Initiating Rapid Neurite Extension and Formation Functional Neuronal Connections
Abstract
Source: M. H. Magdesian et al., Rewiring Neuronal Circuits: A New Method for Fast Neurite Extension and Functional Neuronal Connection, J. Vis. Exp. (2017)
This video demonstrates a technique for the rapid initiation and extension of a neuron and creation of new connections with a target neuron using a microfluidic device and micromanipulation. This could be used for therapies that aim to reconnect neuronal circuits after trauma or in neurodegenerative diseases.
Protocol
1. Standardization of Neuronal Cultures Using Microfluidic Devices: Device Assembly
- Select a suitable microfluidic device for the desired experiment. To connect neurons within the same population, use the Neuro Devices (Figure 1), and to connect neurons in different populations, use the Co-Culture Devices (Figure 2).
- Clean and prepare the desired number of sterile coverslips or glass bottom dishes. For best results on plastic surfaces, use 35 mm dishes, on glass, use 25 mm coverslips or 35 mm glass bottom dishes. Select glass thickness based on the imaging system, for instance, 0.15 mm.
- Coat the dishes or coverslips with 0.5-1 mL of 100 µg/mL PDL for 2 h or overnight at room temperature.
NOTE: Protocol can be paused here and resumed the following day if desired. Furthemore, dishes can be coated with borate buffer-diluted PDL, Poly-L-Lysine (PLL), laminin or any other cell adhesion molecule. - Wash the dishes twice with water (do not use phosphate-buffered saline (PBS) as salt crystals may block the channels), remove all the liquid and let it dry in a sterile environment such as a biosafety cabinet for 5-10 min or until the surface is completely dry.
NOTE: Be careful to ensure that the coverslips are absolutely dry, as any remaining liquid will interfere with the adherence of the microfluidic systems. - Place microfluidic devices with patterns facing up under UV light in a sterile environment (biosafety cabinet) for 10 min. Be sure to follow sterile procedures when working in the biosafety cabinet10.
- Using tweezers, place a microfluidic device with a pattern facing down in contact with the clean coverslip/dish. Use the tweezers to softly press the device so that it adheres to the glass.
NOTE: The transparency of the adhered region will be visible when looking against the light. Make sure all corners are in contact with the glass. Do this to all microfluidic devices. See Figure 1a. - To fill the single population device with medium, point the pipette towards the channels and add 50 µL of complete cell medium supplemented with serum-free B-27 (volume ratio 1:50) and 500 µg/mL penicillin/streptomycin/glutamine (collectively called NBM) to the right upper well, and then add another 50 µL to the well lying diagonally to it. Do this for all devices, making sure that the medium flows between wells. Next, add 50 µL of medium to the remaining two wells. See Figure 3a.
- To fill the multiple population device with medium, point the pipette towards the channels and add 30 µL of complete NBM to the right wells, refer to Figure 2a. Do this for all devices, making sure that the medium flows between wells. Next, add 50 µL of medium to the remaining four wells.
- Place the devices in a bigger plate with an open dish with autoclaved water (wet chamber) and place in the incubator (37 °C, 5% CO2, and 95% humidity) for 1-2 h while preparing the cell culture. See Figure 3b.
2. Plating Neurons in Microfluidic Systems
- Following a protocol, obtain dissociated hippocampal or cortical neurons from Sprague Dawley rat embryos (either gender).
- Resuspend embryonic neurons in NBM at a concentration of 1-2 million neurons/mL. Verify cell concentrations in the microscope using a hemocytometer and following reference8. Adjust the cell concentration according to the desired cell density. To increase the chances of obtaining single hippocampal axons per channel, plate 10,000 neurons per device. To have multiple axons in the same channel, plate 60,000 neurons per device.
Note: These numbers vary according to the neuronal type used. - Remove the medium from the microfluidic devices without emptying the wells. Leave approximately 5 µL in each.
- To plate cells in the single population device, add 50 µL of NBM to the lower right well. At this point, the medium flows by itself to fill the other lower well. Add 20 µL of the concentrate cell solution into the top right well of the microfluidic device, as indicated in Figure 1b.
- To plate cells in the multiple population device add 20 µL of the concentrate cell solution into each of the right wells in Figure 2a.
- Check in the microscope if cells are inside the chambers and place the devices in the incubator for 15-30 min to promote cell attachment to the substrate.
- Check in the microscope if there are enough cells in the chambers. If more are needed, repeat steps 2.4 and 2.5.
- Add 50 µL of NBM to the 2 top wells of the single population device and 20 µL of NBM into the same well as the cells were injected in the multiple population device. The media protrudes slightly to form a positive meniscus giving the wells a muffin top aspect. Again, see Figure 3a.
- Maintain the cells at 37 °C, 5%CO2, and 95% humidity.
3. Maintaining the Neuronal Cultures
- Remove NBM (roughly 30 µL with a pipette) from the cells and apply new pre-warmed NBM the day following their introduction to the devices (that is 1 d after step 2).
- Check every 2 days if there is enough medium in each channel. If the muffin top is low just add more medium to the top wells.
- Culture cells for at least 7 d before removal of the microfluidic devices. The cells can survive in these devices for several weeks. Remove the devices 1-2 days before experiments are performed on samples.
4. Removal of Microfluidic Devices
- 1 – 2 d before removal of microfluidic devices, add 2 mL of NBM prewarmed to 37 °C to each sample dish, flooding the chambers, and maintain the devices in the incubator.
- Use sterile tweezers and one tip to remove the microfluidic devices from the coverslips leaving a patterned configuration of neurons. Use the tip to hold the coverslip in place and the tweezers to clasp the edge of the device at the bottom left corner of the well. Delicately apply torsion, raising the device up with the tweezers so that it peels off the coverslip. See Figure 3c–3d.
- Every 2-3 days, replace half the NBM until the sample is used for experiments.
- Before performing rewiring experiments on the sample, verify that neurites in the single population device channels and the neuronal populations in the multiple population device are isolated by examining the gaps between them in the microscope to ensure there are no filaments linking neuronal populations.
5. Preparing PDL-coated Beads
- Add 2 x 50 µL drops of either 4, 10 or 20 µm polystyrene beads diluted in water (1:500) to 1 mL of PDL (100 µg/mL). Leave for at least 2 h at room temperature.
NOTE: Protocol can be paused here and resumed the following day. - Centrifuge the solution at 8,820 x g for 1 min. Carefully remove the supernatant without disturbing the beads accumulated at the bottom of the container.
- Wash the beads twice with 1 mL of sterile 10 mM HEPES pH 8.4 solution.
- Resuspend the PDL-coated beads in 200 mL of 10 mM HEPES pH 8.4 solution.
6. Preparing Micropipettes
- Prepare pipettes from glass capillary tubes (1 mm inner diameter, 1.5 mm outer diameter) using a horizontal electrode puller. Adjust settings so the outer tip of the pulled micropipette is ~ 2-5 µm. Before pulling, ensure the glass tubes are clean.
- Fix pipettes to glass slides for storage, and ensure that the tip does not contact the surface of the slide as the tip is fragile. Store at room temperature in a covered container to protect from dust. Use pipettes the same day they are pulled.
7. PDL-bead Adhesion to Neurons
- Add 40-60 µL of PDL-coated beads prepared in step 5 to a cell culture prepared in step 4. Center the pipette tip over the neurons, which are faintly visible on the coverslip, and add the beads (See Figure 4).
- Return the sample to the incubator for 1 h to promote the formation of synaptic contacts.
- After the incubation, remove any unadhered beads by gently washing the culture with pre-warmed NBM.
8. Preparing Physiological Saline Solution (for Room Temperature Experiments)
- Prepare a physiological saline solution. This is to regulate the cell environment outside the incubator.
- Verify osmolarity and pH levels.
- Continuously infuse solution with O2 to minimize pH fluctuations while conducting experiments.
- Heat to room temperature.
- Set up the perfusion system by inserting one end of a plastic tube (optional dimensions) in an O2-infused physiological solution and fixing the other end to a needle inserted in the sample holder. Place the tubing and solution higher than the sample (See Figure 5).
- Disconnect the tube from the needle and connect it to a syringe. Use the syringe to exert pressure and draw liquid, filling the tube. Seal with a roller clamp and reconnect the needle.
9. Bead Micromanipulation
- Install the sample in an experimental set-up such that cells can be accessed from above by two micropipettes mounted in micromanipulators and accessed optically below, for instance, with the 40X-phase objective (numerical aperture of 0.6) of an inverted optical microscope. In this configuration, mount a CCD camera for image capture on the side port of the microscope. Connect each pipette to 1 mL syringes via plastic tubing. At this step, replace NBM with a physiological saline solution (1-2 mL) (See Figure 5).
- During experiments, continuously perfused cells with the physiological saline solution prepared in step 8 at a rate of 0.5-1 mL/min.
- Select a PDL bead NOT attached to a neuron in the field of view. Align the bead with a micropipette tip by focusing on the bead and then up to the micropipette. Bring the tip down as close as possible to the bead by monitoring it through the microscope.
- Apply negative pressure with the 1 mL syringe connected to the pipette to pick up the bead. Maintain negative pressure throughout the experiment.
10. Pulling Neurites
- Select a PDL bead attached to a neuron in the field of view and attach it to the second micropipette using suction as described in steps 9.3-9.4.
- Pull the PDL-bead-neuron complex slowly (~0.5 µm/min), moving either the micromanipulator or the sample stage by 1 µm and pausing for 5 min to allow neurite initiation.
- Repeat step 10.2 twice.
Note: The first 3 µm have to be pulled very slowly to guarantee experimental success, which occurs over 95% of the time. - Pull the PDL-bead-neuron complex by slowly (~0.5 µm/min) moving either the micromanipulator or the sample stage by 2 µm and pause for 5 min to allow neurite elongation.
- After successful initiation and neurite extension for the first 5 µm, pull the neurite at 20 µm/min over millimeter-scale distances.
Note: Pulling can be performed continuously or in steps and at varying rates. See Figure 6b-6c.
11. Connecting Neurons
- Select a region rich in neurites and lower the PDL-bead-neurite complex so that it physically contacts it. Use other beads to gauge tip height above the coverslip surface. See Figure 6d.
- Leave the PDL-bead-neurite complex in contact with the target neurite while manipulating the second micropipette. Lower the second pipette with the second PDL-bead on top of the newly formed neurite about 20 µm from the first bead. Use the second PDL-bead to push the new neurite filament toward the target cell.
- Hold both beads in place for at least 1 h. Verify the absence of focal swelling, a thickening of the neurites contacting the bead, with the microscope16.
- During this time, use perfusion to slowly change the medium of the sample from physiological saline to pre-warmed, CO2-equilibrated NBM.
- Release the bead from the second pipette by releasing suction. If the new neurite remains attached, release the first bead as well. See Figure 6e .
- Gently remove saline solution and replace with NBM (~2 mL).
- Carefully place the sample back in the incubator to strengthen the neuronal connection for future experiments. This connection is stable for >24
Representative Results

Figure 1: Standardization of Neuronal Cultures using Microfluidic Devices7. (a) Device assembly: when the microfluidic devices are properly assembled on a dry surface all chambers are visible. (b) Cell plating: plate the cells on the top right well and cells should move towards the left well. (c) Cell density: just after plating, check in the microscope if the concentration of cells is adequate. (d) After 1 d in culture, hippocampal neurons are well adhered close to the microchannels and start forming neurites.
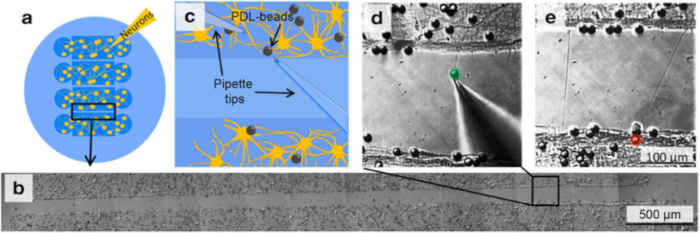
Figure 2. Initiation, Elongation and Connection of New Neurites to Connect two Isolated Populations using Multiple Population Device and Micromanipulation. (a) The device isolates 4 neuronal populations between 3 gaps of 100 or 200 µm each. (b) Following removal of the device, select one gap and certify that no neurites connect the 2 individual populations. (c) Schematic of the experimental set-up as should be visible in the optical microscope, indicates the position of two micropipettes and the presence of PDL-coated beads. (d) By applying negative pressure to a pipette, a PDL-bead adhered to one neuronal population is pulled with the pipette tip, thereby initiating a new neurite. By maintaining the negative pressure in the pipette, the PDL-bead-neurite complex (green) can be pulled, elongating the neurite. (e) Pipette micromanipulation guides extension of the new neurite over the gap and the formation of a connection with a new neuronal population. To ensure the adhesion of the new neurite to the second population, a PDL-bead (red) is positioned with a second pipette on top of both the extended neurite and neuronal population.
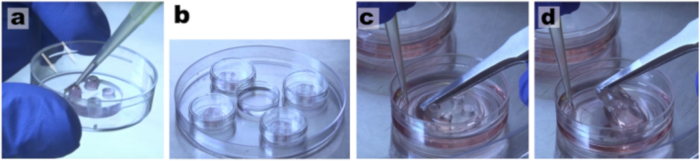
Figure 3: Maintenance of healthy neuronal cultures for several weeks. (a) Add medium every 2-3 d and keep a positive meniscus in the upper wells of the microfluidic chambers so cells will have a constant supply of nutrients. (b) Keep cells inside a larger plate with a dish containing water to reduce medium evaporation. (c) Use sterile tip and tweezers to (d) easily peel off the microdevices.
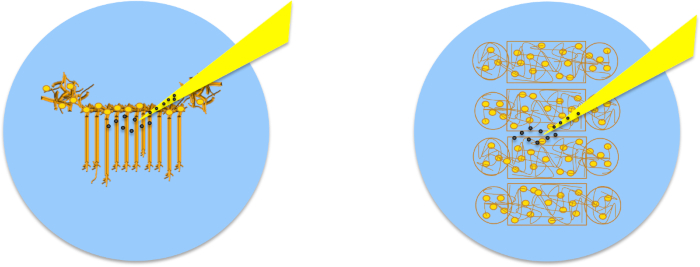
Figure 4: Location of Pipette Depositing Beads into Cultures. Once the microfluidic device has been removed, neurons are visible on the coverslip. When depositing beads, position the pipette tip so that it is in the center of the cells.
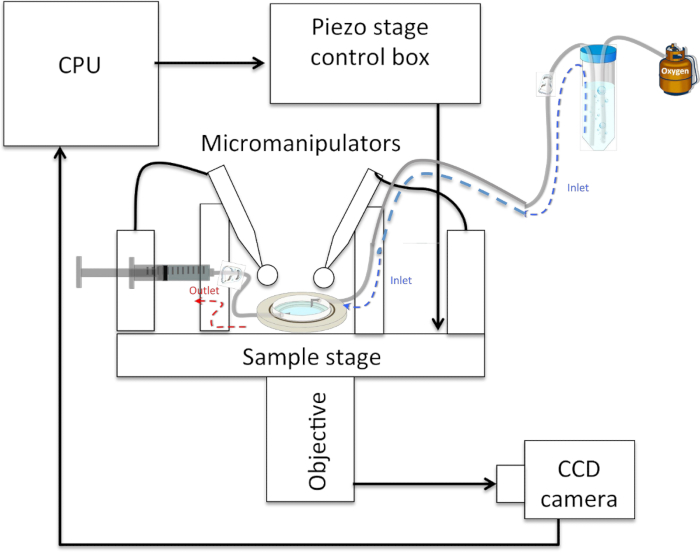
Figure 5: Schematic of a Typical Neurite-pulling Set-up. The sample rests on a (piezo-actuated) stage and can be accessed from above by 2 micropipettes held in micromanipulators and connected to 1 mL syringes via plastic tubing. The sample is accessed optically from below by an objective connected to a CCD camera that sends images to a CPU. An inlet tube feeds oxygenated physiological saline solution to the sample, which rests below it, and an outlet tube connected to a syringe allows the withdrawal of solution in the event of overflow.
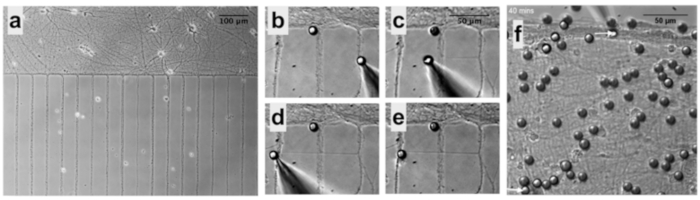
Figure 6: PDL-bead Adhesion to Neurons and Pipette Micromanipulation. (a) Neurons remain organized in patterns after removal of microfluidic chambers allowing easy identification of soma and neurites. (b) Micromanipulation of PDL-beads adhered to neurites enables neurite initiation, extension (c) and connection (d), followed by release of the PDL-bead from the pipette (e). (f) After contacting two isolated neuronal populations (bottom arrow), a second PDL-bead and pipette micromanipulator (top arrow) is used to establish a second adhesion point, a few hundreds of microns apart form the first contact point, and guarantee functional connections.
Açıklamalar
The authors have nothing to disclose.
Materials
| Neuro devices | Ananda Devices | Commercially available at http:// www.anandadevices.com | |
| No. 1 Glass Coverslip 25 mm Round | Warner Instruments | 64-0705 | |
| 35 mm cell culture dish, NonPyrogenic, Sterile | Corning Inc | 430165 | |
| 95 mm x 15 mm Petri Dish, Slippable Lid, Sterile Polystyrene | Fisherbrand | FB0875714G | |
| Neurobasal Medium | Life Technologies | 21103-049 | Extracellular solution |
| B-27 Supplement (50X), serum free | Thermo Fisher Scientific | 17504044 | Extracellular solution |
| Pennicilin, Streptomyocin, Glutamine | Thermo Fisher Scientific | 11995-065 | Extracellular solution |
| 2 – 20 μL Pipettors Aerosol Resistant Tips | Thermo Fisher Scientific | 2149P | |
| Dumont Dumostar Tweezers 11 cm | World Precision Instruments | 500233 | |
| Dissection tools | Braun, Aesculap | ||
| Poly-D-lysine Hydrobromide | Sigma-Aldrich | P6407 | |
| Micro particles based on polystyrene, 10 μm | Sigma-Aldrich | 72986 | |
| Horizontal Pipette Puller | Sutter Instruments | Brown-Flaming P-97 | |
| Micromanipulators, PCS-5000 Series MC7600R | SD Instruments | MC7600R | |
| 1 mL Syringe | BD Luer-Lok | 309628 | |
| Inverted Microscope | Olympus | IX71 | |
| Objective UIS2 | Olympus | LUCPLFLN 40X | |
| CCD Camera | Photometrics | Cascade II: 512 |

