Assessing Pupil-linked Changes in Locus Coeruleus-mediated Arousal Elicited by Trigeminal Stimulation
Summary
To verify whether trigeminal effects on cognitive performance involve locus coeruleus activity, two protocols are presented that aim to evaluate possible correlations between the performance and task-related pupil size changes induced by chewing. These protocols may be applied to conditions in which locus coeruleus contribution is suspected.
Abstract
Current scientific literature provides evidence that trigeminal sensorimotor activity associated with chewing may affect arousal, attention, and cognitive performance. These effects may be due to widespread connections of the trigeminal system to the ascending reticular activating system (ARAS), to which noradrenergic neurons of the locus coeruleus (LC) belongs. LC neurons contain projections to the whole brain, and it is known that their discharge co-varies with pupil size. LC activation is necessary for eliciting task-related mydriasis. If chewing effects on cognitive performance are mediated by the LC, it is reasonable to expect that changes in cognitive performance are correlated to changes in task-related mydriasis. Two novel protocols are presented here to verify this hypothesis and document that chewing effects are not attributable to aspecific motor activation. In both protocols, performance and pupil size changes observed during specific tasks are recorded before, soon after, and half an hour following a 2 min period of either: a) no activity, b) rhythmic, bilateral handgrip, c) bilateral chewing of soft pellet, and d) bilateral chewing of hard pellet. The first protocol measures level of performance in spotting target numbers displayed within numeric matrices. Since pupil size recordings are recorded by an appropriate pupillometer that impedes vision to ensure constant illumination levels, task-related mydriasis is evaluated during a haptic task. Results from this protocol reveal that 1) chewing-induced changes in performance and task-related mydriasis are correlated and 2) neither performance nor mydriasis are enhanced by handgrip. In the second protocol, use of a wearable pupillometer allows measurement of pupil size changes and performance during the same task, thus allowing even stronger evidence to be obtained regarding LC involvement in the trigeminal effects on cognitive activity. Both protocols have been run in the historical office of Prof. Giuseppe Moruzzi, the discoverer of ARAS, at the University of Pisa.
Introduction
In humans, it is known that chewing quickens cognitive processing1,2 and improves arousal3,4, attention5, learning, and memory6,7. These effects are associated with shortening of the latencies of cortical event-related potentials8 and an increase in the perfusion of several cortical and subcortical structures2,9.
Within cranial nerves, the most relevant information sustaining cortical desynchronization and arousal is carried by trigeminal fibres10, likely due to strong trigeminal connections to the ascending reticular activating system (ARAS)11. Among ARAS structures, the locus coeruleus (LC) receives trigeminal inputs11 and modulates arousal12,13, and its activity covaries with pupil size14,15,16,17,18. Although the relation between LC resting activity and cognitive performance is complex, task-related enhancement of LC activity leads to arousal-associated19 pupil mydriasis20 and enhanced cognitive performance21. There is reliable covariation between LC activity and pupil size, and the latter is currently considered a proxy of central noradrenergic activity22,23,24,25,26.
Asymmetric activation of sensorimotor trigeminal branches induces pupil asymmetries (anisocoria)27,28, confirming the strength of the trigemino-coerulear connection. If the LC participates in the stimulating effects of chewing on cognitive performance, it may affect parallel task-related mydriasis, which is an indicator of LC phasic activation during a task. It may also affect performance, so a correlation can be expected between chewing-induced changes in performance and mydriasis. Moreover, if trigeminal effects are specific, chewing effects should be larger than those elicited by another rhythmic motor task. In order to test these hypotheses, two experimental protocols are hereby presented. They are based on combined measurements of cognitive performance and pupil size, performed before and after a short period of chewing activity. These protocols utilize a test consisting in finding target numbers displayed in numeric attentive matrices29, alongside with non-target numbers. This test verifies attentive and cognitive performance.
The overall goal of these protocols is to illustrate that trigeminal stimulation elicits specific changes in cognitive performance, which cannot be ascribed aspecifically to the generation of motor commands and are related to pupil-linked changes in LC-mediated arousal. Applications of the protocols extend to all behavioral conditions in which performance can be measured and involvement of the LC is suspected.
Protocol
All steps follow the guidelines of the Ethical Committee of the University of Pisa.
1. Participant Recruitment
- Recruit a subject population according to the specific goal of the study (i.e., normal subjects and/or patients, males and/or females, young people and/or elders).
2. Material Preparation
- Prepare a soft pellet; use commercially available chewing gum (Table of Materials; initial hardness = 20 Shore OO).
- Prepare a hard pellet; use silicon rubber pellets (Table of Materials; constant hardness = 60 Shore OO)30.
- Prepare an anti-stress ball for a handgrip task. Use a polyurethane foam-made ball (Table of Materials; constant hardness = 30 Shore OO)30.
- Prepare a tangram puzzle (Table of Materials; number of pieces = seven) for performing the haptic task.
3. Flowchart of the Experiment
- Flowchart of protocol 1
- Evaluate baseline performance (see section 4.1) in the cognitive (matrices) test (T0, control).
- Evaluate pupil size (see section 4.2) at rest (no activity requested from the subject) (T0, control).
- Evaluate pupil size during a haptic task based on tangram (T0, control).
- Remove one of the pieces from the puzzle and place it in the subject's hand.
- Ask the subject to put the piece back into the puzzle, without looking at the puzzle.
- Ask each subject to perform three specific activities for 2 min or to rest for 2 min, according to steps 3.1.4.1–3.1.4.4. Ask the subjects to perform these activities in separate sessions occurring on different days (2–3 days between sessions).
- Ask the subject to chew a self-administered soft pellet for 2 min, letting him/her spontaneously choose both the rate of chewing and side of the mouth on which to chew. After 1 min of chewing, ask him/her to change the chewing side (and the pellet).
- Ask the subject to chew a self-administered hard pellet for 2 min. After 1 min, ask him/her to change the chewing side (but not the pellet).
- Ask the subject to perform a rhythmic squeezing of an anti-stress ball (handgrip exercise) for 2 min at the rate and on the hand of their choosing. After 1 min, ask the subject to switch hands.
- Ask the subject to rest (no activity) for 2 min.
- Just after the end of each step (3.1.4.1–3.1.4.4), evaluate performance in the matrices test and pupil size at rest and during the haptic task (T7).
NOTE: The term "at rest" means that the subject during the pupil size measurement is relaxing. The term "during haptic task" means that the subject during the pupil size measurement is performing the task based on tangram. - Thirty minutes following the end of each step (3.1.4.1–3.1.4.4), evaluate performance and pupil size at rest and during the haptic task (T37).
- Flowchart of protocol 2
- Evaluate pupil size while the subject is resting (T0, control; see section 4.3).
- Evaluate baseline performance in the cognitive (matrices) test while simultaneously testing pupil size (T0, control).
- Ask each subject to perform three specific activities for 2 min or to rest for 2 min, according to steps 3.2.3.1–3.2.3.4. Ask the subjects to perform these activities in separate sessions occurring on different days (2–3 days between sessions).
- Ask the subject to chew a self-administered soft pellet for 2 min, letting him/her spontaneously choose both the rate of chewing and side of the mouth on which to chew. After 1 min of chewing, ask him/her to change the chewing side (and the pellet).
- Ask the subject to chew a self-administered hard pellet for 2 min. After 1 min, ask him/her to change the chewing side (but not the pellet).
- Ask the subject to perform a rhythmic squeezing of an anti-stress ball (handgrip exercise) for 2 min at the rate and on the side of their choosing. After 1 min, ask the subject to switch hands.
- Ask the subject to relax (no activity) for 2 min.
- Just after the end of each step (steps 3.2.3.1–3.2.3.4), evaluate pupil size at rest and both performance and pupil size in the matrices test (T7).
- Thirty minutes following the end of each step (steps 3.2.3.1–3.2.3.4), evaluate pupil size at rest and both performance and pupil size in the matrices test (T37).
4. Measured Variables in Protocols 1 and 2
- Cognitive performance
NOTE: In both protocols 1 and 2, measure cognitive performance using a test based on a modified version of the Spinnler-Tognoni numeric matrices test29.- Display three numerical matrices (10 x 10) printed on paper to the subject. Then, ask the subject to sequentially scan the matrix lines, while ticking with a pencil as many of the target numbers as possible (60 targets out of 300 total displayed numbers) indicated above each matrix (Figure 1) within 15 s.
- Utilize matrices with different positions of target numbers at T0, T7, and T37 to avoid the introduction of confounders related to learning processes.
- Evaluate offline the performance index (PI), scanning rate (SR), and error rate (ER) as follows: PI = (target numbers underlined in 15 s)/15; SR = (target + non-target numbers scanned in 15 s)/15; ER = (target numbers missed + non-target numbers underlined in 15 s)/15.
- Pupil size in protocol 1
- Prepare the subject for the pupil size measurement with a corneal topographer-pupillographer (Table of Materials), which prevents vision of the environment, using one of the following two acquisition procedures.
- Record a single camera shot of the pupil (Figure 2A,B) with a constant illumination level of 40 lux, pressing the specific button on the corneal topographer-pupillographer. Maintain an optimal working distance of 56 mm between the camera and pupil.
NOTE: A single measurement is sufficient, due to the low level of variability of pupil size measured at constant illumination. - Perform a continuous recording of the pupil (sampling rate = 5 Hz; Figure 2C,D) in the continuous acquisition modality. Discard the first 20–50 measurements (4–10 s), since during this lapse of time, the pupil diameter is growing (the acquisition starts by switching off pupil illumination at 40 lux). Average the remaining measurements.
- Record a single camera shot of the pupil (Figure 2A,B) with a constant illumination level of 40 lux, pressing the specific button on the corneal topographer-pupillographer. Maintain an optimal working distance of 56 mm between the camera and pupil.
- Record pupil size of the left and right eyes separately at rest (steps 3.1.2, 3.1.5, and 3.1.6).
- Record pupil size during the haptic task (steps 3.1.3, 3.1.5 and 3.1.6; left and right separately). When using the single shot modality (step 4.2.1.1), acquire the photo during the second of two task repetitions, at the beginning of puzzle surface exploration. In the continuous mode of recording (step 4.2.1.2), start the acquisition when the piece of the puzzle has been placed in the hand of the subject.
- Evaluate offline left and right pupil size at rest and during the haptic task by direct acquisition of the values (in mm) displayed by the software. Calculate the task-related mydriasis by subtracting the pupil size at rest from pupil size during the haptic task, and obtain all average left-right values.
- Prepare the subject for the pupil size measurement with a corneal topographer-pupillographer (Table of Materials), which prevents vision of the environment, using one of the following two acquisition procedures.
- Pupil size in protocol 2
- Prepare the subject for the pupil size measurement using a wearable pupillometer/eye tracker (Figure 3A), endowed with a 3D-printed glass frame structure, using the following procedure.
- Have the subject wear the wearable pupillometer. Adjust the position of the two infrared cameras (Figure 3A-2,3) mounted on bars stemming from the frame (Table of Materials), so that the eyes are within the field of view of the cameras and in focus.
- Acquire images of the pupils (sampling rate = 120 Hz), which are processed online by the software supplied with the wearable pupilometer and provides pupillary diameter (in mm) using a geometrical model of the "average" human ocular. Disregard blink artifacts.
- Record continuously the environmental illumination level using a calibrated logarithmic light sensor mounted on the wearable pupillometer frame. Use a frontal RGB camera mounted on the wearable pupillometer (Figure 3A-1) to record the subject field of view (sampling rate = 30 Hz) useful to study gaze behaviour.
- Record simultaneously the size of the two pupils at rest for 20 s (Figure 3B).
- Record the size of the pupils while the subject performs the Spinnler-Tognoni test, so to have pupil size and cognitive performance simultaneously recorded (steps 3.2.2, 3.2.4, and 3.2.5).
- Evaluate offline left and right pupil size at rest and during the Spinnler-Tognoni test, by averaging acquired values (n = 2,400) for each pupil. Calculate the task-related mydriasis by subtracting the pupil size at rest from the pupil size during the matrices test, then all the average left-right values.
- Prepare the subject for the pupil size measurement using a wearable pupillometer/eye tracker (Figure 3A), endowed with a 3D-printed glass frame structure, using the following procedure.
- Gaze position
NOTE: Reconstruct online the fixation point using the images of the two pupils obtained from section 4.3. Process the acquired frames in real-time and estimate the gaze fixation point using a previously calculated transfer function31 specific for each subject wearing the eye tracker.- If necessary, when performing protocol 2, reconstruct gaze position from the pupil images. To do this, add four computer detectable vision markers (ArUco or AprilTag libraries of the instrument software) to four corners of the matrices sheet used in section 4.1.
- Allow the calibration system (embedded in the eye tracker software as for the pupil headset used) to acquire the data, and evaluate the parameters of the transfer function that map the fixation point, starting from images of the two pupils. As an example, ask the subject to gaze at a predefined sequence of points that are shown in his/her field of view (i.e., the four corners of the matrices sheet and at the center of the sheet itself), which are recorded simultaneously by the additional RGB camera mounted on the frame and facing the field of view.
- Record pupil size during the matrices test.
- Calculate offline gaze position that appears as a mark on every frame of the subject's field of view. Use the four markers to track gaze position over the matrices across frames.
5. Statistical Analysis
- Analyze pupil size at rest and during the task, task-induced mydriasis, PI, SR, and ER under four conditions (no activity, handgrip, soft pellet, hard pellet) for three times (T0, T7, T37) using repeated measures ANOVA and statistics software package.
- Analyze changes in variables with respect to baseline values (T0) under four conditions, (no activity, handgrip, soft pellet, hard pellet) for two times (T7, T37) using repeated measures ANOVA.
- When running ANOVA, if the software indicates that the data distribution is not spherical, take the p-value corresponding to the Greenhouse-Geisser ε correction from the outputted statistic table.
- Correlate the changes in performance (PI, SR, ER) at T7 and T37 with those observed in task-related mydriasis by linear regression analysis.
Representative Results
Figure 4 shows a representative example of the results obtained when protocol 1 was applied to a single subject (46 years old, female). PI was increased soon after having chewed (T7) both a hard (from 1.73 numb/s to 2.27 numb/s) and soft pellet (from 1.67 numb/s to 1.87 numb/s) (Figure 4A). However, 30 min later (T37), the increased performance persisted only for the hard pellet. On the other hand, both a lack of activity and the handgrip exercise had a negative effect on performance, which dropped from 1.73 numb/s to 1.67 numb/s and from 1.6 numb/s to 1.53 numb/s, with a tendency to recover observed 30 min later, during the last experimental evaluation.
As observed in Figure 4B, qualitatively similar changes were observed for the task-related mydriasis. In this instance, measurements consisted of individual samples taken randomly when the subject was resting. During the haptic task, two samples were recorded, but the first was discarded. Alternatively, in the continuous acquisition mode of the instrument, 100 samples were recorded in 20 s, with the first 20–50 measurements disregarded, and the remaining were then averaged following the removal of blink artefacts (Figure 2C,D). Individual samples closely reflect the average value, due to the fact that pupil size reaches a very stable level 4–10 s following the switching off of eye illumination (Figure 2C,D). Data illustrated in Figure 4 and Figure 5 have been replicated in a population of 30 subjects, and both the chewing- and handgrip-induced changes were statistically confirmed. On the other hand, when the subjects were not involved in any activity, there were no modifications in cognitive performance and mydriasis30 both at T7 and T37.
Despite the fact that 1) performance and mydriasis were recorded in different tasks and 2) the 12 experimental points illustrated in Figure 5A,B were recorded on 4 separate days, it is remarkable that a strong correlation was observed between performance and task-related mydriasis (r = 0.939, p < 0.0005, y = 1.166x – 0.417). As can be inferred from Figure 5A, this relation was due to the modifications induced by chewing hard and soft pellets. Even more surprisingly, a correlation was evident also when the corresponding changes with respect to baseline values were considered (r = 0.924, p < 0.001, y = 1.210x + 0.101; Figure 5B).
Among the 30 subjects analyzed in the study of Tramonti Fantozzi et al.30, PI and mydriasis were significantly correlated in 26 of them, with slopes of the corresponding regression lines ranging from 0.310–1.327 numb/s/mm. The corresponding changes were significantly correlated in 22 subjects (range of slopes: 0.390–1.408).
Even stronger evidence of LC involvement in the stimulating effects of chewing on cognitive performance can be obtained by correlating the chewing-induced changes in PI with the change in mydriasis observed only during the execution of the matrices test. This can be achieved under the more natural conditions of protocol 2, in which subjects perform the matrices test while pupil size is simultaneously recorded (Figure 6).

Figure 1: Example of Spinnler-Tognoni numerical matrices. The test consists in identifying the target numbers indicated above each matrix, which have been ticked by the subject. Please click here to view a larger version of this figure.
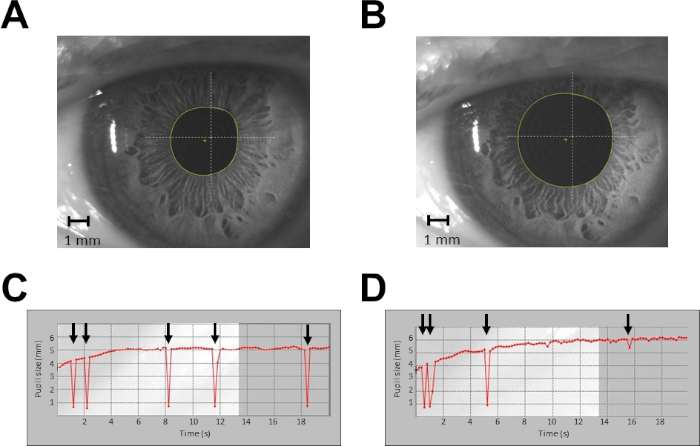
Figure 2: Example of pupil size recordings from a single subject in protocol 1. (A) Recording of pupil size at rest, single shot. (B) Recording of pupil size during haptic task, single shot. (C) Continuous recording of pupil size at rest for 20 s. (D) Continuous recording of pupil size during haptic task for 20 s. Arrows indicate blinking artefacts. In (C) and (D), data taken from time 0 to time 4 s are discarded from the analysis. Please click here to view a larger version of this figure.
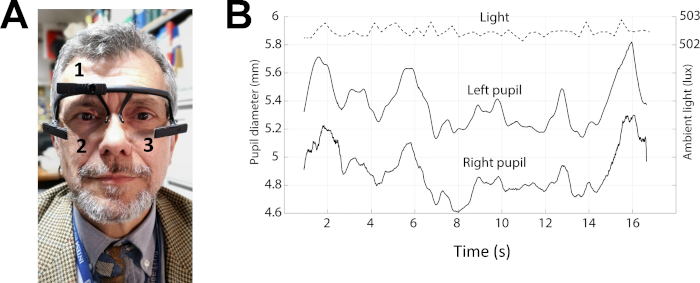
Figure 3: Example of pupil size recordings in protocol 2. (A) Photo of a subject wearing the pupillometer. The numbers 1–3 indicates the position of the three cameras, which allow behaviour (1) and pupil size (2-3) recordings. (B) Top trace: level of the environmental lightening. Middle and bottom traces: left and right pupil size during performance of the Spinnler-Tognoni matrices test. Please click here to view a larger version of this figure.
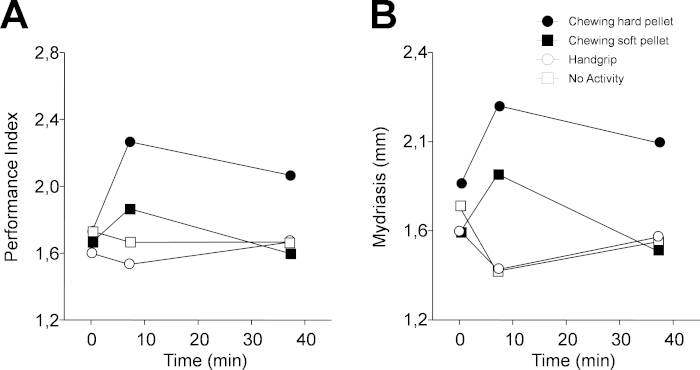
Figure 4: Changes in performance and task-related mydriasis induced by different sensorimotor activities in protocol 1. (A) Changes in PI. (B) Changes in task-related mydriasis. In (A) and (B), dots, black squares, circles and white squares represent data relative to chewing hard pellet, chewing soft pellet, handgrip, and no activity, respectively. Each activity was performed for 2 min from time 5 min to time 7 min. Please click here to view a larger version of this figure.
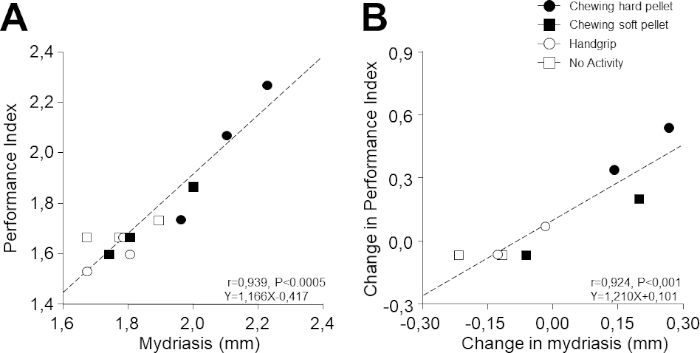
Figure 5: Relation between PI and task-related mydriasis. (A) PI values obtained at different times during the different activities illustrated in Figure 4 are plotted as a function of the corresponding values of task-related mydriasis. (B) Changes in PI with respect to T0 (evaluated as a difference) have been plotted as a function of the corresponding changes in task-related mydriasis. In (A) and (B), dots, black squares, circles, and white squares represent data relative to chewing hard pellet, chewing soft pellet, handgrip, and no activity, respectively. Dashed lines are regression lines of all the data points. Please click here to view a larger version of this figure.
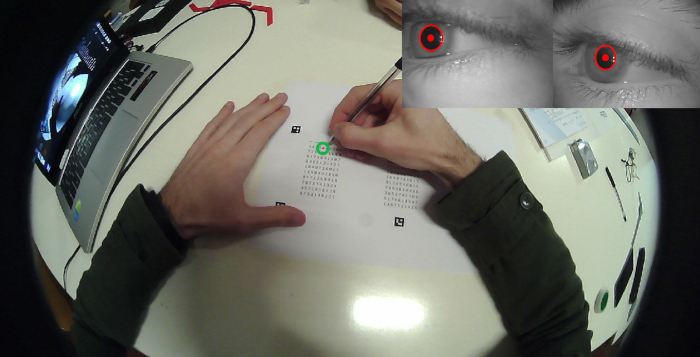
Figure 6: Simultaneous recording of performance and task-related mydriasis. Single frame view of a subject performing the attentive matrices test, taken from the camera mounted on the pupilometer frame. The inset on the right upper corner shows the simultaneous images of both pupils. The green circle represents the fixation point. The red spot and circles drown on the pupil are the pupil centre and contour, as evaluated by the tracking system operating on the eye's videos. Please click here to view a larger version of this figure.
Discussion
The protocols presented in this study address the acute effects of sensorimotor trigeminal activity on cognitive performance and the role of the LC in this process. This topic has some relevance, considering that 1) during aging, the deterioration of masticatory activity correlates with cognitive decay32,33,34; people that preserve oral health are less prone to neurodegenerative phenomena; 2) malocclusion and teeth extraction induces neurodegenerative effects in animals at hippocampal and cortical level35,36,37,38,39; 3) the LC exerts trophic action on the brain, regulates neurovascular coupling, and inhibits neuroinflammation and accumulation of beta-amyloid11,40; 4) there is evidence that neurodegenerative diseases can be triggered by neurodegenerative processes at the LC level11,40.
Protocol 1 allows the defining of specific effects of chewing with respect to a) learning processes elicited by successive repetitions of the task and b) other kinds of ordinary motor activity. Moreover, it establishes the presence/absence of a correlation between changes in performance and mydriasis, with the latter considered an indicator of phasic LC activation during task. This evidence strongly suggests involvement of the LC in the effects of sensorimotor trigeminal activation. Such a protocol has been successfully applied by Tramonti Fantozzi et al.30. As seen in the results section, it may also be utilized to assess the degree of dependence of performance on pupil changes linked to LC-mediated arousal at the level of individual subjects. Gaining this measurement (performance/LC activation) represents a new and important neuropsychological variable that can be studied in relation to gender, age, drug administration, and any behavioral condition.
The main limitation of protocol 1 is that pupil size measurements are performed at constant lightening, impeding vision and precluding the assessment of mydriasis elicited during matrices scanning. This obliges the recording of mydriasis during a different task. This problem is resolved by performing protocol 2, in which a wearable pupillometer endowed with a light sensor is introduced. In this way, it is possible to contextually record both cognitive performance and mydriasis during the same task, providing even more compelling evidence about the effects of sensorimotor activity on LC and performance. This also helps to address studies aimed at relating LC activation to behavioral conditions. For a correct application of protocol 2, care must be taken to keep a constant level of environmental lighting and preliminary calibration of wearable instruments.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The research was supported by grants of the University of Pisa. We thank Mr. Paolo Orsini, Mr. Francesco Montanari, and Mrs. Cristina Pucci for valuable technical assistance, as well as the I.A.C.E.R. S.r.L. company for supporting Dr. Maria Paola Tramonti Fantozzi with a fellowship. Finally, we thank the OCM Projects company for preparing hard pellets and performing hardness and spring constant measurements.
Materials
| Anti-stress ball | Artengo, Decathlon, France | TB600 | |
| Chewing gum | Vigorsol, Perfetti, Italy | Commercially available product | |
| Infrared Camera-Wearable pupillometer | Pupil Labs, Berlin, Germany | Pupil Labs headset | |
| Pupillographer | CSO, Florence, Italy | MOD i02, with chin support | |
| Silicon rubber | Prochima, Italy | gls50 | |
| Software for pupil detection – wearable pupillometer | Pupil Labs, Berlin, Germany | Pupil Labs headset | |
| Tangram Puzzle | Città del Sole srl, Milano, Italy | Tangram Puzzle | |
| Wearable pupillometer | Pupil Labs, Berlin, Germany | Pupil labs model | Dimension of the frame: 13.5 x 15.5cm |
References
- Hirano, Y., et al. Effects of chewing on cognitive processing speed. Brain and Cognition. 81 (3), 376-381 (2013).
- Hirano, Y., Onozuka, M. Chewing and cognitive function. Brain and Nerve. 66 (1), 25-32 (2014).
- Allen, A. P., Smith, A. P. Effects of chewing gum and time-on-task on alertness and attention. Nutritional Neuroscience. 15 (4), 176-185 (2012).
- Johnson, A. J., et al. The effect of chewing gum on physiological and self-rated measures of alertness and daytime sleepiness. Physiology & Behavior. 105 (3), 815-820 (2012).
- Tucha, O., Mecklinger, L., Maier, K., Hammerl, M., Lange, K. W. Chewing gum differentially affects aspects of attention in healthy subjects. Appetite. 42 (3), 327-329 (2004).
- Allen, K. L., Norman, R. G., Katz, R. V. The effect of chewing gum on learning as measured by test performance. Nutrition Bulletin. 33 (2), 102-107 (2008).
- Smith, A. Effects of chewing gum on mood, learning, memory and performance of an intelligence test. Nutritional Neuroscience. 12 (2), 81-88 (2009).
- Sakamoto, K., Nakata, H., Kakigi, R. The effect of mastication on human cognitive processing: a study using event-related potentials. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 120 (1), 41-50 (2009).
- Hirano, Y., et al. Effects of chewing in working memory processing. Neuroscience Letters. 436 (2), 189-192 (2008).
- Roger, A., Rossi, G. F., Zirondoli, A. Le rôle des afferences des nerfs crâniens dans le maintien de l’etat vigile de la preparation “encephale isolé”. Electroencephalography and Clinical Neurophysiology. 8 (1), 1-13 (1956).
- De Cicco, V., et al. Trigeminal, Visceral and Vestibular Inputs May Improve Cognitive Functions by Acting through the Locus Coeruleus and the Ascending Reticular Activating System: A New Hypothesis. Frontiers in Neuroanatomy. 11, 130 (2017).
- Samuels, E. R., Szabadi, E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Current Neuropharmacology. 6 (3), 235-253 (2008).
- Carter, M. E., et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nature Neuroscience. 13 (12), 1526-1533 (2010).
- Rajkowski, J., Kubiak, P., Aston-Jones, G. Correlations between locus coeruleus (LC) neural activity, pupil diameter and behaviour in monkey support a role of LC in attention. Society for Neuroscience Abstracts. 19, 974 (1993).
- Rajkowski, J., Kubiak, P., Aston-Jones, G. Locus coeruleus activity in monkey: phasic and tonic changes are associated with altered vigilance. Brain Research Bulletin. 35 (5-6), 607-616 (1994).
- Alnæs, D., et al. Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. Journal of Vision. 14 (4), (2014).
- Murphy, P. R., O’Connell, R. G., O’Sullivan, M., Robertson, I. H., Balsters, J. H. Pupil diameter covaries with BOLD activity in human locus coeruleus. Human Brain Mapping. 35 (8), 4140-4154 (2014).
- Joshi, S., Li, Y., Kalwani, R. M., Gold, J. I. Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron. 89 (1), 221-234 (2016).
- Bradshaw, J. Pupil size as a measure of arousal during information processing. Nature. 216 (5114), 515-516 (1967).
- Gabay, S., Pertzov, Y., Henik, A. Orienting of attention, pupil size, and the norepinephrine system. Attention, Perception & Psychophysics. 73 (1), 123-129 (2011).
- Usher, M., Cohen, J. D., Servan-Schreiber, D., Rajkowski, J., Aston-Jones, G. The role of locus coeruleus in the regulation of cognitive performance. Science (New York, NY). 283 (5401), 549-554 (1999).
- Laeng, B., et al. Invisible emotional expressions influence social judgments and pupillary responses of both depressed and non-depressed individuals. Frontiers in Psychology. 4, (2013).
- Silvetti, M., Seurinck, R., van Bochove, M. E., Verguts, T. The influence of the noradrenergic system on optimal control of neural plasticity. Frontiers in Behavioral Neuroscience. 7, 160 (2013).
- Hoffing, R. C., Seitz, A. R. Pupillometry as a glimpse into the neurochemical basis of human memory encoding. Journal of Cognitive Neuroscience. 27 (4), 765-774 (2015).
- Kihara, K., Takeuchi, T., Yoshimoto, S., Kondo, H. M., Kawahara, J. I. Pupillometric evidence for the locus coeruleus-noradrenaline system facilitating attentional processing of action-triggered visual stimuli. Frontiers in Psychology. 6, 827 (2015).
- Hayes, T. R., Petrov, A. A. Pupil Diameter Tracks the Exploration-Exploitation Trade-off during Analogical Reasoning and Explains Individual Differences in Fluid Intelligence. Journal of Cognitive Neuroscience. 28 (2), 308-318 (2016).
- De Cicco, V., Cataldo, E., Barresi, M., Parisi, V., Manzoni, D. Sensorimotor trigeminal unbalance modulates pupil size. Archives Italiennes De Biologie. 152 (1), 1-12 (2014).
- De Cicco, V., Barresi, M., Tramonti Fantozzi, M. P., Cataldo, E., Parisi, V., Manzoni, D. Oral Implant-Prostheses: New Teeth for a Brighter Brain. PloS One. 11 (2), e0148715 (2016).
- Spinnler, H., Tognoni, G. Italian standardization and classification of Neuropsychological tests. The Italian Group on the Neuropsychological Study of Aging. Italian Journal of Neurological Sciences. 8, 1 (1987).
- Tramonti Fantozzi, M. P., et al. Short-Term Effects of Chewing on Task Performance and Task-Induced Mydriasis: Trigeminal Influence on the Arousal Systems. Frontiers in Neuroanatomy. 11, 68 (2017).
- Kassner, M., Patera, W., Bulling, A. Pupil: An Open Source Platform for Pervasive Eye Tracking and Mobile Gaze-based Interaction. arXiv.org. , (2014).
- Gatz, M., et al. Potentially modifiable risk factors for dementia in identical twins. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2 (2), 110-117 (2006).
- Okamoto, N., et al. Relationship of tooth loss to mild memory impairment and cognitive impairment: findings from the Fujiwara-kyo study. Behavioral and Brain Functions. 6, 77 (2010).
- Weijenberg, R. A. F., Lobbezoo, F., Knol, D. L., Tomassen, J., Scherder, E. J. A. Increased masticatory activity and quality of life in elderly persons with dementia–a longitudinal matched cluster randomized single-blind multicenter intervention study. BMC Neurology. 13, 26 (2013).
- Kato, T., et al. The effect of the loss of molar teeth on spatial memory and acetylcholine release from the parietal cortex in aged rats. Behavioural Brain Research. 83 (1-2), 239-242 (1997).
- Onozuka, M., et al. Impairment of spatial memory and changes in astroglial responsiveness following loss of molar teeth in aged SAMP8 mice. Behavioural Brain Research. 108 (2), 145-155 (2000).
- Watanabe, K., et al. The molarless condition in aged SAMP8 mice attenuates hippocampal Fos induction linked to water maze performance. Behavioural Brain Research. 128 (1), 19-25 (2002).
- Kubo, K. Y., Iwaku, F., Watanabe, K., Fujita, M., Onozuka, M. Molarless-induced changes of spines in hippocampal region of SAMP8 mice. Brain Research. 1057 (1-2), 191-195 (2005).
- Oue, H., et al. Tooth loss induces memory impairment and neuronal cell loss in APP transgenic mice. Behavioural Brain Research. 252, 318-325 (2013).
- Mather, M., Harley, C. W. The Locus Coeruleus: Essential for Maintaining Cognitive Function and the Aging Brain. Trends in Cognitive Sciences. 20 (3), 214-226 (2016).

