Ocular Kinematics Measured by In Vitro Stimulation of the Cranial Nerves in the Turtle
Özet
This protocol describes how to use an in vitro isolated turtle head preparation to measure the kinematics of their eye movements. After removal of the brain from the cranium, cranial nerves can be stimulated with currents to quantify rotations of the eye and changes in pupil sizes.
Abstract
After animals are euthanized, their tissues begin to die. Turtles offer an advantage because of a longer survival time of their tissues, especially when compared to warm-blooded vertebrates. Because of this, in vitro experiments in turtles can be performed for extended periods of time to investigate the neural signals and control of their target actions. Using an isolated head preparation, we measured the kinematics of eye movements in turtles, and their modulation by electrical signals carried by cranial nerves. After the brain was removed from the skull, leaving the cranial nerves intact, the dissected head was placed in a gimbal to calibrate eye movements. Glass electrodes were attached to cranial nerves (oculomotor, trochlear, and abducens) and stimulated with currents to evoke eye movements. We monitored eye movements with an infrared video tracking system and quantified rotations of the eyes. Current pulses with a range of amplitudes, frequencies, and train durations were used to observe effects on responses. Because the preparation is separated from the brain, the efferent pathway going to muscle targets can be examined in isolation to investigate neural signaling in the absence of centrally processed sensory information.
Introduction
Rationale for Using Red-eared Slider Turtles in Electrophysiological Experiments:
Red-eared slider turtles (Trachemys scripta elegans), are considered one of world's worst invasive species1 and can indicate that an ecosystem is in trouble. The reason why red-eared slider turtles are so successful is poorly understood but it may in part be due to their tolerant physiology and possession of nervous tissues that can survive under hypoxic conditions2,3,4. Using them for experimentation does not threaten their numbers and with minimal efforts, electrophysiological preparations can remain viable over extended durations, as long as 18 hours5,6. The benefit is similar to the advantage of using invertebrate animals such as crayfish7, which also have the ability to withstand low levels of oxygen8.
Techniques for Measuring Eye Movements:
Approaches to measure eye movements in frontal-eyed animals using non-human primates have been well developed9. The eye rotates in the orbit around three axes: horizontal, vertical, and torsional. The magnetic search coil method is generally considered the most reliable for measuring rotations, but is invasive, requiring small coils to be inserted into the scleras of animals10,11. Video-based systems also can measure rotations and have the advantage of being non-invasive. The development of better cameras along with innovative image processing have enhanced their functionality making video-based systems an attractive alternative to consider12,13,14.
The techniques developed for measuring eye movements in nonmammals have been much less significant. Measures are either low resolution or describe only some of the rotations15,16,17,18. The lack of development can be partially blamed on the difficulty in training nonmammals to follow visual targets. Although eye movements have been well studied in red-eared slider turtles19,20,21,22,23,24,25,26,27,28,29,30, because of the challenge in training animals to track targets, the precise kinematics of their eye movements is poorly understood.
Red-eared slider turtles are generally considered lateral-eyed vertebrates, but because they can fully retract their heads into their shell31, significant occlusion of the lateral visual fields by the carapace occurs32. The result is that their visual line of sight is forced toward the front, making them behave more like frontal-eyed mammals. Therefore, their use as a model for developing approaches for measuring eye movements also offers a unique evolutionary perspective.
The protocol described in this work uses an in vitro isolated head preparation to identify the kinematics of the eye movements in red-eared slider turtles. Brains are dissected from the skulls leaving the cranial nerves intact. Heads are placed into a gimbal to calibrate eye movements and evoke responses by electrical stimulation of the cranial nerves innervating the eye muscles. Measures of rotations by the eyes are done by a video-based system, using software algorithms, which track the dark pupil and the markings of the iris. The preparation provides the opportunity to measure kinematics of both extraocular (i.e., horizontal, vertical, and torsional rotations)32 and intraocular (i.e., pupil changes)33 movements.
Model System for Analysis of Efferent Neural Pathways:
More generally, the approach provides investigators the chance to study how efferent neural signals generate eye movements when muscles start from their relaxed states and in the absence of integrated sensory information processed by the brain32,33. Therefore, the eye kinematics can be examined in a model system in which they are solely processed by the efferent neural pathway leaving the brain and synapsing onto the muscles.
Protocol
NOTE: Red-eared slider turtles, both male and female, were purchased from a vendor. Turtles were housed in a warm animal suite containing two 60-gallon tubs equipped with brick islands for sunning under 250-W infrared lights. The environment was maintained on a 14/10-h light/dark cycle with the water temperature at 22 °C. Lights were turned on at 6:00 am and turned off at 8:00 pm. The tanks equipped with filtering systems were cleaned weekly, and turtles were fed ad libitum every other day. The care of red-eared slider turtles and all of the following experimental procedures described here32,33 were approved by the Institutional Animal Care and Use Committee (IACUC) at Lafayette College.
1. Equipment Setup
- Prepare turtle Ringer's solution. Add the following to distilled water in this order: sodium chloride 96.5 mM (58.44 g/mol), potassium chloride 2.6 mM (74.56 g/mol), magnesium chloride 2.0 mM (203.31 g/mol), sodium bicarbonate 31.5 mM (84.01 g/mol), dextrose 20.0 mM (180.16 g/mol), concentrated hydrochloric acid to adjust the pH to 7.51, and calcium chloride 4.0 mM (110.98 g/mol) (see Table of Materials). Mix the solution while adding each salt.
CAUTION: Concentrated HCl is hazardous (skin, eyes, inhalation, and ingestion hazards). - Make tips for the suction electrodes from 5-cm-long capillary glass (see Table of Materials), by fire-polishing to different sizes of inner diameters in order to accommodate cranial nerves of varying thickness.
- Use a small file to etch a line across a piece of capillary glass. Place in tissue paper and break in half.
- Slowly roll one of the ends of the capillary glass into the flame of a Bunsen burner. Periodically examine the tip for size, smoothness, and symmetry using a dissection scope and a fiber optic light source (see Table of Materials).
NOTE: For turtles with head widths between 20 and 30 mm, optimal fitting inner diameter sizes typically range from 0.4 to 0.8 mm for the oculomotor nerve (nIII), 0.3 to 0.6 mm for the trochlear nerve (nIV), and 0.2 to 0.4 mm for the abducens nerve (nVI).
- Clean and organize Rongeurs, a blunt dissection probe, microscissors, fine forceps, curved forceps, and a scalpel handle with blades (see Table of Materials) for the dissection.
NOTE: Sterilization of instruments is optional.
2. Anesthesia and Euthanasia
- Place the turtle in an ice bucket for 60 min to cryoanesthetize it.
- Euthanize the turtle by decapitation using a small animal guillotine (see Table of Materials).
- Gently pry the jaws of the animal open with a small weighing spatula so that a hook can be inserted and turned to fit under the tip of the upper jaw.
- Pull with steady pressure to extend the animal's head through the guillotine. Swiftly decapitate the animal.
- Place turtle head in a dissection dish. Have enough turtle Ringer’s solution on hand to irrigate the tissue. Oxygenize the solution with 95/5% O2/CO2 (see Table of Materials).
- Maintain the tissue at 4 °C by placing ice around the outside of the dish.
3. Dissection
- Use the dissection scope with a fiber optic light source to carry out the dissection.
- Remove the lower jaw. Place a blunt dissection probe through the mouth to provide easier handling of the head. Cut the joint connecting the dentary bone to the cranium with a scalpel. Use rongeurs to pull the lower jaw away from the cranium. Use rongeurs to pull off the skin and muscles from their attachments at the dorsal and lateral regions of the cranium.
- Remove the vertebral column.
- Identify the vertebral column at the caudal end of the cranium. Bend the vertebral column ventrally to expose the spinal cord. Use microscissors to snip the spinal cord. Use rongeurs to remove the vertebral column and other tissue from the cranium by pulling caudally.
- Remove the brain from the cranium after cutting the cranial nerves.
- Starting at the foramen magnum, use rongeurs to cut two incisions on the dorsal cranium. Make cuts superficial to avoid damaging the brain beneath.
- Use rongeurs to carefully pull off the dorsal cranium. Use microscissors to remove the meninx to expose the rest of the brain. Remove enough meninx until the olfactory bulbs, in the anterior cranial cavity, can be identified (see Figure 1A). Continue to irrigate the brain with turtle Ringer's solution, as necessary.
- Use curved forceps to gently pull the cerebrum caudally and produce slight tension on the cranial nerves. Carefully cut away and remove the olfactory bulbs and cerebrum with curved forceps.
- Use microscissors to gently push the midbrain toward the midline to expose the cranial nerves; nIII, about 0.6 mm, can be seen in front of nIV, and the diameter of nIV will be slightly less than nIII. Cut nIII and nIV where they attach to the midbrain (see Figure 1B). Repeat this on the other side.
- Cut the left and right optic nerve (nII) with microscissors. Then tilt the brainstem to one side. Observe nVI emerging from the ventral surface near the junction of the pons and the medulla (see Figure 1C); the diameter of nVI is small and approximately 0.3 mm. Cut both the left and right nVI.
- Remove the remaining parts of the brainstem from the turtle with fine forceps and microscissors. Once the cranium is empty, examine the cranial cavity floor. Identify nIII, nIV, and nVI.
- Remove the upper and lower eye-lids with fine forceps and microscissors.
4. Calibration of Eye Movements
- Use a rigid table (see Table of Materials) to support the placement of the gimbal and other instruments. Place the turtle head into the gimbal chuck so that the dorsal surface of the head is parallel to the horizon using a small bubble-level resting across the skull. Roughly position one of the eyes at the center of the gimbal's horizontal and vertical rotations.
- Place the infrared camera, fitted with an infrared light emitting diode (LED), which is part of the video-based eye tracking system (see Table of Materials), at a viewing distance of approximately 12 cm from the turtle's eye.
- Angle the camera 45 degrees above the line of sight of the eye. The LED should be at the 11 o’clock position when looking at the camera lens. Center the LED along the optical axis of the eye. The camera will be slightly off axis (as viewed from above the eye).
- Adjust the distance of the camera from the eye so that the camera view is maximally filled by the eyeball. Ensure that the corners of the eyes (canthi) are at the edges of the horizontal view.
- Connect the camera to the video-based eye tracking system to process the data. Split the signal to a DVD-recorder to capture the raw video. Focus the camera to obtain a clear image of the eye. Take care to fine-position the eye at the center of the camera view using the three degrees of linear adjustment (x, y, z) provided with the gimbal.
- Detect the dark pupil by setting the threshold and contrast appropriately using the program provided with the video-based eye tracking system.
- Using the mouse, click on the "Video" menu and under "Mode" select "High Precision" to capture images at a sampling rate of 30 Hz (resolution of 640 pixels x 480 lines). Also under "Video", select "Dark Pupil" for "Pupil Type" and "Ellipse (rotated ellipse)" for "Pupil Segmentation Method".
- In the "EyeCamera" window, click on the "Pupil Search Area Adjustment" icon (small vertical rectangle with a dot in the center). Use the mouse to drag out a rectangle that limits an area around the pupil. Avoid dark areas that could be confused with the pupil.
- In the "Controls" window, confirm that boxes for "Auto Image" and "Positive-Lock Threshold-Tracking" are checked. Click on "Auto-Threshold" to optimize the density of scanning, which will show as green dots over the dark pupil.
- Calibrate the video-display of the video-based eye tracking program to the rotations of the gimbal 12.5 ° (+/-) around its horizontal axis and 10 ° (+/-) around its vertical axis.
- In the "Controls" window, click on "Display". Check the boxes under both "Gaze" and "Stim" for "Gaze Point", "Calib Region", and "Geometry Grid". After checking the boxes under "Geometry Grid", a window will pop up and say, "The stimulus display geometry must be measured before the geometry grid can be displayed. Do you wish to do this now?" Select "Y" for yes.
- Using the mouse, click on the "Windows" menu and select "Stimulus". The "Stimulus" window will open showing a vertical and horizontal line crossing at the center of the display. Measure the lengths of the lines to the nearest mm. Press "Esc" on the keyboard to exit the "Stimulus" window.
- Using the mouse, click on the "Stimulus" menu and select "Geometry Setup". Input the lengths of the lines that were just measured. Adjust the viewing distance so that the degrees/line equal 25 ° for the horizontal line and 20 ° for the vertical line. Click on the "Store" button and close the window.
- In the "EyeSpace" window, select the number of calibration "Data Point" to be "9". With the turtle's eye positioned at the center, click on the center data point and click the "Re-present" button.
NOTE: The "Stimulus" window will open, and "Get Ready" will appear at the center of the screen. A box will appear at the center location and then disappear. On its disappearance, the "Stimulus" window will close. The center position should now be calibrated. - Repeat the procedure by rotating the gimbal right/left, +12.5 °/-12.5 °, and up/down +10 °/-10 ° to calibrate the remaining data points.
- To calibrate torsional rotation, use the template fitting algorithm provided with the video-based eye tracking program. The algorithm sets a zero position based on markings of the iris and computes an angle of rotation when the markings become offset from the centroid of the pupil.
- Using the mouse pointer, click on the "Windows" menu and select "Torsion". Click the "START" button in the "Torsion" window. In the "EyeCamera" window, an arc will appear over the image of the eye.
- Adjust the radius, the angle, and the length of the arc using the sliders at a location where irregular markings are present in the iris. Check the boxes for "Real-time graphics" and "Auto-Set after adjust". If necessary, adjust the brightness and contrast in the Controls window and re-threshold the dark pupil (see step 4). Click the "Set Template" button.
- Place a ruler in the same focal plane as the pupil and record the width of the full camera view. The value will be used later to determine the actual width of the pupil.
5. Positioning of Suction Electrode on Cranial Nerve to Evoke Eye Movements
- Carefully place a pin reference electrode into the connective or muscle tissue remaining on the head.
- Place the suction electrode (see Table of Materials) on the cranial nerve using a micromanipulator and dissection scope mounted on a boom. Use a fiber optic light source to view and guide the placement.
- Match the size of a nerve to a capillary glass tip. Trial and error is necessary to obtain a best fit around the diameter of a nerve (see step 1.2 for size recommendations). Place the glass tip onto the suction electrode. Fill the suction electrode with Ringer's solution and adjust the volume within the syringe to about half of its capacity.
- Carefully move the glass tip of the electrode using the micromanipulator to a position above the cut-end of the selected nerve. Ensure that Ringer's solution fills the cranium and the tip is below its surface. If necessary, use modeling clay to dam up locations where the Ringer's solution is leaking out of the cranium.
- Pull back on the plunger of the syringe.
NOTE: The vacuum will draw the nerve into the end of the capillary tip. A good fit is indicated by the nerve remaining within the tip with little or no additional vacuum applied.
6. Stimulation of Cranial Nerve and Analysis of Eye Movements
- Use a general purpose nerve/muscle stimulator with a current isolation device (see Table of Materials) to stimulate the cranial nerve via the suction electrode.
- Connect the suction electrode to the current isolation device using a cable. Connect the lead from the pin reference electrode to the ground connection of the isolation device.
- Select parameters of the currents using the dials and switches on the stimulator and isolation device. Use a range of currents from 1 to 100 µA, with frequency of 10 to 400 Hz. Use 1- or 2-ms pulses in trains lasting 100, 500, or 1,000 ms.
- Record the timing of stimulations.
NOTE: Transistor-transistor-logic (TTL) pulses are synchronized with the deliveries of the currents from the stimulator and communicate in real-time via a cable to input channels of the video-based eye tracking system. A software module provided with the video-based eye tracking program controls the communication.- To visualize the timing of the current applications and their influence on eye movements, click on the "PenPlots" menu. Select "X Gaze Position", "Y Gaze Position", "Torsion", and "Pupil Width" to show real time raw data plots for X and Y eye positions, torsion, and pupil width. Also select the "Seconds & Markers" from the "PenPlots" menu to show a timing plot with tick marks, which appear at 1 s intervals.
NOTE: A capital letter "T" will appear marking the onset of the TTL pulse, occurring simultaneously with the current application. - To store the data of eye movements evoked by currents, click on the "File" menu and select "New Data File" under "Data". Input a file name and press "Enter". Saving data can be paused and then restarted using the combination of key commands, "Ctrl" + "p". When an experimental session is completed, select "Close Data File" off "File" menu under "Data".
- To help keep track of the type of currents applied, click on the "Windows" menu and select "Data Pad". The "KeyPad/DataMarker" window will appear. Click on a letter or a number to identify parameters of the current stimulations being delivered to the nerve.
NOTE: For example, "X" could stand for 10 µA. Clicking on "X" stores its entry into the data file in real time for post hoc analysis. It also appears on the "PenPlot" for "Seconds & Markers" for ongoing observation.
- To visualize the timing of the current applications and their influence on eye movements, click on the "PenPlots" menu. Select "X Gaze Position", "Y Gaze Position", "Torsion", and "Pupil Width" to show real time raw data plots for X and Y eye positions, torsion, and pupil width. Also select the "Seconds & Markers" from the "PenPlots" menu to show a timing plot with tick marks, which appear at 1 s intervals.
- Analyze data from the eye tracker system.
- Open the saved data file, which is in a text delimited format, into a spread sheet program of choice to organize the data and carry out statistical analysis.
- Convert the pupil width values stored in the file to real sizes in mm.
- Convert the values of X and Y eye positions and torsions to units of degree and use conventions for describing eye movements; therefore, positive directions of rotations: intorsion, elevation, and adduction; and negative directions of rotations: extorsion, depression, and abduction.
- Copy the header information onto a new worksheet. This will include values for the screen size (width and height) and the viewing distance. Eight columns of data collected at a rate of 30 Hz will follow below the header information.
- Go back to the worksheet containing the raw data. Do a "Find" for "X" in the last column titled "Marker" to locate where stimulation was applied with 10 µA. Find incidence of "T", marking the beginning of the current stimulation. Copy 15 rows (0.5 s) of data occurring before "T" and 90 frames after "T" (3.0 s); i.e., 3.5 s total. Paste the data in the new worksheet below the header information.
- Insert a blank column after "PupilWidth". In the blank column, convert to the calibrated dimension in mm:
Horizontal pupil diameter = "PupilWidth" × the dimension of camera view width - Insert 2 blank columns after both "X_Gaze" and "Y_Gaze". Normalize positions to the dimensions of the viewing screen: coordinates (0, 0) at the top left of the screen extending to (1, 1) at the bottom right. In the first blank column, translate positions to a coordinate system having (0, 0) at the center of the screen. Include the conversion to the dimensions of the screen in mm:
X = (0.5 × width) – ("X_Gaze" × width); Y = (0.5 × height) – ("Y_Gaze" × height)
NOTE: The sequence of operation is for the left eye. The sequence will need to be reversed for the right eye in order to follow convention of negativity for abduction and positivity for adduction. - In the second columns, use trigonometry functions to convert to angles (°) of rotation:
horizontal rotation = arctan (X/viewing distance);vertical rotation = arctan (Y/viewing distance) - Torsion is already shown in units of degrees, but to conform to convention of positivity for intorsion, if measuring is done on the left eye, multiply by -1. For the right eye, no multiplication is necessary. The program codes clockwise rotation as positive.
- Plot the pupil diameter and rotations as a function of time.
- Open the saved data file, which is in a text delimited format, into a spread sheet program of choice to organize the data and carry out statistical analysis.
Representative Results
Figure 1 shows stills of images taken from a video describing the dissection. Images provide typical locations of the nerves prior to cutting from the brain.
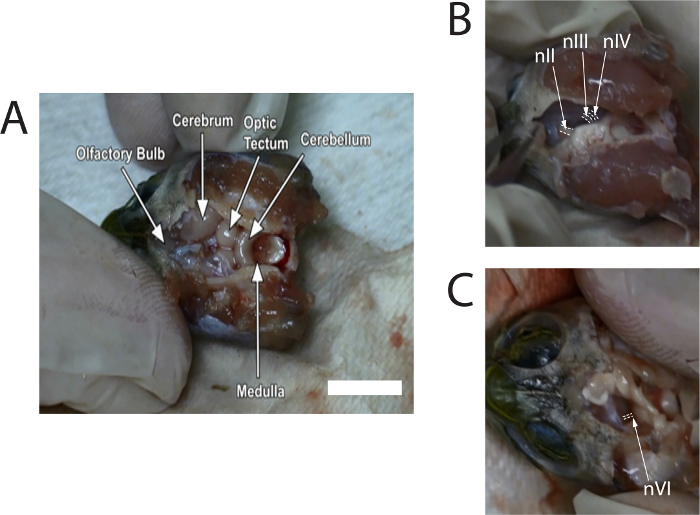
Figure 1: Stills of images captured from the video of the dissection to show locations of the optic nerve (nII), oculomotor nerve (nIII), trochlear nerve (nIV), and abducens nerve (nVI). (A) Image with labeled regions of the brain after removal of the meninx. White scale bar = 10 mm. (B) Image shows location of nII, nIII, and nIV where they connect to the right side of the midbrain (after removal of the olfactory bulbs and cerebrum). (C) Image shows location of nVI where it connects to the left side of the brainstem (after removal of the midbrain). White dashed lines are drawn around the nerves. Please click here to view a larger version of this figure.
Figure 2 shows the mean change in pupil diameter occurring after stimulating nIII. Although extrinsic eye movements also are observed, which change the location of the pupil, the measure of the action by the sphincter pupillae of the iris remains reliable. Measures are from one preparation and show the typical variability of responses to repeated current stimulations. Mean pupil diameter is reduced from 1.95 ± 0.01 mm to 1.60 ± 0.01 mm. The narrow standard deviation indicates a successful fit of the electrode to the nerve. When measuring among different preparations (N = 5), typical variability is ± 0.08 mm. Although still relatively narrow, the value is about eight times greater than the variability observed from a single preparation.
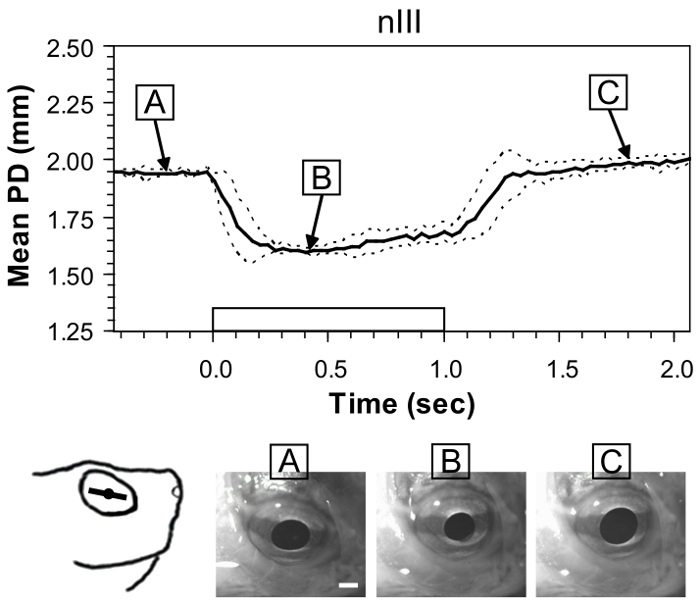
Figure 2: Example of pupil constriction evoked by stimulating the oculomotor nerve (nIII) in the whole-head preparation. Black trace is the mean pupil diameter (PD) from six stimulations in one preparation, and dashed lines show ± standard deviation (SD). Rectangular waveform on the x-axis denotes onset and offset of a 100-Hz train of 1-ms pulses with an amplitude of 50 µA. The sketch at the lower left shows the orientation ofthe iris line in the eye prior to stimulation,and images at the bottom show still frames from a representative trial before(A), during (B), and after stimulation (C). White scale bar = 1 mm. This figure has been reprinted with permission33. Please click here to view a larger version of this figure.
Measures of pupil responses do not require calibration of eye rotations; however, if measuring extrinsic movements, placement of the head in a gimbal must be carefully done to allow comparisons among preparations (Figure 3). When the head is placed in the gimbal with the dorsal surface of the skull parallel with the horizon, the iris line is offset from the horizon toward the nostril. Figure 3D shows a typical offset (28.6°) in a preparation. For three different preparations the mean offset was 30.1 ± 9.0°. An angle of offset within the standard deviation indicates acceptable fit within the gimbal.
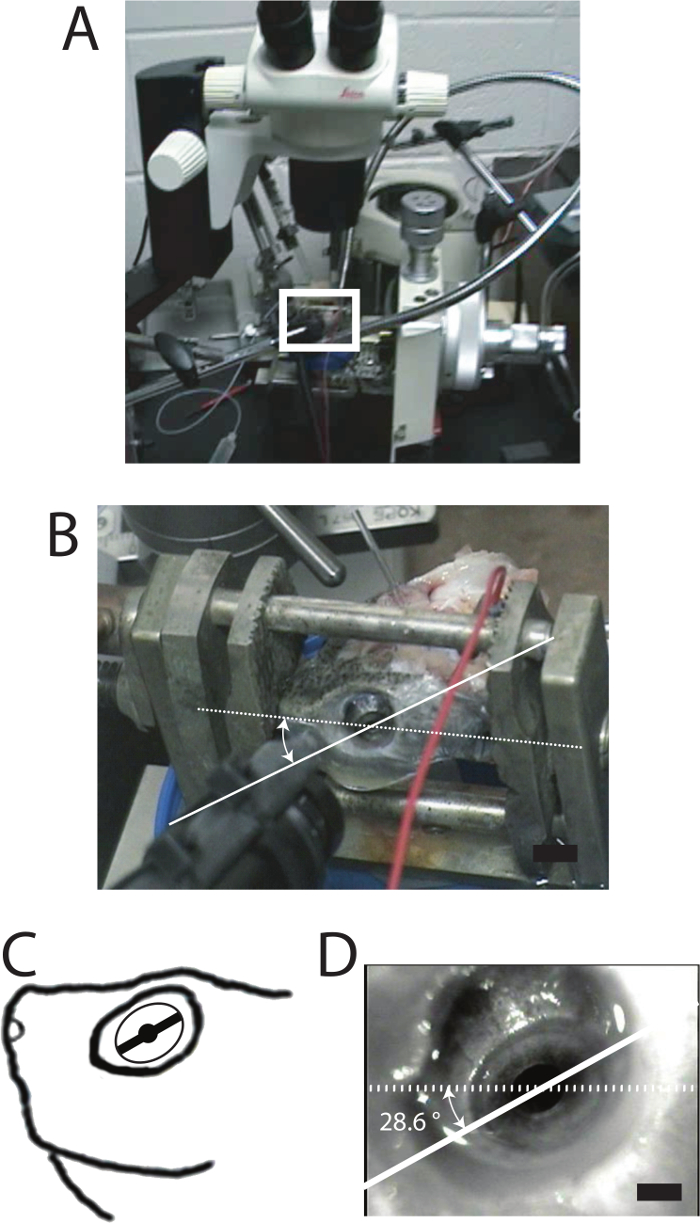
Figure 3: An isolated turtle head preparation positioned in the gimbal. (A) Region of the white rectangle shows the location of the turtle head in a photograph of the equipment setup. (B) Magnified view of the white rectangle (seven times). Dotted white line is drawn on the image from nostril to pupil center and is level with the horizon. A solid white line is superimposed and parallel to the iris line. (C) Cartoon of the left side of the head. (D) Image of the eye captured by the camera of the video-based eye tracking system. Black scale bar: 5 mm in B; 1 mm in D. This figure has been modified with permission32. Please click here to view a larger version of this figure.
Figure 4 shows the mean rotations of the eyes from ten preparations evoked by stimulation of the nIV going to the superior oblique muscle. Typical peak rotations for intorsion, elevation, and abduction (in response to 70 µA, orange trace) are 10.0 ± 5.7°, 2.8 ± 1.2°, and 3.0 ± 2.1° respectively. Magnitudes measured for rotations in single preparations to repeated stimulations are similar but with less variability (e.g., N = 5), 14.8 ± 1.0° for intorsion, 3.2 ± 0.2° elevation, and 2.4 ± 0.2° for abduction. The pattern of variabilities among different preparations compared to a single preparation is comparable (within one logarithmic unit) to what is observed for measures of pupil changes: 6 times greater for both intorsion and elevation, 11 times greater for abduction.
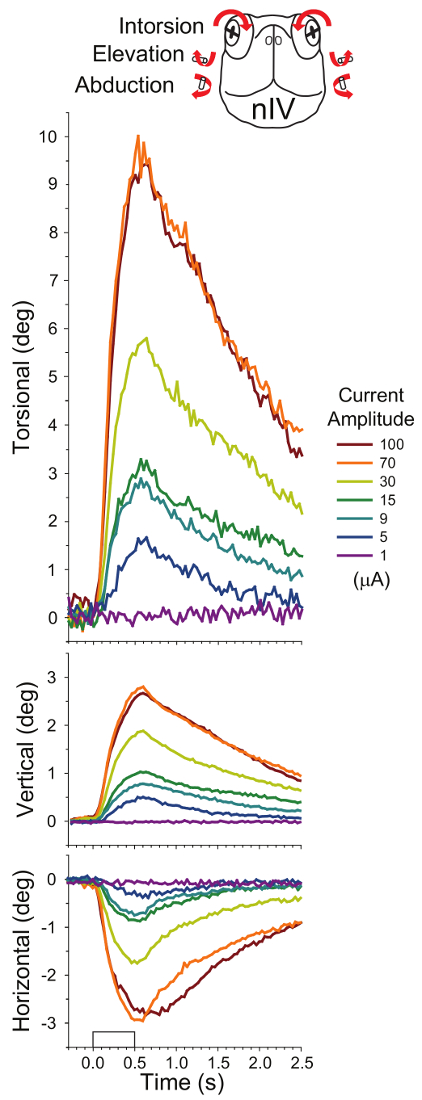
Figure 4: Mean eye rotations evoked after stimulating the left trochlear nerve (nIV) with 500-ms 100-Hz trains of 2-ms pulses in 10 preparations (six applied to the left side and four applied to the right side, five trials measured for each). The rectangular waveform on the x-axis of the bottom plot denotes timing of the stimulus. Responses from left and right eyes were not significantly different from each other. Intorsion was accompanied by elevation and abduction (see overlying cartoon of the head-on view of the turtle, whose arrows summarize the components of the eye movements). The eye movement amplitude roughly corresponds to the seven current amplitudes applied to nIV (see code in legend box). This figure has been reprinted with permission32. Please click here to view a larger version of this figure.
In contrast to nIV, which goes only to the superior oblique muscle, nIII and nVI innervate multiple muscles so movements are more challenging to interpret. Resultant eye movements depend on what motor units are recruited (Figure 5) (See Discussion). Consequently, variability from preparation to preparation can become significant. For example, nVI evokes intorsion, elevation, and abduction (Figure 5C). Abduction is likely from the motor units promoting the action of the lateral rectus; intorsion and elevation instead result from other motor units going to targets such as the retractor bulbi or the nictitating membrane.
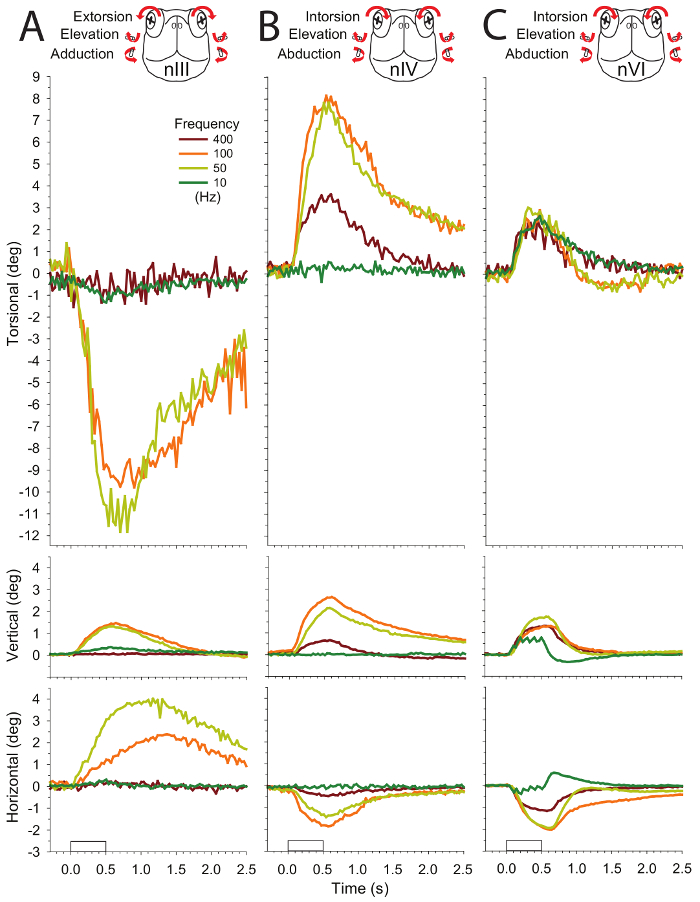
Figure 5: Mean eye movement responses evoked by stimulating three cranial nerves with 10-, 50-, 100-, and 400-Hz trains in four preparations. All of the stimulus trains were 500 ms in duration, consisting of 2-ms pulses with amplitude of 70 µA, a value above the threshold. Eye movements are displayed as before, with columns A–C showing responses to stimulation of nIII, nIV, and nVI, respectively. This figure has been reprinted with permission32. Please click here to view a larger version of this figure.
Because nIII innervates several targets (superior rectus, inferior rectus, inferior oblique, medial rectus, and the sphincter pupillae), analysis of the evoked movements are the most complex. For the example shown in Figure 5A, extorsion, adduction, and elevation occurs. Based on this, a reasonable interpretation is that the actions are mostly from the inferior oblique with possible contribution from the medial rectus. Superior rectus and inferior rectus may cancel each other. A higher variability of the actions for nIII likely stems from the greater branching of the nerve and the location of the cut for fitting the electrodes.
Discussion
Critical Steps:
The critical steps within this protocol are the following: 1) the dissection and the care taken to maintain the viability of the transected nerves; 2) the matching of sizes by the suction electrodes to cranial nerves to provide consistent responses; and 3) the placement of the head in the gimbal to provide adequate calibration of the rotations of the eye.
Troubleshooting:
The dissection can be challenging, but after completing it a few times, the steps should become relatively straight forward. If nerves appear non-responsive, the most likely cause is a failure of the dissection. During the removal of the brain only minimal tensions and pressures should be applied to the nerves during their cutting. Touching the nerves with metallic surgical instruments should also be avoided as much as possible, and so glass hooks and plastic forceps can substitute for metal tools if troubles persist34. Although the turtle tissues have the ability to survive variable levels of oxygen, the use of freshly made turtle Ringer's solution bubbled with 95/5% O2/CO2 and chilled is advisable; however, this is less critical. After all, the advantage of using the preparation is the survivability under varying oxygen levels and temperature fluctuations2,3,4,35. More important is the periodic irrigation of the preparation with turtle Ringer's solution to avoid drying out the tissue.
Making a set of tips of varying sizes for the suction electrode in advance of carrying out an experiment will improve chances of a good fit to the nerve. Similar to the dissection, fire polishing the tips takes some practice. Preserving a head with nerves from a prior experiment and using it as a reference while making the tips can help. Arranging the tips from small to large diameter size and storing them on some modeling clay allows for quick fitting during an experiment. For smaller tips, more time rolling the tips into flames is required. The tips should be free of cracks and possess smooth edges to allow for a good seal onto the nerve.
The orientation of the head in the gimbal must be adequate. If not, the axis of the measurements will be skewed. For example, a rotation to the right that is greater than the same increment to the left, indicates that the center of the eye is offset and needs to be shifted to the right. Some trouble also can occur in maintaining a reliable signal from the pupil during the rotations. Adjusting the illumination of the pupil by the infrared LED slightly can help achieve better results.
Limitations:
A shortcoming of the preparation is the difficulty in identifying the kinematics of actions by specific muscles. This is especially true for movements measured after stimulations of nIII and nVI. One way to resolve individual muscle actions is to make systematic transections of nerve branches in order to isolate a pathway traveling to a particular muscle. For example, we have been successful in cutting nIII distal to the ciliary ganglia and stimulating the short ciliary nerve to isolate the action of the sphincter pupillae33,36. Another approach is to mount a strain-gauge system on both the agonist and antagonist muscles and then correspond the movements with the activities of specific muscles37,38 . For that method, isolation of a pathway traveling to a specific muscle would not be as necessary.
The preparation also allows for passive stretching associated with visco-elastic elements acting in opposite directions on the corresponding muscles (e.g., lateral rectus versus medial rectus muscles, or sphincter dilator versus sphincter pupillae)39. To parse out visco-elastic elements, the responses obtained by stimulating transected nerves as described here can be compared to those obtained using a preparation in which the subcortical areas and the cerebellum are sparred; therefore this keeps the functional neural integrators intact, and then it is possible to stimulate single motoneuron areas within the brainstem innervating each muscle40.
Significance with Respect to Other Vertebrate Models:
Although cryoanesthesia is acceptable for turtles, this approach cannot be approved with work in warm-blooded animals41,42. Therefore, a pharmacological agent such as pentobarbital or ketamine is necessary. The agents, however, confound eye movements43. Nonetheless, the approach is useful for reptiles and could be applied to other lateral-eyed non-mammalians, such as amphibians and fish44,45, because it allows comparison of behaviors generated by an isolated efferent neural pathway to those made by an intact animal32,33.
Future Applications:
The preparation could serve as an assay for nervous tissue survivability. A protocol could be designed to systematically address how various factors influence eye movements. Approaches could be as simple as testing different temperatures or pharmacological agents including analgesics. Because movements are more difficult to interpret for nIII and nVI, their use is more limited. Therefore, nIV would likely be the best choice for manipulation.
However, to resolve individual muscle kinematics resultant from stimulating nIII and nVI, modification of the preparation would be necessary, such as making a systematic transection of branches of nerves or including strain gauges on targeted muscle sets. Lastly, measuring responses in a preparation where neural integrators are kept intact would enable determination of how visco-elastic elements could possibly be limiting quantifications of kinematics.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
The authors thank Mrs. Paulette McKenna and Lisa Pezzino in this study for secretarial support, and Mr. Phil Auerbach for technical support. The authors also thank Drs. Michael Ariel and Michael S. Jones (Saint Louis University School of Medicine) for introducing us to the in vitro isolated head preparation. Funding for support of this collaboration was provided by the Department of Biology (Robert S. Chase Fund), the Academic Research Committee, and the Neuroscience Program at Lafayette College. Lastly, this work is dedicated to Mr. Phil Auerbach, who passed away 28 September 2016; he decommissioned a scanning electron microscope and recognized the usefulness of its 5-axis stage for use in this protocol. His friendship and resourcefulness will be missed greatly.
Materials
| Red-eared slider turtles | Kons Scientific | Trachemys scripta elegans | Large size (carapace length 15-20 cm) |
| Sodium chloride | Sigma-Aldrich Co. LLC. | S5886 | |
| Potassium chloride | Sigma-Aldrich Co. LLC. | P5405 | |
| Magnesium choride | Sigma-Aldrich Co. LLC. | M7304 | |
| Sodium bicarbonate | Sigma-Aldrich Co. LLC. | S5761 | |
| Dextrose | Sigma-Aldrich Co. LLC. | C5767 | |
| Concentrated hydrochloric acid | Sigma-Aldrich Co. LLC. | H7020 | |
| Calcium chloride | Sigma-Aldrich Co. LLC. | C7902 | |
| pH meter | Oakton | pH 6+ | |
| Suction stimulation electrode | A-M Systems | 573000 | Bipolar suction electrode. Note that 573000 has been replaced with 573050. |
| Capillary glass | A-M systems | 626000 | Single-barrel borosilicate capillary glass without microfilament, length 10 cm, outside diameter 1.0 mm, inner diameter 0.50 mm |
| Alternative suction stimulation electrode | A-M Systems | 573050 | Bipolar suction electrode. Requires larger diameter capillary glass: 627000, outside diameter 1.2 mm, inner diameter 0.68 mm |
| Stereoscope | Lieca | GZ7 | Magnification range, 10x – 70x |
| Fiber optic light source | Amscope | HL250-A | 150W Fiber optical microscope illuminator light box |
| Rongeurs | Carolina Biological Supply Company | 625654 | stainless steel, straight spring, 5.25" |
| Blunt dissection probe | Carolina Biological Supply Company | 627405 | Huber mall probe, double-ended probe and seeker, 6" |
| Microscissors | Carolina Biological Supply Company | 623555 | Iris microdissecting scissors, stainless steel, 0.5" blades, 4.75" long |
| Fine forceps | Sigma-Aldrich Co. LLC. | F6521 | Jewelers forceps, dumont No. 5, inox alloy, 4.25" |
| Curved forceps | Sigma-Aldrich Co. LLC. | Z168696 | Medium tip, curved forceps, stainless steel, 4" |
| Scalpel handle | Sigma-Aldrich Co. LLC. | S2896 | Scalpel handles, No. 3, stainless steel |
| Scalpel blade | Sigma-Aldrich Co. LLC. | S2771 | Scalpel blades, No. 11, steel |
| Guillotine | Harvard Apparatus | 73-1918 | Kleine guillotine type 7575 |
| Spatula | Sigma | Z648299 | Micro spoon and spatula weighing set. Use small spatula: 5.9” long x 0.07” diameter handle with square end: 0.17” x 1.3” long, other end round: 0.17” x 1.27” long |
| Hook | Autozone | 98069 | SureBilt hook and pick set. Use grinder to dull sharp points of hook to prevent injury to animals mouth. |
| 95/5% O2/CO2 | Airgas, Inc. | X02OX95C2003102 | 5% Carbon dioxide balance oxygen certified standard gas mixture, size 200 Cylinder, CGA-296 |
| Regulator | Airgas, Inc. | Y11244D296-AG | Single stage brass 0-100 psi analytical cylinder regulator CGA-296 with needle outlet. Use brass adjustable airline pipe valve to go from 3/8", inner diameter, vinyl airline tubing connected to regulator to a 3/16", inner diameter, airline connection going to airstone or glass pasteur pipette. |
| Adjustable airline pipe valve | Doctors Foster and Smith | CD-12061 | Brass valve |
| Rigid table | Unknown | Unknown | Auto-clave door laid on top of a sturdy table. Nine 5" diameter tennis balls isolate vibrations from the top surface of the table. |
| 5" tennis ball | Petco Animal Supplies, Inc. | 712868 | Petco Jumbo Pet Tennis Ball: balls are unsliced and held within an integrated frame on the underside part of the autoclave door. |
| Alternative vibration isolation table | Newport Corporation | INT1-36-6-N | Rigid vibration control system, integrity 1: Surface dimensions, 3' x 6' |
| Gimbal | ISI, International Scientific Instruments, Inc. | Stage from SUPER III-A Scanning EM | 5-axis eucentric stage: X, Y, and Z linear movements, ±20 mm, 0.1 mm precision; Rotations, vertical, ±10°, and horizontal, ±12.5°, with 1.25° precision. Note: from decommission instrument. |
| Chuck for gimbal | Unknown | Unknown | Chuck from an old microtome of unknown manufacture was machined to fit the shaft of the specimen holder of the Scanning EM stage |
| Alternative gimbal | ThorLabs, Inc. | GN2/M with MBT602/M | Dual-axis goniometer (GN2/M) mounted on 3-axis microblock stage with thumbscrew adjusters (MBT602/M): design a chuck to hold turtle head with eye at 12.7 mm above top surface of goniometer (distance to point of rotation) |
| Video-based eye tracking system | Arrington Research, Inc. | ViewPoint EyeTracker, PC-60 | Tracking method: Infrared video by dark pupil; Black and white camera (Item BC02): 30 Hz, 640 x 480; System requirements: Windows 2000, XP, 7, 8, 8.1, 10; Visual range: Horizontal +/- 44°; vertical +/- 20°; Accuracy ~0.5°; Spatial resolution ~0.15°; Pupil size resolution ~0.03 mm; Eye data: X, Y position of gaze, pupil height and width, torsion, delta time, total time, and regions of interest (ROI); Real-time communication (Item 0022): 4-Channel AnalogOut with eight TTL input channels to mark codes into the data file |
| Multi-position magnetic base | Harbor Freight Tools | Pittsburg, item #5645 | Magnetic holder reaches up to 12" and produces 45 lbs. of magnetic pull. Use to position camera. Machine thread holes onto the end of the rod to mount cameras. |
| Micromanipulator | Kopf | 900 | 5 axis manipulation for mount of suction electrode: X, Y, Z linear travel, 2 axis of rotation |
| Dissection scope on boom | Lieca | GZ6 | Magnification range, 6.7x – 40x |
| Nerve/muscle stimulator | Astro-Med Grass Telefactor | Grass S88 | Dual pulse voltage stimulator: two output channels that can be operated independently or synchronized to generate non-isolated constant voltage pulses (10 mv to 150 V). Pulses can be single (10 μsec to 10 sec), repetitive (0.01 Hz to 1 KHz), and trains (1 ms to 10 s) and synchronized with TTL inputs and output. Send TTL outputs via the output channels of a DB25 connector to the TTL input channels of the ViewPoint EyeTracker. Note: Astro-Med Grass Telefactor is no longer in business. |
| Current isolation device | Astro-Med Grass Telefactor | PSIU6 | Current stimulus isolation unit: enables safe delivery of constant currents by the S88 to the preparation. The PSIU6 connects by a BNC cable to one of the output channels of the S88. Multiplier switches on the PSIU6 allow the S88 to generate a wide array of current amplitudes ranging from 0.1 µA to 15 mA. |
| Alternative nerve/muscle stimulator with isolation | A-M Systems | 2100 | Isolated Pulse Stimulator: Unit has built-in isolator to produce constant currents. |
Referanslar
- Kikillus, K. H., Hare, K. M., Hartley, S. Minimizing false-negatives when predicting the potential distribution of an invasive species: A bioclimatic envelope for the red-eared slider at global and regional scales. Anim Conserv. 13, 5-15 (2010).
- Lutz, P. L., Rosenthal, M., Sick, T. J. Living without oxygen: turtle brain as a model of anaerobic metabolism. Mol Physiol. 8, 411-425 (1985).
- Lutz, P. L., Milton, S. L. Negotiating brain anoxia survival in the turtle. J Exp Biol. 207, 3141-3147 (2004).
- Storey, K. B. Anoxia tolerance in turtles: Metabolic regulation and gene expression. Comp Biochem Physiol A-Mol Integr Physiol. 147 (2), 263-276 (2007).
- Granda, A. M., Dearworth, J. R., Subramaniam, B. Balanced interactions in ganglion-cell receptive fields. Vis Neurosci. 16, 319-332 (1999).
- Dearworth, J. R., Granda, A. M. Multiplied functions unify shapes of ganglion-cell receptive fields in retina of turtle. J Vis. 2 (3), 204-217 (2002).
- Nesbit, S. C., Van Hoof, A. G., Le, C. C., Dearworth Jr, J. R. Extracellular recording of light responses from optic nerve fibers and the caudal photoreceptor in the crayfish. J Undergrad Neurosci Educ. 14 (1), A29-A38 (2015).
- McMahon, B. R. Respiratory and circulatory compensation to hypoxia in crustaceans. Resp Phsiol. 128 (3), 349-364 (2001).
- Leigh, R. J., Zee, D. S. . The neurology of eye movements. , (1999).
- Robinson, D. A. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng. 10, 137-145 (1963).
- Judge, S. J., Richmond, B. J., Chu, F. C. Implantation of magnetic search coils for measurement of eye position: an improved method. Vis Res. 20, 535-538 (1980).
- Ong, J. K. Y., Halswanter, T. Measuring torsional eye movements by tracking stable iris features. J Neurosci Meth. 192, 261-267 (2010).
- Kimmel, D. L., Mammo, D., Newsome, W. T. Tracking the eye non-invasively: simultaneous comparison of the scleral search coil and optical tracking techniques in the macaque monkey. Front Behav Neurosci. 6 (49), 1-17 (2012).
- Otero-Millan, J., Roberts, D. C., Lasker, A., Zee, D. S., Kheradmand, A. Knowing what the brain is seeing in three dimensions: A novel, noninvasive, sensitive, accurate, and low-noise technique for measuring ocular torsion. J Vis. 15 (14), 1-15 (2015).
- Demski, L. S., Bauer, D. H. Eye movements evoked by electrical stimulation of the brain in anesthetized fishes. Brain Behav Evol. 11, 109-129 (1975).
- Gioanni, H., Bennis, M., Sansonetti, A. Visual and vestibular reflexes that stabilize gaze in the chameleon. Vis Neurosci. 10, 947-956 (1993).
- Straka, H., Dieringer, N. Basic organization principles of the VOR: lessons from frogs. Prog Neurobio. 73 (4), 259-309 (2004).
- Voss, J., Bischof, H. -. J. Eye movements of laterally eyed birds are not independent. J Exp Biol. 212 (10), 1568-1575 (2009).
- Ariel, M. Independent eye movements in the turtle. Vis Neurosci. 5, 29-41 (1990).
- Ariel, M., Rosenberg, A. F. Effects of synaptic drugs on turtle optokinetic nystagmus and the spike responses of the basal optic nucleus. Vis Neurosci. 7, 431-440 (1991).
- Balaban, C. D., Ariel, M. A “beat-to-beat” interval generator for optokinetic nystagmus. Biol Cybern. 66, 203-216 (1992).
- Keifer, J. In vitro eye-blink reflex model: Role of excitatory amino acid receptors and labeling of network activity with sulforhodamine. Exp Brain Res. 97, 239-253 (1993).
- Keifer, J., Armstrong, K. E., Houk, J. C. In vitro classical conditioning of abducens nerve discharge in turtles. J Neurosci. 15, 5036-5048 (1995).
- Rosenberg, A. F., Ariel, M. A model for optokinetic eye movements in turtles that incorporates properties of retinal slip neurons. Vis Neurosci. 13, 375-383 (1996).
- Ariel, M. Open-loop optokinetic responses of the turtle. Vis Res. 37, 925-933 (1997).
- Anderson, C. W., Keifer, J. Properties of conditioned abducens nerve responses in a highly reduced in vitro brainstem preparation from the turtle. J Neurophysiol. 81, 1242-1250 (1999).
- Keifer, J. In vitro classical conditioning of the turtle eyeblink reflex: Approaching cellular mechanisms of acquisition. Cerebell. 2, 55-61 (2003).
- Zhu, D., Keifer, J. Pathways controlling trigeminal and auditory nerve-evoked abducens eyeblink reflexes in pond turtles. Brain Behav Evol. 64, 207-222 (2004).
- Jones, M. S., Ariel, M. The effects of unilateral eighth nerve block on fictive VOR in the turtle. Br Res. 1094, 149-162 (2006).
- Jones, M. S., Ariel, M. Morphology, intrinsic membrane properties, and rotation-evoked responses of trochlear motoneurons in the turtle. J Neurophysiol. 99 (3), 1187-1200 (2008).
- Krenz, J. G., Naylor, G. J. P., Shaffer, H. B., Janzen, F. J. Molecular phylogenetics and evolution of turtles. Mol Phylogenet Evol. 37 (1), 178-191 (2005).
- Dearworth, J. R., et al. Role of the trochlear nerve in eye abduction and frontal vision of the red-eared slider turtle (Trachemys scripta elegans). J Comp Neur. 52, 3464-3477 (2013).
- Dearworth, J. R., et al. Pupil constriction evoked in vitro by stimulation of the oculomotor nerve in the turtle (Trachemys scripta elegans). Vis Neurosci. 26, 309-318 (2009).
- Mead, K., et al. IFEL TOUR: a description of the introduction to FUN electrophysiology labs workshop at Bowdoin College, July 27-30, and the resultant faculty learning community. J Undergrad Neurosci Educ. 5, A42-A48 (2007).
- Jackson, D. C., Ultsch, G. R. Physiology of hibernation under the ice by turtles and frogs. J Exp Zool A Ecol Genet Physiol. 313 (6), 311-327 (2010).
- Romano, J. M., Dearworth, J. R. Pupil constriction evoked by stimulation of the ciliary nerve in the red-eared slider turtle (Trachemys scripta elegans). J Penns Acad Sci. 85, 4-8 (2011).
- Miller, J. M., Robins, D. Extraocular-muscle forces in alert monkey. Vis Res. 32, 1099-1113 (1992).
- Gamlin, P. D., Miller, J. M. Extraocular muscle motor units characterized by spike-triggered averaging in alert monkey. J Neurosci Meth. 204, 159-167 (2011).
- Quaia, C., Ying, H. S., Optican, L. M. The Viscoelastic properties of passive eye muscle in primates. III: Force elicited by natural elongations. PLOS ONE. 5, A236-A254 (2010).
- Anderson, S. R., et al. Dynamics of primate oculomotor plant revealed by effects of abducens microstimulation. J Neurophys. 101, 2907-2923 (2009).
- Maxwell, J. H., Harless, M., Morlock, H. Anesthesia and surgery. Turtles: Perspective and Research. , 127-152 (1979).
- AVMA Panel on Euthanasia. American Veterinary Medical Association. J Am Vet Med Assoc. 218 (5), 669-696 (2001).
- Clarke, R. J. Shaping the pupil’s response to light in the hooded rat. Exp Br Res. 176, 641-651 (2007).
- Bennett, R. A. A review of anesthesia and chemical restraint in reptiles. J Zoo Wild Med. 22 (3), 282-303 (1991).
- Bickler, P. E., Buck, L. T. Hypoxia Tolerance in Reptiles, Amphibians, and Fishes: Life with Variable Oxygen Availability. Ann Rev Physiol. 69, 145-170 (2007).

