1. Isoelectric focusing (IEF) is well-suited for high resolution separation of complex biomolecules where carrier ampholytes travel according to their charge and establish the pH gradient in an electric field3. The first commercial carrier ampholyte for IEF was developed by Olof Vesterberg in 1964 and patented4,5. Carrier ampholytes are aliphatic oligo-amino oligo-carboxylic acid molecules of varying length and branching6. Subsequently, Vesterberg and others improved the carrier ampholytes for their expanded use in separating biomolecules6,7.
Methods to separate biomolecules include agarose and polyacrylamide gel electrophoresis, two-dimensional gel electrophoresis (2-DE), isoelectric focusing, capillary electrophoresis, isotachophoresis and other chromatographic techniques (e.g., TLC, FPLC, HPLC)2. Liquid-phase IEF performed in an instrument called a “Rotofor” was invented by Milan Bier8. He pioneered the concept and design of this instrument and contributed extensively to the theory of electrophoretic migration. His team also developed a mathematical model of electrophoretic separation process for computer simulations9.
The liquid-phase IEF apparatus is a horizontally rotating cylindrical cell consisting of a nylon core divided into 20 porous compartments and a circulating water cooling ceramic rod. The porous chambers allow molecules to migrate through the aqueous phase between the electrodes and permit collection of purified samples under vacuum in fractions. This purification system can provide up to 1000-fold purification of a specific molecule in <4 hours. A valuable feature of this instrument is that it can be applied as a first step for purification from a complex mixture or as a final step to achieve purity10. If the molecule of interest is a protein, another advantage is that its native conformation will be maintained during the separation.
The use of liquid-phase IEF has been reported widely for proteins, enzymes and antibody purification6,10,11,12,13,14. Here we describe the use of this approach for separating and purifying small molecules and peptides from the medicinal plant Gymnema sylvestre. This protocol will help researchers concentrate and purify active small molecules from a plant extract for downstream applications at low cost. In addition, we also demonstrate that enrichment of proteins from a complex protein extract from Candida albicans fungus15 in this IEF-based system as a second example.
Protocol
1. Setup and prerunning of standard liquid-phase IEF unit
- Assemble the liquid-phase IEF electrodes (anode-red button and cathode-black button) with their respective exchange membranes according to the instruction manual (see Table of Materials). Equilibrate the anion exchange membranes with 0.1 M NaOH and the cation exchange membranes with 0.1 M H3PO4 at least for 16 h when new membranes are used.
- Store the membranes in electrolytes (0.1 M NaOH or 0.1 M H3PO4) between runs and do not allow to dry out.
- Assemble the inner and outer portion of the electrode by aligning three oblong holes in the ion-exchange gaskets. Fill the electrodes with respective electrolytes (~25-30 mL) to prevent their membranes from drying out.
- Do not add excess (more than 1/3rd of electrode chamber volume) electrolyte that may build up pressure inside the electrode and cause leakage.
- Cover the sample collection ports with sealing tape that comes with the instrument assembly parts (see Table of Materials). The sample collection ports side can be identified by the two vertical metal aligning pins. Alternatively, use standard sealing tape to seal the ports.
- Assemble all parts of the focusing chamber assembly in sequence (anode electrode, nylon membrane core, focusing chamber and cathode electrode) over the ceramic cooling finger.
- Fill the focusing chamber with precooled distilled water (total volume of 60 mL for the standard IEF cell) using a 50 mL syringe.
- Connect the liquid-phase IEF instrument to a circulating cooling water at 4 ºC and prerun the unit at 15 W and 3,000 V for 3-5 min or until the voltage stabilizes.
NOTE: Generally, within one minute, the voltage will reach the maximum set values. Prerunning with distilled water helps to remove the residual ions from the focusing chamber and the nylon membrane core. - Switch off the power source and remove the water from the cell using the fraction collector. Re-seal the collection ports with sealing tape.
NOTE: Now the instrument is ready for use in step 2.4.
2. Separation and purification of small molecules and peptides from Gymnema sylvestre extract
- Measure 0.6 g of plant extract and dissolve in distilled water (60 mL) by mixing in a roller tube for 5 min.
NOTE: Any biological sample that is soluble and free of salt can be used for separation and purification using this IEF instrument. Samples with buffering salt concentration up to 10 mM can be used with slightly decreased resolution. We are interested in the saponin family of triterpenoid compounds, the gymnemic acids, from G. sylvestre plant for their unique antifungal properties16. - Centrifuge the solubilized plant extract at 10,000 x g for 5 min to remove insoluble particles.
- Transfer the supernatant (~60 mL) to an 80 mL centrifuge tube, and mix it with 0.6 mL of ampholyte (pH 3-10) to 1% (v/v).
- Follow steps 1.1 – 1.7 to prepare the liquid-phase IEF unit. The chamber is now ready to load the sample.
- Use a 50 mL syringe with a 1-1/2 inch 19 G blunt end needle (comes with the instrument) and load the prepared sample with ampholyte (60 mL total) in the cell through sample collection ports.
- Remove air bubbles from the sample cell by removing the focusing cell from the stand and tapping the electrode chamber to dislodge the bubbles. The presence of air bubbles will cause voltage and current fluctuations and affect the run.
- Connect the unit to the water coolant (4 °C) and start fractionation with the power supply at a constant 15 W.
- Run the apparatus for 3 h or until the voltage reaches a constant value.
NOTE: As the sample starts focusing, the voltage will start climbing gradually until it reaches a constant value. - After the run, prepare to collect the fractions in the harvest box (containing 20 plastic tubes, 12 mm x 75 mm, 5-6 mL volume) connected to the vacuum pump by pressing the harvest button ON in the IEF unit.
- Align the 20-collection pins with the 20-collection ports of the focusing cell that is sealed with the tape.
- Push the collection pins through the sealing tape and turn the vacuum pump ON simultaneously (see Figure 2B, 2C & 2D).
NOTE: About 3 mL fractions from each chamber will be collected into the tubes for each time. Fractions can be used for subsequent downstream applications (SDS-PAGE for peptide quantification, TLC and bioassay for small molecules).
3. Separation and purification of proteins from C. albicans
- Grow single colony of C. albicans in yeast-peptone-dextrose (YPD) broth16 at 30 °C with shaking (200 rpm) for overnight.
- Collect the yeast cells by centrifugation (10,000 x g for 5 min).
- Suspend C. albicans yeast cells in ammonium carbonate (1.89 g/L) buffer containing 1% (v/v) beta-mercaptoethanol (β-ME) (1/10th of the culture volume) and rotate in a roller tube for 1 h at 5 °C15.
- Remove the yeast cells by centrifugation (10,000 x g for 5 min) and filter the protein extract through a 0.45 µm filter.
NOTE: Protein samples from C. albicans cytosol, membrane or cell wall can be prepared and used for IEF fractionation. Similarly, proteins from bacteria or other biological samples (animal tissue extract) can be used after removing any salt by appropriate methods (e.g., by dialysis or using desalting columns). - Dialyze the protein extract in a dialysis tubing (3,500 MWCO) against water for 15 h at 4 °C. Estimate the protein concentration by the Bradford dye-binding method using gamma globulin as a standard17.
- Use 500 mg of total protein in 60 mL of water containing 1% (v/v) ampholyte (pH 5-8) for the fractionation.
NOTE: Since a broad-range ampholyte (pH 3-10) does not enrich certain non-glucan attached cell wall proteins of C. albicans well, use a narrow-range (pH 5-8) ampholyte. An ampholyte concentration of up to 2% can be used if the sample protein concentration is more than 2 mg/mL; this minimizes protein aggregation during focusing. Always keep the samples, ampholytes, and water precooled on ice. - Repeat steps 1.1- 1.7 to prepare the IEF unit and use the protein solution from step 3.6 to load into the IEF cell.
- After 4 h of focusing at a constant 15 W, harvest protein fractions (1-20) as described above (steps 2.9-2.11) and analyze on 12.5% SDS-PAGE after reducing and boiling the protein samples18.
- Stain the resolved proteins by staining the SDS-PAGE gel with the Coomassie blue dye (0.01%) solution for 2-3 h at room temperature on a rocker. Destain the gel and record the gel image using a gel imager.
NOTE: Coomassie blue dye (0.01%) can be prepared by mixing 0.01 g of Coomassie blue powder in a destaining solution containing 40% methanol and 10% acetic acid.
4. Bioactivity of purified small molecules from plant extract Gymnema sylvestre
- Grow C. albicans in yeast cells as in step 3.1.
- To prepare cell suspension, dilute overnight culture of C. albicans yeast cells (1/1000 dilution) into a fresh RPMI cell culture medium supplemented with 50 mM glucose.
NOTE: C. albicans converts from yeast growth to hyphae under hyphal inducing conditions (RPMI at 37 °C). Gymnemic acid small molecules are shown to inhibit the conversion of yeast cells into hyphae under hypha inducing conditions16. We aimed to determine if liquid-phase-IEF-separated G. sylvestre extract contain these bioactive molecules. - From the prepared cell suspension, add 90 μL to each well of the 96-well plate.
- From each fraction (1-20 harvested fractions of G. sylvestre extract obtained from liquid-phase IEF, Figure 4), add 10 μL into the wells with 90 μL of the above cell suspension (step 4.2). Perform the assay in triplicate.
- Add 10 μL of water that contains ampholyte (1%) into separate wells with 90 μL of C. albicans yeast cell suspension as a negative control.
NOTE: Ampholytes have bioactivity potential and so it is essential to include an ampholyte control while performing any bioassay19. - Incubate the 96-well plate at 37 °C for 12 h and observe the inhibition of C. albicans yeast-to-hypha conversion under the microscope16. Also, determine the percentage of inhibition of yeast-to-hypha conversion.
Representative Results
Separation and purification of small molecules and peptides from Gymnema sylvestre plant extract
Using the preparative liquid-phase IEF method, we fractionated medicinal plant extracts and cell surface proteins from a human pathogenic fungus, C. albicans. A schematic of these fractionation protocols is shown in Figure 1.
From 20 fractions of G. sylvestre extract obtained from liquid-phase IEF, the dark-colored molecules (terpenoid saponins) were found to migrate and be enriched at the anode end (pH 2-3) and the light-yellow clear fractions were observed at the cathode end (pH 8-9) (Figure 2). Aliquots (20 μL) from each fraction (1-20) were resolved on 15% SDS-PAGE after reducing and boiling the samples. A Coomassie blue-stained gel shows the diffused polypeptide band of about 5 kDa that is enriched in fractions 16-19 (Figure 3). It has been reported that the G. sylvestre plant contains a 35 amino acid gurmarin basic polypeptide with the predicted molecular weight of 4,209 Da20. Bacteria, plants and animals contain peptides; many of them are circular (knottins) and stable with wide range of biological activities such as insecticidal and antimicrobial properties21,22.
Biological activity of separated gymnemic acids
The G. sylvestre plant also contains gymnemic acids (terpenoid saponins) as major constituents16,23,24. As expected, these small molecules in fraction 1 and the next few fractions were not detected by SDS-PAGE and Coomassie staining (Figure 3) since they are non-proteinaceous. However, these small molecules can be separated by TLC and detected under UV light (Figure 4A, lane F1). Fraction 10 did not contain a detectable amount of these small molecules on TLC suggesting most of the organic small molecules were enriched in fractions 1-3. Gymnemic acids (GAs) molecules were shown to inhibit C. albicans yeast-to-hypha transition16,25. We assayed all 20 fractions collected in this study for their inhibitory activity against C. albicans yeast-to-hypha conversion and hyphal growth16. The results are shown in Figure 4B,4C. The highest activity is observed in fraction 1, which agrees with the TLC results where several spots can be seen. Isomers of gymnemic acids exist and all have similar biological activities10. These isomers were separated in fraction 1-3 and show inhibition of C. albicans hyphal growth (Figure 4A,4C, fraction 1). The degree of hyphal inhibition was gradually decreased as it goes from 1 to 10. Little or no activity was obtained in fractions 10 and above.
Separation of cell surface proteins from pathogenic fungus, C. albicans
Results from liquid-phase IEF fractionation of C. albicans cell surface proteins are shown in Figure 5. These cell surface proteins play important roles in C. albicans adhesion and pathogenesis26. Several enriched proteins (arrows) in different fractions were observed. This may allow identification of their immunological reactions with Candida infected human serum and/or their identification by mass spectrometry. Similarly, proteins from other cellular fractions (e.g., cytoplasm and cell wall) can be fractionated using this IEF method. The liquid-phase IEF-based purification will allow identification of low abundance proteins from complex biological samples, when coupled with mass spectrometry analysis.
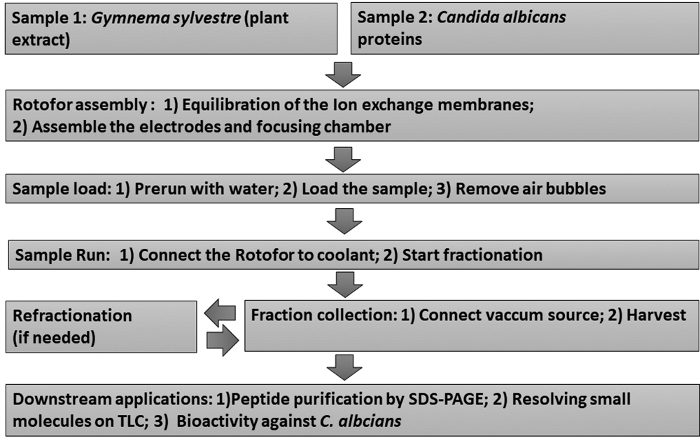
Figure 1: Flow chart showing the experimental workflow. Stepwise liquid-phase IEF fractionation procedures and subsequent downstream assays are depicted. Samples include Gymnema sylvestre leaf extract (sample 1) and Candida albicans non-glucan attached yeast proteins (sample 2). Please click here to view a larger version of this figure.
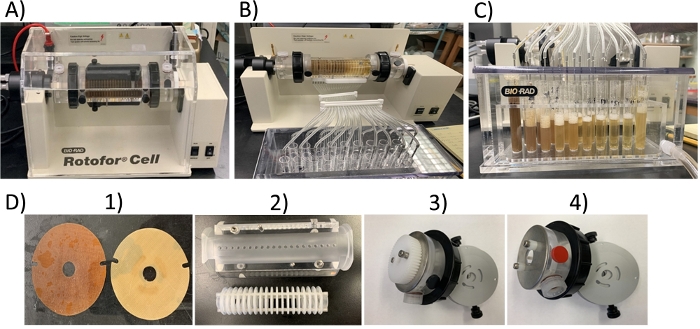
Figure 2: Liquid-phase IEF apparatus setup and fractionation of G. sylvestre plant extract. (A) During the run, (B) during fraction collection, (C) after fraction collection, and (D) liquid-phase IEF apparatus parts, 1) ion exchange membranes, 2) focusing chamber and membrane core, 3) electrode assembly (negative), 4) electrode assembly (positive). Please click here to view a larger version of this figure.
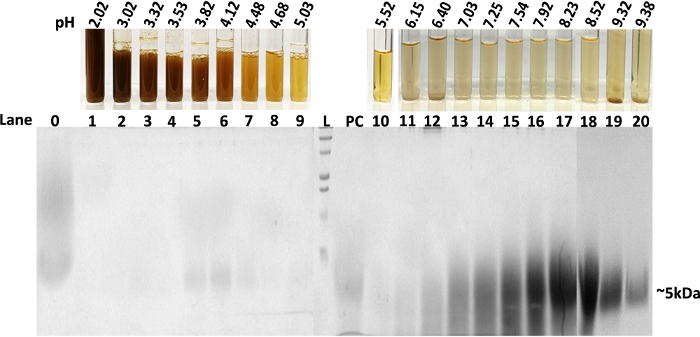
Figure 3: SDS-PAGE separations of the IEF focused plant extract fractions. L- ladder, PC- Positive control (peptide), 0 – input sample, 1-20 separated fractions. SDS-PAGE (15% resolving gel) was stained by Coomassie blue dye to visualize the resolved peptides (~5 kDa) from fractions 1-20. Fractions 1-3 contain small molecules (fraction 1 has darker color indicating enriched compounds) which cannot be stained/detected by Coomassie blue dye. Please click here to view a larger version of this figure.
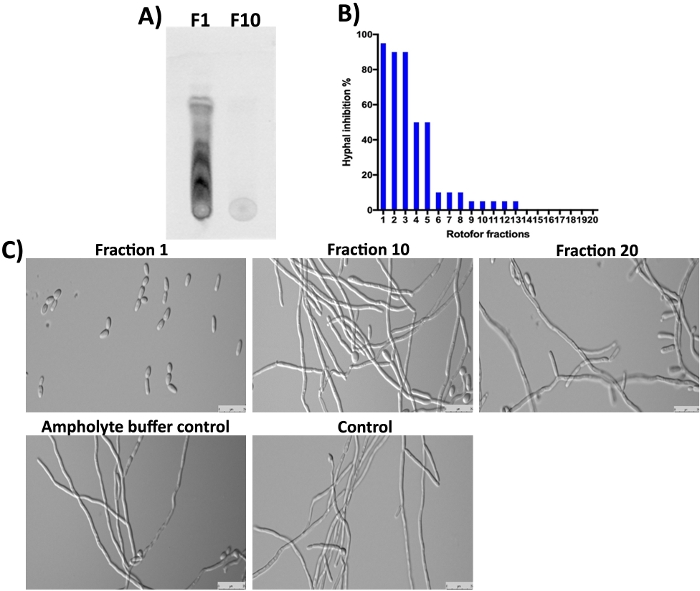
Figure 4: Analysis of IEF fractionated small molecules by TLC and determination of bioactivity against C. albicans. (A) Shows TLC analysis of small molecules from fraction #1 and #10. Activated silica gel plate was used to spot ~5 µL of samples and ran with toluene: chloroform: methanol solvent (5:8:3 ratio) until the solvent front reached the margin. TLC separated compounds were detected under an epifluorescence UV light (310 nm). (B) Shows the % inhibition of C. albicans yeast-to-hypha conversion by different fractions. (C) Demonstrates the cell morphology of C. albicans under hypha inducing conditions. Fraction #1 shows maximum (98%) inhibition of yeast-to-hypha conversion. Other fractions and controls show no inhibition of C. albicans hyphal growth after 12 hours of incubation at 37 °C. Please click here to view a larger version of this figure.
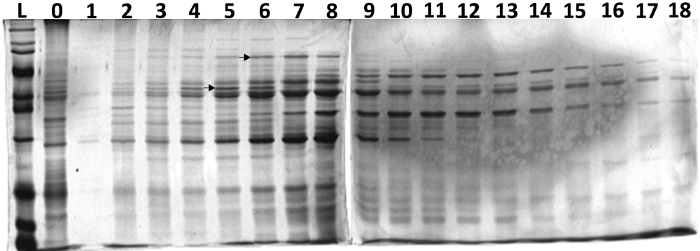
Figure 5: SDS-PAGE analysis of C. albicans cell surface proteins (non-glucan attached). L- ladder, 0 – input sample, 1-18 fractions collected after IEF in a standard liquid-phase IEF cell using a narrow range (pH 5-8) ampholyte. The image shows SDS-PAGE (12.5%) resolved proteins after staining with Coomassie blue dye. Several proteins were enriched in certain fractions (arrows). Please click here to view a larger version of this figure.
Discussion
Small molecules from natural product sources (e.g., plants) include complex secondary metabolites that are highly diverse in chemical structure. They are believed to be involved in plant defense mechanisms. In addition, polypeptides are also present in plant tissues22. These natural product small molecules are rich sources of test molecules for drug discovery and development. However, the difficult and tedious methods required for their isolation and purification limit their use for therapeutic applications. The liquid-phase IEF approach used in this report highlights the ability to separate these small molecules and polypeptides without compromising their bioactivities.
This IEF based method offers several advantages in separating biological molecules including concentration of purified proteins from a complex mixture, maintenance of native conformation during and after their focusing, and collection of samples as individual purified fractions without cross-contamination. When necessary, samples can be re-focused with a narrow pH range to purify protein isoforms. Since a miniature IEF focusing cell (~15 mL) is available, it can be used for smaller volumes of samples as well. The new finding from this report is that organic small molecules and peptides can be separated from a complex plant extract. Though it is difficult to agree that small molecules can be separated from natural product extracts by IEF, it is plausible for those compounds that are amphoteric. The gymnemic acids that were separated from the gurmarin peptide in the G. sylvestre extract appear to be amphoteric as they contain a carboxylic acid group or they behave so at least in the presence of the ampholyte used. Since glycosides are bioactive natural molecules similar to gymnemic acids, the IEF method can be used to separate them from complex natural sources. Similarly, peptides from natural products may also be isolated using this liquid-phase IEF approach.
Some of the limitations in this approach are that not all small molecules can be fractionated by the IEF method as they must be water-soluble and weakly amphoteric, at least. The extract used here was prepared by a 50% methanol extraction of dried plant material but is water-soluble. The use of the IEF method for solvent-soluble and amphoteric compounds remains to be seen as some of the organic solvents are incompatible with the liquid-phase IEF instrument components. The tendency of proteins to precipitate at their isoelectric points (pI) in low ionic strength solutions is well known. However, in a rotating IEF system, protein precipitation is reduced as the focused proteins remain in circulation at their pI point.
If one uses a high concentration of proteins in this IEF separation, precipitation may occur. To minimize protein precipitation and to improve protein focusing, urea can be used up to 3-5 M. Nonionic detergents such as CHAPS, digitonin and low concentration of detergents (0.1-1%) can also be used to reduce protein aggregation during IEF. However, urea and detergents need to be removed before analyzing the proteins for their activity and in some cases, these agents may affect protein functions. A few critical steps to consider during a liquid-phase IEF run include loading the IEF focusing cell without air bubbles, replacing the ion exchange membranes if they were damaged, and replacing the vent buttons after a certain number of repeated uses.
In conclusion, using the liquid-phase IEF method, we have shown the separation of bioactive gymnemic acids and gurmarin polypeptide from the G. sylvestre leaf extract. Further, liquid-phase IEF can be useful to enrich selective proteins from the complex crude extracts of pathogenic microbes.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
We are thankful for the funding sources from the Division of Biology and Johnson Cancer Research Center for BRIEF and IRA awards, respectively to GV. We also thank the K-INBRE postdoctoral award to RV. This work was supported in part by the Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM103418. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health. We thank the anonymous reviewers for their helpful comments.
Materials
| 0.45 µm syringe filter | Fisher scientfic | 09-720-004 | |
| 2-Mercaptoethanol | Sigma | M3148 | |
| Ammonium carbonate | Sigma-Aldrich | 207861-500 | |
| Bio-Lyte 3/10 Ampholyte | Bio-Rad | 163-1113 | |
| Bio-Lyte 5/8 Ampholyte | Bio-Rad | 163-1192 | |
| Compact low temperature thermostat | Lauda -Brinkmann | RM 6T | Set water cooling to 4 oC and it can be run even at 0 oC as when it passes through the Rotofor cooling core, the circulating water temperature will be around 5 or more depending on the voltage. |
| Coomassie Brilliant Blue R | Sigma-Aldrich | B7920 | |
| Dialysis tubing (3,500 MWCO) | Spectrum Spectra/Por | 132112T | |
| Gymnema plant leaf extract powder (>25% Gymnemic acids) | Suan Farma, NJ USA | ||
| Incubator | Lab companion | SI 300R | |
| Microscope | Leica | DM 6B | |
| Mini protean electrophoresis | Bio-Rad | ||
| pH meter | Mettler Toledo | S20 | Useful to determine the pH of the Rotofor (liquid-phase IEF) fractions |
| Rotofor | Bio-Rad | 170-2972 | http://www.bio-rad.com/webroot/web/pdf/lsr/literature/M1702950E.pdf (Rotofor Instruction manual for assembling the unit) |
| RPMI-1640 Medium | HyClone | DH30255.01 | |
| Sealing tape | Bio-Rad | 170-2960 | Scotch tape may also be used. |
| Sorvall legend micro 17 centrifuge | Thermo scientific | 75002432 | |
| TPP tissue culture plate -96 well flat bottom | TPP | TP92696 |
Referanslar
- Jankowska, U., et al. Optimized procedure of extraction, purification and proteomic analysis of nuclear proteins from mouse brain. Journal of Neuroscience Methods. 261, 1-9 (2016).
- Pergande, M. R., Cologna, S. M. Isoelectric Point Separations of Peptides and Proteins. Proteomes. 5 (1), (2017).
- Stoyanov, A. IEF-based multidimensional applications in proteomics: toward higher resolution. Electrophoresis. 33 (22), 3281-3290 (2012).
- Vesterberg, O. A. Y. . Method of Isoelectric Fractionation. , (1964).
- Vesterberg, O., Svensson, H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chemica Scandinavica. 20 (3), 820-834 (1966).
- Righetti, P. G. . Isoelectric Focusing: Theory, Methodology and Applications. , 1 (1983).
- Vesterberg, O. Synthesis and Isoelectric Fractionation of Carrier Ampholytes. Acta Chemica Scandinavica. 23, 2653-2666 (1969).
- Bier, M. Recycling isoelectric focusing and isotachophoresis. Electrophoresis. 19 (7), 1057-1063 (1998).
- Bier, M., Palusinski, O. A., Mosher, R. A., Saville, D. A. Electrophoresis: mathematical modeling and computer simulation. Science. 219 (4590), 1281-1287 (1983).
- Ayala, A., Parrado, J., Machado, A. Use of Rotofor preparative isoelectrofocusing cell in protein purification procedure. Applied Biochemistry and Biotechnology. 69 (1), 11-16 (1998).
- Wagner, L., et al. Isolation of dipeptidyl peptidase IV (DP 4) isoforms from porcine kidney by preparative isoelectric focusing to improve crystallization. Biological Chemistry. 392 (7), 665-677 (2011).
- Hosken, B. D., Li, C., Mullappally, B., Co, C., Zhang, B. Isolation and Characterization of Monoclonal Antibody Charge Variants by Free Flow Isoelectric Focusing. Analytical Chemistry. 88 (11), 5662-5669 (2016).
- Yu, J. J., et al. Francisella tularensis T-cell antigen identification using humanized HLA-DR4 transgenic mice. Clinical Vaccine Immunology. 17 (2), 215-222 (2010).
- Riyong, D., et al. Size and charge antigens of Dirofilaria immitis adult worm for IgG-ELISA diagnosis of bancroftian filariasis. Southeast Asian Journal of Tropical Medicine and Public Health. 41 (2), 285-297 (2010).
- Vediyappan, G., Bikandi, J., Braley, R., Chaffin, W. L. Cell surface proteins of Candida albicans: preparation of extracts and improved detection of proteins. Electrophoresis. 21 (5), 956-961 (2000).
- Vediyappan, G., Dumontet, V., Pelissier, F., d’Enfert, C. Gymnemic acids inhibit hyphal growth and virulence in Candida albicans. PLoS One. 8 (9), 74189 (2013).
- Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 72, 248-254 (1976).
- Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227 (5259), 680-685 (1970).
- Riazi, S., Dover, S., Turovskiy, Y., Chikindas, M. L. Commercial ampholytes used for isoelectric focusing may interfere with bioactivity based purification of antimicrobial peptides. Journal of Microbiological Methods. 71 (1), 87-89 (2007).
- Kamei, K., Takano, R., Miyasaka, A., Imoto, T., Hara, S. Amino-Acid-Sequence of Sweet-Taste-Suppressing Peptide (Gurmarin) from the Leaves of Gymnema-Sylvestre. Journal of Biochemistry. 111 (1), 109-112 (1992).
- Craik, D. J. Chemistry. Seamless proteins tie up their loose ends. Science. 311 (5767), 1563-1564 (2006).
- Craik, D. J., Daly, N. L., Bond, T., Waine, C. Plant cyclotides: A unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. Journal of Molecular Biology. 294 (5), 1327-1336 (1999).
- Stoecklin, W. Chemistry and physiological properties of gymnemic acid, the antisaccharine principle of the leaves of Gymnema sylvestre. Journal of Agricultural and Food Chemistry. 17 (4), 704-708 (1969).
- Liu, H. M., Kiuchi, F., Tsuda, Y. Isolation and structure elucidation of gymnemic acids, antisweet principles of Gymnema sylvestre. Chemical & Pharmaceutical Bulletin (Tokyo). 40 (6), 1366-1375 (1992).
- Veerapandian, R., Vediyappan, G. Gymnemic Acids Inhibit Adhesive Nanofibrillar Mediated Streptococcus gordonii-Candida albicans Mono-Species and Dual-Species Biofilms. Frontiers in Microbiology. 10, 2328 (2019).
- Chaffin, W. L. Candida albicans cell wall proteins. Microbiology and Molecular Biology Reviews. 72 (3), 495-544 (2008).

