Studying the Effects of Inhaled Environmental Pollutants on Olfactory Function in Mice
Summary
This paper provides a detailed description of the buried food test and social odor discrimination experiment to assess the effects of inhaled environmental pollutants exposure on olfactory function in mice.
Abstract
Olfactory impairment is a significant public health problem and independently predicts the risk of neurodegenerative diseases. Inhaled environmental pollutants exposure may impair olfaction; thereby, there is an urgent need for methods to evaluate the effects of inhaled environmental pollutants exposure on olfaction. Mice are ideal models for olfactory experiments because of their highly developed olfactory system and behavioral characteristics. To assess the effects of inhaled environmental pollutants exposure on olfactory function in mice, a detailed buried food test and social odor discrimination experiment is provided, including the experiment preparation, the selection and construction of experimental facilities, the testing process, and indexes of time. Meanwhile, timekeeping equipment, operational details, and the experimental environment are discussed to ensure the success of the assay. Zinc sulfate is used as the treatment to demonstrate the feasibility of the experimental approach. The protocol provides a simple and clear operational process for assessing the effects of inhaled environmental pollutants on olfactory function in mice.
Introduction
Olfactory impairment has emerged as a noteworthy public health concern and is independently associated with an increased risk of neurodegenerative diseases. This condition can adversely affect overall well-being, contribute to the development of depressive symptoms, and result in a diminished quality of life. Its impact is prominently observed in the altered perception of food, hindrance in social communication, and heightened negative feelings1. Various factors, including sinonasal disease, upper respiratory tract infection, and traumatic brain injury, have been considered contributors to olfactory impairment in humans2. Notably, inhalable environmental pollutants such as PM2.5, estimated to range from 2% to 16%, enter the body through inspired air, traverse the nasal cavity, and reach specific olfaction-dedicated regions where they are deposited3,4,5,6,7. Recent findings indicate that inhalable environmental pollutants, including PM2.5 and ammonia, can indeed harm olfactory sensory neurons8,9,10. However, further validation is required to ascertain whether such damage directly leads to olfactory dysfunction. Hence, a meticulous evaluation of the effects of inhalable environmental pollutants on olfactory function is of particular importance.
Currently, numerous research laboratories employ mice as an alternative vertebrate model for behavioral experiments aimed at comprehending changes in olfactory function11,12,13,14. Mice were chosen as the preferred model system for investigating vertebrate chemical communication, and it exhibits a remarkable olfactory sensitivity crucial for foraging and social communication15. Furthermore, the continually evolving array of tools for observing and influencing mouse behavior has rendered this species exceptionally appealing for research on olfactory function16.
In this study, we employed the buried food test and the social odor discrimination experiment to assess olfactory impairment in a mouse model exposed to inhalable environmental pollutants. To enhance the precision of evaluation, we opted for the most representative method for assessing olfactory function. We systematically refined this method to ensure simplicity and clarity, allowing us to gauge the extent of olfactory dysfunction induced by inhalable environmental pollutants effectively.
Protocol
We used male C57BL/6J mice (age: 6-8 weeks; weight: 20-22 g) for all behavioral tests. The mice were subjected to steady conditions (i.e., temperature, 23 ± 1 °C; humidity, 55% ± 5%, and 12/12 h light-dark cycle with lights on at 7:00). All behavioral tests were performed between 10:00 and 17:00. All animal experiments were approved by the Ethics Committee of the Professional Committee of Animal Experiments of Qingdao University. After a 1-week acclimation period, all mice were exposed to inhalable environmental pollutants.
1. Exposure to pollutants
- Intranasal administration by instillation
- Dissolve inhalable environmental pollutants in a 0.9% saline solution. Use 5% zinc sulfate solution once, which has been demonstrated to cause olfactory dysfunction17. For control mice, administer 0.9% normal saline solution.
- Anesthetize the mouse by intraperitoneal injection of 1% sodium pentobarbital18 and assess the depth of anesthesia using the toe-pinch reflex. Apply vet ointment to mouse's eyes to prevent dryness. Position the mouse on its back on an inclined surface, with the head facing downward.
- Using a pipetting gun, administer 10 µL of the solution in one nostril of the mouse, allowing to naturally inhale the solution into the nasal cavity.
- To ensure the well-being of the mouse and prevent any potential discomfort, repeat the inhalation for the other nostril after a 10 min interval.
- At 3 days after intranasal administration by instillation in mice, perform the buried food test.
2. Buried food test
- Carry out food deprivation at 18-24 h before the test by removing all chow pellets from the food hopper of the home cage. Change the bedding materials for mice. Do not remove the water bottle.
- Arrange the operating table as described below.
- At 1 h before the start of the test, take the cage containing the mice to the operating room to rest.
- Arrange the operating room during this period. Use and mark transparent PVC standard mice cages as A as these make a familiar environment. Use and mark the test cage as B, which is a transparent PVC standard squirrel cage. Mark the common cage used to place the mice after the experiment as cage C.
- Cover cages A and B with 3 cm of bedding material and measure them with a ruler. Arrange the cages side by side, with a distance of 0.5 m between each cage (Figure 1).
- Define the experimental area as an area with a radius of 2 m, with the center being the center of the cages. Define the area outside the range of 2 m as the observation area.
- Keep cage C and cages containing untested mice as far away from the experimental area as possible.
- Record the time to find food as described below.
- Select a position at random in cage B, bury the food 1 cm below the surface of the bedding, and smooth the surface of the bedding.
- Put the mouse into cage A for 4 min. Transfer the mice to cage B at the end of the timing, turn on the video device, and return to the observation area.
- When the mouse picks up the food block with its forepaw, stop the video recording. In some cases, mice can be seen eating with their heads bent over the food. This behavior also indicates trial success, even if the mouse is not holding the food with its forepaw.
- Record data. Record the time from contact with the mat at the bottom of cage B until food was found for each mouse. If the mice do not find food after 4 min, record the find time as a delay time of 240 s.
- Put the mouse in cage C after the test.
- Remove the feed from cage B and put it in a sealed bag. Replace the food in cage B and test the next mouse after changing the bedding material, as described below.
- For mice housed in the same cage, test with the same set of bedding materials in cage B. For mice in different cages, clean the cage with alcohol and replace the bedding materials.
- Add feed and water to mice after the experiment. Perform the social odor discrimination experiment 1 day later.
NOTE: Masks need to be worn throughout the process, and transparent gloves need to be changed after each mouse finishes the experiment to avoid odor cross as much as possible. Keep the amplitude of the movements as small as possible to avoid mouse stress.
3. Social odor discrimination experiment
- Urine collection
- Separately collect the urine of sexually mature male mice and female mice and aliquot into tubes in equal volumes19. Pack in 2 mL microcentrifuge tubes with 300 µL of urine in each tube.
- Store the samples at -80 ° C until use. Shake tubes to evenly distribute the sample after thawing. Do not thaw and freeze the sample repeatedly.
- Arrange the operating table as described below.
- At 1 h before the start of the test, take the cage containing the mice to the operating room to rest.
- Arrange the operating room during this period as described in steps 2.2.2-2.2.5.
- Record the time to find urine as described below.
- Make a groove with tape around each of the two wide sides of cage B large enough to hold the microcentrifuge tube containing 300 µL of male and female urine. Place the tubes and keep the two tube covers closed for now.
- Put the mouse in cage A and set a countdown for 4 min. Open the two tubes of cage B at the end of the timing.
- Transfer the mouse to the middle position of cage B, at the same distance from the two tubes, then turn on the video recording equipment and gently and slowly retreat to the observation area.
- When the mouse sniffs the tube wall/mouth or even inside the tube, the tube is successfully smelled. At this time, press the stopwatch and record the time as the time of smelling the tube (M s), then continue to record the residence time (X s) until the mouse moves away from the tube.
- Continue timing the time sniffing the other tube. When the mouse sniffs the tube wall/mouth or even inside the tube, the tube is successfully smelled. Press the stopwatch and record the time it took to smell the other tube (N s), then continue to record the residence time (Y s) until the mouse moves away from the tube.
- Transfer the mouse to cage C at the end of the experiment.
- Change the urine tubes and bedding material of cage B after each mouse test. Put the used rat urine in a clean bag according to sex.
- Change gloves and test the next mouse as described above.
- Perform the experiment in a laboratory without obvious odor. Avoid personal products that emit strong odors. Wear gloves and masks throughout the procedure to avoid odor crossing as much as possible. Keep the amplitude of the movements as small as possible to avoid mouse stress.
Representative Results
Inhalable environmental pollutants impair olfactory function in mice. Atmospheric zinc emitted from incinerators and motor vehicles has been demonstrated to be an inhaled pollutant that can result in allergic lung inflammation20. Zinc sulfate is considered one of the typical compounds to cause olfactory dysfunction21. Therefore, we use zinc sulfate as the treatment to expose mice by intranasal instillation and test using the buried food test and social odor discrimination experiment. In the food burial test, it took longer than 240 s for the zinc sulfate-exposed mice to find the food compared to the control group (Figure 2). Food-deprived mice with normal olfaction typically find the buried food within 1 min. When the mice developed olfactory dysfunction, it took longer to find food or even could not find food. Compared with the control group, the mice in the exposed group took longer to find food, with some instances where they could not find food within 240 s, indicating olfactory function damage.
Similar results were observed in the social odor discrimination experiment. In mice exposed to zinc sulfate, neither male nor female urine could be sniffed during the set observation time (Figure 3A). Beyond the set time, the zinc sulfate-exposed mice briefly sniffed female and male urine, but the sniffing time was significantly less than that of the control mice (Figure 3B). When equal volumes of female and male urine are presented at the same time, male mice usually prefer female urine22. The mice in the exposed group needed a longer time to distinguish between female and male urine or could not even distinguish between them. Specifically, compared with the control group, the mice in the exposed group spent a longer time smelling the urine and spent a shorter time in the vicinity of the urine.

Figure 1: Operation table layout. Cages A and B were kept at a distance of 0.5 m. The video recording equipment was controlled above cage B by the mobile phone holder, and the video field was adjusted to record the internal environment of the cage. Please click here to view a larger version of this figure.
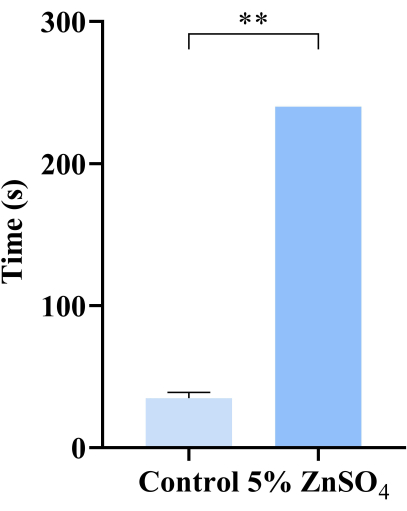
Figure 2: Buried food test results. Statistical analysis of the control mice and mice exposed to zinc sulfate in search of food. Mice in the control group found food within 30 s, and mice exposed to zinc sulfate found food for more than 240 s, which was calculated as 240 s. An independent sample t-test was used to compare the differences between the two groups. Data are presented as mean ± SD (n = 3 mice per group); ** p < 0.01. If any animal did not find the food after 240 s, the time to find food was recorded as 240 s, as was the case with all the treated animals. Please click here to view a larger version of this figure.
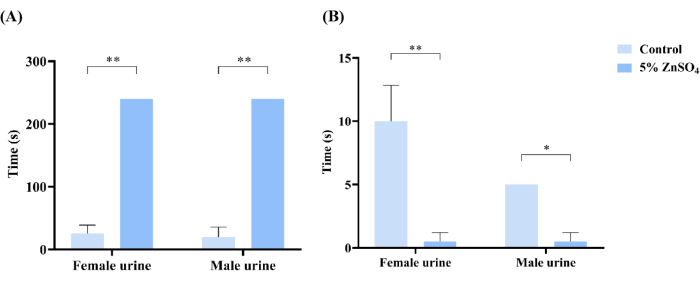
Figure 3: Social odor discrimination experiment. (A) Statistical analysis of the control mice and mice exposed to zinc sulfate in search of urine. Mice in the control group found urine within 30 s, and mice exposed to zinc sulfate spent more than 240 s looking for either female or male urine, which was counted as 240 s. If any animal did not find the urine vial after 240 s, the time to find the vial was recorded as 240 s, as was the case with all the treated animals. (B) The residence time in contact with urine was statistically analyzed for control mice and mice exposed to zinc sulfate. An independent sample t-test was used to compare the differences between the two groups. Data are presented mean ± SD (n = 3 mice per group); ** p < 0.01, * p < 0.5. Please click here to view a larger version of this figure.
Discussion
This article introduces two fundamental protocols designed for the swift assessment of olfactory impairment in mice. Varied inhalable environmental pollutants result in distinct levels of olfactory dysfunction in mice. The buried food test is employed to assess the capability to detect volatile odors, while the social odor discrimination experiment evaluates the animal's capacity to discern and differentiate various social odors. The protocol here serves to evaluate the toxic effects of environmental pollutants on olfactory function.
Inhaled environmental pollutants, including ozone, ammonia, and diesel exhaust, possess odors that may influence the mice's olfactory perception during sample sniffing23,24. It is crucial to note that conducting behavioral experiments in mice immediately after the conclusion of exposure could potentially yield false-negative outcomes22. To address this concern, we implemented measures to enhance experimental reliability. Specifically, cages were thoroughly cleaned, and bedding materials were replaced following the exposure period. Subsequently, behavioral experiments were conducted with a 24 h interval post-exposure22. This procedural adjustment aims to ensure accurate and meaningful results.
In the food burial experiment, a key parameter was the time taken by mice to locate concealed food. It is worth noting that stressed mice tend to exhibit heightened activity and reduced focus during testing, which may potentially lead to false positive results25,26,27,28. Therefore, it is imperative to ensure that the mice remain calm and are not subjected to undue stress during the testing phase. To avoid any potential interference, mice were transferred from their housing to the testing area 1 h prior to the experiment. This step was taken to allow the mice to acclimate and reduce stress. To facilitate accurate time recording, the video recorder was activated when the mice were introduced to experimental cage B, and then observers immediately retreated to the observation area. Observers were positioned at an optimal distance of 2 m from the cage, ensuring clear observation without causing disturbance. The criteria for identifying successful food discovery included instances where mice used their forepaws or bent their heads over the food. Both behaviors were considered indicative of finding food, aligning with widely accepted standards19. In cases where multiple mice within the same dose group needed testing, it was ensured that they were not returned to their original rearing cage but temporarily placed in an ordinary cage C. Only after testing all mice in the same cage were they collectively returned to their original housing. Given the potential odor of the tested foods, precautions were taken to store them in airtight bags beforehand and reseal or place them in airtight containers after use. These measures were implemented to maintain the integrity of the experimental conditions and ensure reliable results.
In social odor discrimination experiments, it is essential to utilize urine samples derived from sexually mature mice19. The determination of sexual maturity is based on the age of the mice, a parameter that varies across different mouse strains. Following collection, it is recommended to store male and female urine separately in a refrigerator at -80 °C. Previous studies employed stopwatches for timing purposes. However, this method had limitations, such as observers being situated at a considerable distance from the experimental cage, leading to potential issues like unclear observations and inaccurate timing19,22,29. To address these challenges, we have enhanced the timing procedures by incorporating video equipment instead of stopwatches. This modification not only facilitates the acquisition of more precise data but also enables the collection of data from multiple experimental groups.
In these experiments, the video device was used to record the behavior of mice, which has the advantage of reducing the frame rate of the video and enabling the observers to record the time of various behavior activities of mice more accurately, making up for the deficiency that the observers could not observe the behavior of mice in the observation area. However, the disadvantage of using video recording equipment is that the analysis time will increase. At the end of the experiment, we needed to watch the video again to record the time of each mouse's various behavioral activities. When the number of mice is large, it is necessary to analyze the video of each mouse, which will increase the labor burden of the laboratory.
Research findings indicate that various inhalable environmental pollutants can negatively impact olfactory function. This paper introduces a swift, uncomplicated, and reasonably precise approach to evaluating the olfactory toxicity associated with inhalable environmental pollutants. This method is crucial in conducting risk assessments for olfactory dysfunction attributed to environmental pollutants in human subjects.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (82204088, 82273669) and the Natural Science Foundation of Shandong Province, China (ZR2021QH209).
Materials
| 0.5-10 μL adjustable micropipette | Eppendorf, Germany | 3123000225 | Intranasal instillation |
| 0.9% saline solution | Solarbio | 7647-14-5 | Dissolve pollutants |
| Anhydrous zinc sulfate | Macklin | 7733-02-0 | Expose mice |
| Centrifuge tube (2 mL) | Biosharp Incorporated | BS-20-M | Place urine |
| Electronic balance | Changzhou Ohaus Co. | EX125DZH | Weight anesthetics and pollutants |
| GraphPad Prism | GraphPad Software | 8.0.1 | statistic analysis |
| Handheld Dust detector | TSI Incorporated | DuatTrak  8532 8532 |
Inhalation-exposed mice |
| Video recording equipment | Apple Inc. | iPhone 6s Plus | The activity time of mice was recorded |
| Vortex mixer | Haimen Kylin-Bell Lab Instruments Co. | Vortex-5 | Mix solution |
References
- Schäfer, L., Schriever, V. A., Croy, I. Human olfactory dysfunction: causes and consequences. Cell Tissue Res. 383 (1), 569-579 (2021).
- Keller, A., Malaspina, D. Hidden consequences of olfactory dysfunction: a patient report series. BMC Ear Nose Throat Disord. 13 (1), 8 (2013).
- Schroeter, J. D., et al. Application of physiological computational fluid dynamics models to predict interspecies nasal dosimetry of inhaled acrolein. Inhal Toxicol. 20 (3), 227-243 (2008).
- Schroeter, J. D., Garcia, G. J., Kimbell, J. S. A computational fluid dynamics approach to assess interhuman variability in hydrogen sulfide nasal dosimetry. Inhal Toxicol. 22 (4), 277-286 (2010).
- Keyhani, K., Scherer, P. W., Mozell, M. M. Numerical simulation of airflow in the human nasal cavity. J Biomech Eng. 117 (4), 429-441 (1995).
- Hahn, I., Scherer, P. W., Mozell, M. M. Velocity profiles measured for airflow through a large-scale model of the human nasal cavity. J Appl Physiol. 75 (5), 2273-2287 (1993).
- Zhang, Z., et al. Exposure to particulate matter air pollution and Anosmia. JAMA Netw Open. 4 (5), e2111606 (2021).
- Ekström, I. A., et al. Environmental air pollution and olfactory decline in aging. Environ Health Perspect. 130 (2), 27005 (2022).
- Adams, D. R., et al. Nitrogen dioxide pollution exposure is associated with olfactory dysfunction in older U.S. adults. Int Forum Allergy Rhinol. 6 (12), 1245-1252 (2016).
- Prah, J. D., Benignus, V. A. Decrements in olfactory sensitivity due to ozone exposure. Percept Mot Skills. 48 (1), 317-318 (1979).
- Shi, Z., et al. Chronic exposure to environmental pollutant ammonia causes damage to the olfactory system and behavioral abnormalities in mice. Environ Sci Technol. 57 (41), 15412-15421 (2023).
- Hernández-Soto, R., et al. Chronic intermittent hypoxia alters main olfactory bulb activity and olfaction. Exp Neurol. 340, 113653 (2021).
- Islam, S., et al. Odor preference and olfactory memory are impaired in Olfaxin-deficient mice. Brain Res. 1688, 81-90 (2018).
- Wang, H., et al. Inducible and conditional activation of ERK5 MAP kinase rescues mice from cadmium-induced olfactory memory deficits. Neurotoxicology. 81, 127-136 (2020).
- Chamero, P., Leinders-Zufall, T., Zufall, F. From genes to social communication: molecular sensing by the vomeronasal organ. Trends Neurosci. 35 (10), 597-606 (2012).
- Mohrhardt, J., et al. Signal detection and coding in the accessory olfactory system. Chem Senses. 43 (9), 667-695 (2018).
- Liu, X., et al. Type 3 adenylyl cyclase in the MOE is involved in learning and memory in mice. Behav Brain Res. 383, 112533 (2020).
- Li, X., et al. Polyhexamethylene guanidine aerosol triggers pulmonary fibrosis concomitant with elevated surface tension via inhibiting pulmonary surfactant. J Hazard Mater. 420, 126642 (2021).
- Yang, M., Crawley, J. N. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. Chapter 8, Unit 8.24 (2009).
- Huang, K. L., et al. Zinc oxide nanoparticles induce eosinophilic airway inflammation in mice. J Hazard Mater. 297, 304-312 (2015).
- Burd, G. D. Morphological study of the effects of intranasal zinc sulfate irrigation on the mouse olfactory epithelium and olfactory bulb. Microsc Res Tech. 24 (3), 195-213 (1993).
- Zou, J., et al. Methods to measure olfactory behavior in mice. Curr Protoc Toxicol. 63, 11.18.1-11.18.21 (2015).
- Ryalls, J. M. W., et al. Anthropogenic air pollutants reduce insect-mediated pollination services. Environ Pollut. 297, 118847 (2022).
- Schiffman, S. S. Livestock odors: implications for human health and well-being. J Anim Sci. 76 (5), 1343-1355 (1998).
- Albrechet-Souza, L., Gilpin, N. W. The predator odor avoidance model of post-traumatic stress disorder in rats. Behav Pharmacol. 30 (2 and 3-Spec Issue), 105-114 (2019).
- Davies, D. A., et al. Inactivation of medial prefrontal cortex or acute stress impairs odor span in rats. Learn Mem. 20 (12), 665-669 (2013).
- Landers, M. S., Sullivan, R. M. The development and neurobiology of infant attachment and fear. Dev Neurosci. 34 (2-3), 101-114 (2012).
- Drobyshevsky, A., et al. Antenatal insults modify newborn olfactory function by nitric oxide produced from neuronal nitric oxide synthase. Exp Neurol. 237 (2), 427-434 (2012).
- Arbuckle, E. P., et al. Testing for odor discrimination and habituation in mice. J Vis Exp. (99), e52615 (2015).

.
