Immunomagnetic Separation of Fat Depot-specific Sca1high Adipose-derived Stem Cells (ASCs)
Özet
We present the techniques required to isolate the stromal vascular fraction (SVF) from mouse inguinal (subcutaneous) and perigonadal (visceral) adipose tissue depots to assess their gene expression and collagenolytic activity. This method includes the enrichment of Sca1high adipose-derived stem cells (ASCs) using immunomagnetic cell separation.
Abstract
The isolation of adipose-derived stem cells (ASCs) is an important method in the field of adipose tissue biology, adipogenesis, and extracellular matrix (ECM) remodeling. In vivo, ECM-rich environment consisting of fibrillar collagens provides a structural support to adipose tissues during the progression and regression of obesity. Physiological ECM remodeling mediated by matrix metalloproteinases (MMPs) plays a major role in regulating adipose tissue size and function1,2. The loss of physiological collagenolytic ECM remodeling may lead to excessive collagen accumulation (tissue fibrosis), macrophage infiltration, and ultimately, a loss of metabolic homeostasis including insulin resistance3,4. When a phenotypic change of the adipose tissue is observed in gene-targeted mouse models, isolating primary ASCs from fat depots for in vitro studies is an effective approach to define the role of the specific gene in regulating the function of ASCs. In the following, we define an immunomagnetic separation of Sca1high ASCs.
Introduction
Stem cell antigen 1 (Sca1, or Ly6A/E) was first identified as a cell surface marker expressed by hematopoietic and mesenchymal stem cells5,6. The stromal vascular fraction (SVF) of adipose tissue obtained from mouse fat depots is a heterogeneous population of cells comprising of fibroblasts, macrophages, vascular endothelial cells, neuronal cells, and adipocyte progenitor cells7. Adipocyte progenitor cells, or adipose-derived stem cells (ASCs) are non-lipid-laden cells that reside in the collagen-rich perivascular extracellular matrix (ECM)8. Approximately 50% of the SVF consist of ASCs, which are characterized as lineage-negative (Lin–) and CD29+: CD34+: Sca1+ 9. Most of these cells are Sca1+: CD24– adipocyte progenitors, which are capable of adipocyte differentiation in vitro; however, only a fraction of cells (0.08% of SVF) constitutes Sca1+: CD24+ cells that are fully capable of proliferating and differentiating into adipocytes in the in vivo conditions9. Despite the potential caveat of using Sca1+ SVF without discriminating CD24+ cells from CD24– cells, isolating Sca1+ ASCs from fat depots using immunomagnetic cell separation is an efficient and practical approach to determine the cell-autonomous phenotype of primary adipocyte progenitor cells.
In the field of obesity and diabetes, tissue fibrosis and inflammation play a critical role in the development and maintenance of type-2 diabetes3. Recently, Tokunaga et al. showed that Sca1high cells isolated from inguinal (or subcutaneous, SQ) and perigonadal (or visceral, VIS) C57BL6/J fat depots exhibit different gene signatures and ECM remodeling in vitro10. MMP14 (MT1-MMP), a prototypical member of the membrane-type matrix metalloproteinase (MMP) family mediates the development of white adipose tissue (WAT) through its collagenolytic activity1.
Examples of experiments that may be conducted with the cells isolated and enriched through the following protocol include three-dimensional culture, differentiation studies, collagen degradation assays, and RNA sequencing10,11. Degradation assays should be conducted with acid-extracted collagen to ensure the preservation of telopeptide11,12. The following protocol will demonstrate the methods to isolate primary vascular stromal cells from different fat depots and enrich adipocyte progenitor cells using immunomagnetic cell separation. The validity of the cell sorting will be assessed with flow cytometry and through using Sca1-GFP mice that express GFP in Sca1+ cells, driven by a Sca1 promoter13.
Protocol
Ethics Statement: The University of Michigan Committee on Use and Care of Animals (UCUCA) has approved all methods and protocols in accordance with the Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, National Research Council). Mice are maintained in a University of Michigan vivarium and are given free access to food and water and kept on a 12 hr dark/light cycle.
1. Preparations
- Prepare primary culture media with DMEM, 10% FBS, 1x P/S/G, and 1x antibiotics/antifungals. This will be used to keep tissues in before digestion. Place stock and aliquot in 37 ºC water bath.
- Prepare five milliliters of Type III collagenase solution at 5mg/ml dissolved in HBSS (+Ca, +Mg) for each type of fat pad being isolated, up to five mice. For example, when isolating SQ and VIS from a single genotype, make 10 ml, if wild-type and mutant SQ and VIS, make 20 ml. Adjust pH to 7.4 and sterile filter. Aliquot into 50 ml tubes. Set aside away from light.
- Use 70% ethanol to disinfect surgical field. Wipe down and cover with a blue pad. Spray some ethanol on the blue pad.
- Use the 22 G needles to pin down an absorbent pad to the styrofoam board and trim with scissors. Spray the pad with ethanol then set the board on the blue pad and cover with another blue pad until beginning dissection.
- Fill two 50 ml tubes with ethanol and place in a stand adjacent to the surgical field. Place the scissors and forceps in the ethanol.
- Fill another 50 ml tube with PBS plus 1x anti-anti for rinsing ethanol off surgical tools.
- In the hood, label 60 mm dishes for each tissue type and fill with culture media warmed to 37 ºC. Place the plates adjacent to the surgical area.
2. Isolation of Subcutaneous (SQ) Fat Pads
- Euthanize mouse with an overdose of isoflurane and pneumothorax.
- Gently spray mouse with 70% ethanol and lay supine. Pin the paws to the foam board with 22 G needles.
- Make a small cut in the skin of the lower abdomen. Holding the top of the cut with the forceps, take the scissors and separate the skin from the peritoneum.
- Once the skin is separated from the peritoneum from the abdomen to the thorax, reflect the skin away from the peritoneum towards the head by making lateral cuts in the skin along the side of the body.
- Reflect the remaining skin holding the inguinal fat away from the body and pin down to the board with 22 G needles.
- Switch to fine scissors and forceps. Grab the inguinal fat pad with the forceps at the origin near the peritoneum and snip away between the skin and inguinal fat progressing towards the groin avoiding contaminating the SQ with skin.
- Place the insolated inguinal fat pad in the labeled 60 mm dish.
3. Isolation of Visceral (VIS) Fat Pads
- In a similar fashion to step 2.5, cut the peritoneum open to expose the visceral fat pads and gut.
- Move the gut away toward the thorax.
- Grab the VIS fat pad at the distal end and gently pull up. Dissect out the VIS fat pad while being careful to exclude epididymal (or uterine, if using female mice) tissue.
- Place in labeled dish and repeat step 3.3 for the remaining VIS pad.
4. Collagenase Digestion of Fat Pads
- Move 60 mm dishes to the tissue culture hood and aspirate off media.
- Mince the fat pads with curved scissors then add to collagenase solution. Be sure to rinse the scissors and forceps in between tissue types with ethanol and PBS.
- Shake at 300 rpm and 37 ºC for 10-20 min until tissues are digested. It is acceptable if some pieces of SQ are still visible. This will be addressed in a later step.
- Add 25 ml of culture media to the collagenase solution to stop the collagenase treatment, and pipette up and down 10 times with a 10 ml serological pipette to disperse the digested tissues.
- Strain cells using a 100 µm cell strainer. Centrifuge for 10 min at 300 x g.
- Carefully decant off media not to disturb the pellet and add 5 ml of sterile water to the pellet and gently pipette to suspend cells. Wait 2 min to lyse erythrocytes.
- Add 25 ml of media with 10% FBS and strain cells through a new 100 µm cell strainer.
- Centrifuge for 10 min at 300 x g. Decant media and resuspend pellet in 1 ml of culture media.
- Count cells with a hemocytometer using 1:1 trypan blue.
- Plate 1 x 106 cells in one well on a 6-well plate.
5. Magnetic Cell Separation
- After about 4 to 6 hr of cell adhesion, rinse cells twice with HBSS (-Ca, -Mg). Dissociate adherent cells using 0.05% trypsin. Dilute trypsin cell suspension in 1 ml separation buffer and spin for 5 min at 300 x g.
- Aspirate supernatant. Resuspend cells in 500 µl buffer. Remove a 10 µl aliquot and count cells using a hemocytometer.
- Spin cells for 5 min at 300 x g.
- Aspirate supernatant completely. Resuspend cells in 90 µl buffer. Add 10 µl anti-Sca1-FITC primary antibody. Mix well and incubate for 10 min at 4 ºC in the dark.
- Wash cells with 1 ml buffer and centrifuge cells for 5 min at 300 x g. Remove supernatant completely.
- Resuspend cells in 80 µl buffer. Add 20 µl anti-FITC microbeads. Mix well and incubate for 15 min at 4 ºC in the dark.
- Wash cells with 1 ml buffer and centrifuge cells for 5 min at 300 x g.
- Remove supernatant and resuspend pellet in 500 µl buffer.
- Place column in the magnetic holder and rinse column with 500 µl buffer.
- Add cell suspension to column. Avoid bubbles while pipetting.
- Collect the unlabeled Sca1– cells and wash column 3 times with 500 µl buffer. Only add new buffer when the reservoir is empty. Avoid bubbles while pipetting.
- Remove column from the magnetic holder and place into a 15 ml conical tube.
- Add 1 ml buffer to column and elute Sca1+ by pushing supplied plunger through the column reservoir.
- Spin down cell fractions for 5 min at 300 x g. Resuspend cells in 1 ml culture media.
- Remove a 10 µl aliquot from the labeled and unlabeled fractions and count cells using a hemocytometer.
- Plate each cell fraction in 1 well of a 6-well plate.
6. Verification of Immunomagnetic Separation of Sca1high ACSs with Flow Cytometry
- Rinse cells twice with HBSS (-Ca, -Mg) and dissociate cells using 0.05% trypsin.
- Centrifuge cells at 300 x g for 5 min. Resuspend cells in 1 ml culture media and count using a hemocytometer.
- Obtain >106 cells in 1 ml culture media and centrifuge at 300 x g for 5 min.
- Remove supernatant and repeat step 6.3 two more times.
- Resuspend cells with 1 ml of 2% goat serum + 2% BSA and block for 30 min at RT.
- Centrifuge cells at 300 x g for 5 min.
- Remove blocking solution.
- Add rat IgG2a Alexa Fluor 647 (0.25 µg, 1:400) or anti-Sca1 Alexa Fluor 647 (0.25 µg, 1:400), in 100 µl of PBS with 2% goat serum and 2% BSA + PBS at 4 °C for 30 min in the dark.
- Add 1 ml of cold PBS to cells. Centrifuge 300 x g for 5 min at 4 °C .
- Remove supernatant and repeat step 6.9 two more times.
- Resuspend cells in 1 ml PBS. Pass cell suspensions through a 100 µm cell strainer in preparation for flow cytometric analysis.
Representative Results
Enrichment of Sca1high ASCs from Different Fat Pads.
The vascular stromal cells isolated from SQ fat display fibroblast-like, stretched cell shape regardless of Sca1 expression level (Figure 1A). On the other hand, VIS (eWAT-derived) Sca1high and Sca1low cells demonstrate distinct difference in their cell shape. Like SQ (iWAT-derived) Sca1high cells, VIS (eWAT-derived) Sca1high cells display stretched, fibroblast-like cell shape, whereas VIS Sca1low cells demonstrate epithelioid shape. Sca1high cells isolated from Sca1-GFP mice are easily identified as GFP-positive cells in tissue culture (Figure 1B). When these cells were assessed with flow cytometry, most of the GFP positive cells were confirmed to express Sca1 proteins on the cell surface as detected with Anti-Sca1 antibody (Figure 1C). Sca1high cells derived from inguinal fat pad maintain increased capacity of adipocyte differentiation whereas eWAT-derived Sca1high cells are more difficult to differentiate into adipocyte with conventional adipogenic mix10.
Fat Depot-dependent Gene Expression of Sca1highASCs (Figure 2).
Genome-wide transcriptome analyses with RNA sequencing demonstrated the enrichment of genes related to extracellular matrix proteins and modifiers (GO:0031012, GO:0005578) in those Sca1high ASCs10. Coupled with real-time PCR analyses, we were able to demonstrate the differential expression of the collagenolytic MMPs (MMP2, MMP8, MMP13, MMP14) between iWAT- and eWAT-derived Sca1high ASCs. When fluorescein-labeled type I collagen gels were used to assess pericellular degradation activity, we observed the markedly increased collagen remodeling activity mediated by VIS Sca1high ASCs10.
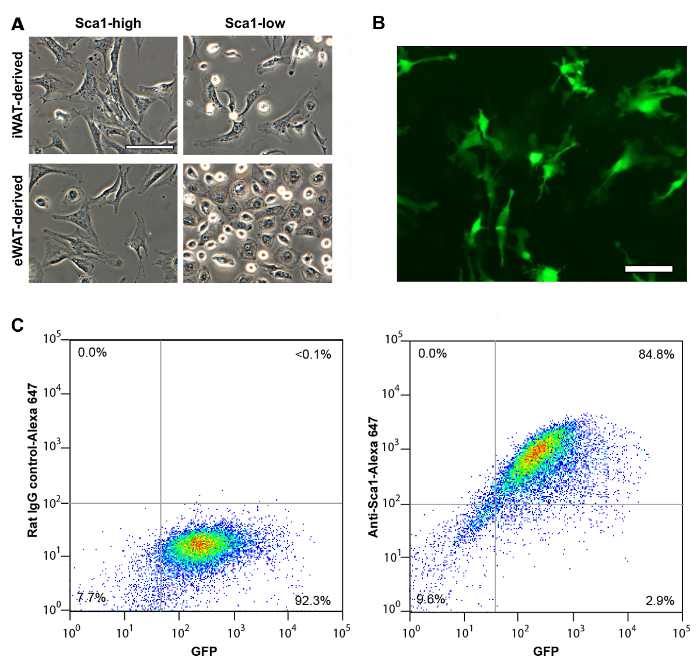
Figure 1: Immunomagnetic Separation of Murine Sca1high ASCs from Different Fat Depots. (A) Sca1high and Sca1low ASCs isolated from SQ (iWAT) and VIS (eWAT). Scale = 100 µm. (B) Sca1-GFP cells isolated from iWAT of Sca-GFP mice. Scale = 100 µm. (C) Cell surface expression of Sca1 in Sca1-GFP-positive cells assessed with flow cytometry. (left) control rat IgG (right) anti-Sca1 antibody. X-axis, GFP intensity. Y-axis, Alexa-Fluor 647. Panel A shown previously in Tokunaga, M. et al., (2014). Please click here to view a larger version of this figure.
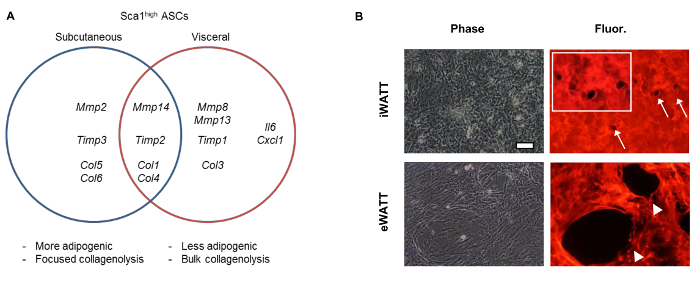
Figure 2: Fat Depot-Dependent Expression of Collagenolytic MMPs, TIMPs, and Collagens. (A) Differential gene expression of ECMs and ECM modifiers in Sca1high ASCs isolated from different fat depots. (B) Increased collagen degradation activity of VIS (eWAT-derived) Sca1high ASCs. Degradation of collagen is shown as the disappearance of fluorescent signals (arrows and arrowheads). Inset is an enlarged image of focused collagen degradation mediated by individual cell of SQ ASCs. Cells were cultured for 72 hr. Data shown previously in Tokunaga, M. et al., (2014). Please click here to view a larger version of this figure.
Discussion
Herein we demonstrate the isolation and immunomagnetic cell separation of murine ASCs from different fat pads and their use for in vitro experiments. The presented method is effective for the quick isolation of large number of Sca1-positive ASCs, which is advantageous over the technically complex and expensive FACS-mediated isolation of ASCs9,14. Unlike FACS, immunomagnetic cell separation does not allow the use of multiple antigen for the identification of a target cell population. Nonetheless, if the surface antigen is well-characterized, the use of immunomagnetic separation increases the number of cells to be analyzed without relying on the use of FACS equipment15, which is still not readily accessible to many biological researchers at small institutes without core facilities.
There are some critical aspects of the procedure that must be observed to ensure a successful outcome. The procurement of adipose-derived stem cells requires the surgical isolation of mouse adipose tissue depots. Therefore, the speed and accuracy of tissue retrieval is imperative to yield a high number of viable cells. Additionally, the maintenance of clean surgical fields and instruments are vital to the outcome of the procedure by preventing microbial contamination of cell and tissue samples. Dissected adipose tissues must be enzymatically dissociated in a type II collagenase solution. ECM composition differs between SQ and VIS fat pads10,16, where VIS contain less collagens than SQ. Therefore, the duration of VIS fat pad digestion requires approximately half the amount of time as SQ. It is acceptable to stop tissue digestion after the stated incubation period even if small particles of tissue are still present in the collagenase solution. These pieces may be mechanically dissociated with pipetting once culture media has been added to inhibit collagenase activity. While it is possible to proceed directly to immunomagnetic sorting following SVF isolation, our group seeds 1 x 106 unsorted cells on plastic plates for about 4 to 6 hr before continuing with cell sorting. Despite multiple filtration steps during SVF isolation, debris will still be present within the cell suspension. Plating the cells before proceeding to immunomagnetic cell separation provides an opportunity to wash debris and unattached cells away, thus allowing magnetic sorting to only be applied to adherent cells that contain adipogenic progenitor cells.
While Sca1 is not found in human genome, the identification and validation of alternative cell surface antigens expressed on fat depot-dependent human adipose stem cells17, when coupled with this cell separation technique, may help us define the biology of human adipose stem cells.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
This work is supported by NIH DK095137 (to THC). We thank the current and former lab members who contributed to the development and sophistication of the described methods.
Materials
| Type 3 Collagenase | Worthington Biochemical | LS004182 | Tissue digestion |
| DMEM | Gibco | 11965-092 | High-glucose culture medium |
| Pen/Strep/Glutamine (100x) | Gibco | 10378-016 | Media antibiotic |
| Anti-anti (100x) | Gibco | 15240-062 | Media antifungal |
| FBS | Gibco | 16000-044 | |
| PBS (1x, pH 7.4) | Gibco | 10010-023 | |
| Trypsin (0.05%) | Gibco | 25300-054 | |
| Cell strainer | BD Bioscience | 352360 | 100-μm cell strainer |
| 60mm plates | BD Falcon | 353004 | |
| Scissors | FST | 14001-12 | Large |
| Scissors | FST | 14091-11 | Fine, curved tip |
| Large Forceps | FST | 11000-12 | |
| Fine Forceps | Any vendor | ||
| 25G 5/8” needles | BD | 305122 | |
| 22G 1.5” needles | BD | 305159 | |
| 15 ml conical tubes | BD Falcon | 352097 | |
| 50 ml conical tubes | BD Falcon | 352098 | |
| MACS separation columns | Miltenyi Biotec | 130-042-201 | |
| Anti-Sca1 microbead kit (FITC) | Miltenyi Biotec | 130-092-529 | FITC-anti-Sca1 1ºAb and anti-FITC microbeads 2ºAb |
| AutoMACS running buffer | Miltenyi Biotec | 130-091-221 | |
| MiniMACS separator | Miltenyi Biotec | 130-042-102 | |
| MACS MultiStand | Miltenyi Biotec | 130-042-303 | |
| Blue chux pads | Fisher | 276-12424 | |
| Absorbent pads | Fisher | 19-165-621 | |
| Styrofoam board | Use from 50ml tubes | ||
| 70% ethanol | |||
| Isoflurane | Any vendor | ||
| Rat IgG2a Alexa Fluor 647 | Invitrogen | R2a21 | |
| Rat IgG2a anti-mouse Sca1 Alexa Fluor 647 | Invitrogen | MSCA21 | |
| Rat IgG2a R-PE | Invitrogen | R2a04 | |
| Rat IgG2a anti-mouse F4/80 R-PE | Invitrogen | MF48004 | |
| Round-bottom tube | BD Falcon | 352058 | |
| HBSS (–Ca, –Mg) | Gibco | 14175-095 | |
| HBSS (+Ca, +Mg) | Gibco | 14025-092 | For collagenase solution |
| Type I collagen (2.7 mg/ml in 37mm acetic acid | Prepare in house12 | ||
| 10x MEM | Gibco | 11430-030 | |
| 1M HEPES | Gibco | 15630-080 | |
| 0.34N NaOH | Prepare in house | ||
| Cover slips | Corning | 2870-22 | |
| Alexa Fluor 594 carboxylic acid, succinimidyl ester, mixed isomers | Invitrogen | A-20004 | |
| 0.89M NaHCO3 | Gibco | 25080-094 |
Referanslar
- Chun, T. H., et al. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 125 (3), 577-591 (2006).
- Chun, T. H., et al. Genetic link between obesity and MMP14-dependent adipogenic collagen turnover. Diabetes. 59 (10), 2484-2494 (2010).
- Chun, T. H. Peri-adipocyte ECM remodeling in obesity and adipose tissue fibrosis. Adipocyte. 1 (2), 89-95 (2012).
- Sun, K., Tordjman, J., Clement, K., Scherer, P. E. Fibrosis and adipose tissue dysfunction. Cell Metab. 18 (4), 470-477 (2013).
- Spangrude, G., Heimfeld, S., Weissman, I. Purification and Characterization of Mouse Hematopoietic Stem Cells. Science. 241, 58-62 (1988).
- Welm, B. E., et al. Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol. 245 (1), 42-56 (2002).
- Gesta, S., Tseng, Y. H., Kahn, C. R. Developmental origin of fat: tracking obesity to its source. Cell. 131 (2), 242-256 (2007).
- Tang, W., Zeve, D., Suh, J. M., Bosnakovski, D., Kyba, M., Hammer, R. E., Tallquist, M. D., Graff, J. M. White fat progenitor cells reside in the adipose vasculature. Science. 322, 583-586 (2008).
- Rodeheffer, M. S., Birsoy, K., Friedman, J. M. Identification of white adipocyte progenitor cells in vivo. Cell. 135 (2), 240-249 (2008).
- Tokunaga, M., et al. Fat depot-specific gene signature and ECM remodeling of Sca1(high) adipose-derived stem cells. Matrix Biol. 36, 28-38 (2014).
- Chun, T. H., Inoue, M. 3-D adipocyte differentiation and peri-adipocyte collagen turnover. Methods Enzymol. 538, 15-34 (2014).
- Rajan, N., Habermehl, J., Cote, M. F., Doillon, C. J., Mantovani, D. Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nat Protoc. 1 (6), 2753-2758 (2006).
- Ma, X., Robin, C., Ottersbach, K., Dzierzak, E. The Ly-6A (Sca-1) GFP Transgene is Expressed in all Adult Mouse Hematopoietic Stem Cells. Stem Cells. 20 (6), 514-521 (2002).
- Berry, R., Rodeheffer, M. S. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 15 (3), 302-308 (2013).
- Jeffery, E., Church, C. D., Holtrup, B., Colman, L., Rodeheffer, M. S. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol. 17 (4), 376-385 (2015).
- Mori, S., Kiuchi, S., Ouchi, A., Hase, T., Murase, T. Characteristic Expression of Extracellular Matrix in Subcutaneous Adipose Tissue Development and Adipogenesis; Comparison with Visceral Adipose Tissue. Int J Biol Sci. 10 (8), 825-833 (2014).
- Ong, W. K., et al. Identification of Specific Cell-Surface Markers of Adipose-Derived Stem Cells from Subcutaneous and Visceral Fat Depots. Stem Cell Reports. 2 (2), 171-179 (2014).

