Two-Photon Laser-Mediated Ablation of Osteoblasts in Developing Zebrafish Larvae
Summary
This protocol describes a two-photon laser ablation approach carried out in zebrafish larvae, which serves as a model to study bone regeneration and the effects of the immune response to ablation.
Abstract
Zebrafish (Danio rerio) have an outstanding capacity to regenerate different organs and appendages. Bone regeneration in zebrafish has been studied using different methods such as fin amputation, scale plucking, skull trepanation, and microscopic approaches. Using a confocal laser scanning setup equipped with a two-photon laser, a laser ablation method was developed as a lesion paradigm to ablate bone-forming cells (osteoblasts) in the developing opercle of zebrafish larvae. The method described here allows the ablation of cells in a precise manner, as the area, shape, and depth can be finely adjusted. In addition, this method allows imaging of the area before and just after the ablation, so that short-term effects of the injury can be analyzed. In this experimental setup, the immune response after ablation of osteoblasts in the injured area was studied. An increase in the recruitment of macrophages was observed after ablation, indicating the relevance of their presence during bone regeneration.
Introduction
Zebrafish regenerate diverse organs such as the retina, brain, heart, and pancreas1. In addition, zebrafish regenerate skeletal elements, which is why they have been used to study the regeneration of fins, scales, and calvariae (skull caps)2. Different experimental paradigms have been used to study tissue and bone regeneration, such as fin resection (amputation), fin fracture, skull trepanation3,4, cryoinjury5,6, or genetic ablation7,8,9. Recently, laser ablation approaches have been widely used in zebrafish to study the healing and regeneration response after injury10,11,12,13,14.
Laser-mediated ablation has been used in different areas of biological research, such as mechanobiology, developmental biology, regeneration research, and tumor surgery10,11,12,15,16,17,18,19. There are various methodologies of laser ablation, such as using YAG (yttrium aluminum garnet), UV (ultraviolet), or 2p (two-photon) lasers20. Laser ablation allows the removal of single cells or larger portions of tissues in a very accurate manner, enabling the study of different processes, such as the response of the immune system to tumor ablation21 or during zebrafish opercle regeneration. In the latter example, ablation was confirmed by the disappearance of the nuclear and cytoplasmic fluorophore signal and necrosis staining10,22.
It is well known that the innate immune response and its regulated kinetics are essential for appropriate tissue regeneration. Neutrophils and macrophages are the first cells to be recruited to the injury site, where they perform different roles, such as cytokine and growth factor release, cellular debris removal, and extracellular matrix remodeling23. This recruitment and subsequent macrophage functionalization have also been observed in amputated zebrafish fins24 and the developing opercle, which was subjected to laser 'nanodissection' leading to osteoblast ablation10. In the latter experiment, recovery of the osteoblast number, normal opercle development, and recruitment of innate immune cells (neutrophils, macrophages, and osteoclast-like cells) to the injured area was observed after UV laser and two-photon laser-mediated ablation10. Experiments in zebrafish in which the immune system was pharmacologically suppressed by using glucocorticoids showed an impairment of regeneration upon misregulated immunity, supporting the functional role of the immune system in tissue repair3,10,25,26.
Here, a two-photon laser-mediated ablation methodology to study the biology of bone regeneration in developing zebrafish bones is described. In particular, the effect of the osteoblast ablation approach in the opercle is shown in terms of the immune response, which is investigated by monitoring the recruitment of macrophages to the ablation site.
Protocol
The study protocol received approval from the Landesdirektion Sachsen, permit numbers 25-5131/564/2, DD25-5131/450/4, 25-5131/496/56, DD25.1-5131/354/87. The zebrafish strains used were maintained according to national law and under standardized conditions as previously described27,28. The details of all the reagents and the equipment used in the study are listed in the Table of Materials.
1. Preparation of materials and solutions
- Maintain the embryos in 1x E3 media. Prepare the 1x E3 media by diluting 170 mL of a 60x E3 stock in 10 L of deionized water and adding 2 mL 0.1% methylene blue.
- Prepare the 60x E3 media stock by mixing 17.2 g of NaCl, 0.76 g of KCl, 2.90 g of CaCl2·2 H2O, 4.90 g of MgSO4·7 H2O in 1 L of deionized autoclaved water.
- For the larvae embedding, prepare aliquots of 1% low melting point agarose (LMA) in advance and keep them at room temperature.
- To prepare aliquots, dissolve LMA in 1x E3 media, e.g., 500 mg of LMA in 50 mL of 1x E3 in an Erlenmeyer flask, heat the solution in the microwave until the agarose completely dissolves, and dispense it into 1 mL aliquots in 1.5 mL tubes.
2. Zebrafish breeding and embryo collection
NOTE: Double transgenic zebrafish larvae reporting the presence of committed osteoblasts [osterix:nGFP = Tg(Ola.Sp7:NLS-GFP)zf132]29 and macrophages [mpeg:mCherry = Tg(mpeg:mCherry)gl23] were used here30 (Figure 1A).
- Set up parental zebrafish with the desired single transgenes in standard mating cages the evening before mating.
NOTE: Standard mating cages allow the eggs to pass to a lower compartment where the parental zebrafish cannot reach them.- Keep males and females separated until mating using a divider in the mating cage. Remove the divider in the morning of the next day to initiate mating.
- At noon, at the latest, remove parental zebrafish from the breeding tanks and collect the embryos, straining the water from the mating cage through a sieve.
NOTE: In case the exact age of the larvae is decisive, harvest embryos in regular intervals of approximately 30 min. Clutch mates should be used in experiments with different experimental groups. - Place the embryos in a 100 mm diameter dish containing 1x E3 media and keep them in an incubator at 28 °C31.
- Remove unfertilized eggs and dead embryos from the dish using a plastic pipette with the help of a stereomicroscope and change 1x E3 media daily.
- Before the experiment, sort larvae positive for the reporter transgenes using a fluorescence stereomicroscope.
3. Larvae embedding in low-melting agarose
- Melt LMA aliquots in a thermoblock or water bath at 65 °C. Once the LMA is melted, keep it at 42 °C until embedding (Figure 1B). Add 50 µL of 0.4% MS-222 to the melted 1 mL of LMA aliquot to achieve a 0.02% MS-222 concentration for anesthesia.
- Anaesthetize 4 dpf (days post fertilization) larvae (or older larvae) in 0.02% MS-222 E3 media.
- Transfer the anesthetized larvae to a glass-bottom microwell dish using a 3 mL plastic Pasteur pipette and remove the excess E3 media with the same pipette (Figure 1B). Avoid drying up of the larvae.
NOTE: More than one larva can be embedded in a dish simultaneously; it is recommended to embed 3 to 5 larvae to allow sufficient time for placement. - Take the melted LMA agarose from the water bath/thermoblock and add a (cooling) drop of LMA to the dish, covering the glass bottom where the larvae were positioned. Pay attention that the LMA is not too hot but still liquid.
- Then, place the larvae into the desired lateral position before the agarose solidifies. Here, ensure a lateral position in which the opercle is as close as possible to the glass bottom of the dish as a preparation for the inverse microscope setup (Figure 1B).
- Let the agarose solidify and then add E3 media (containing a maximum of 0.02% MS-222) to the dish to avoid drying of the agarose (Figure 1B).
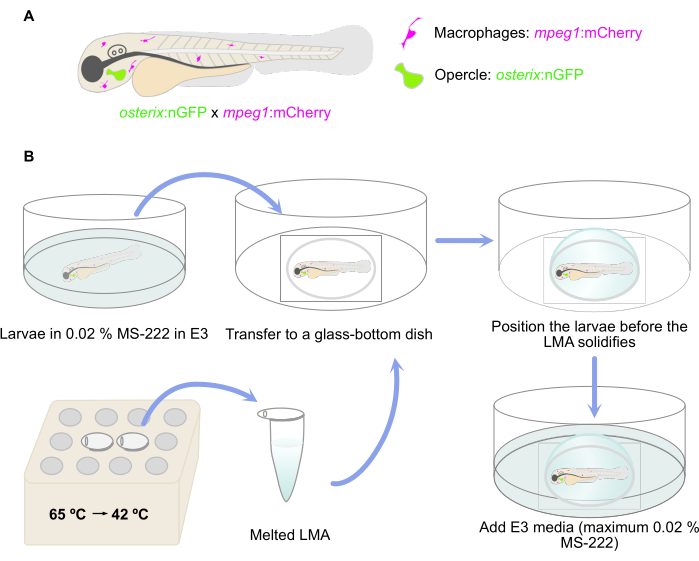
Figure 1: Schematic representation of the embedding procedure. (A) Double transgenic Tg(osterix:nGFP; mpeg1:mCherry)29,30 zebrafish larvae with green labeled opercular osteoblasts and macrophages labeled in red were used. (B) The workflow of the embedding procedure, as described in step 3 of the protocol. Please click here to view a larger version of this figure.
4. In vivo imaging of the larvae and laser ablation
- To laser-ablate osteoblasts in the developing opercle of transgenic zebrafish Tg(osterix:nGFP; mpeg1:mCherry)29,30, use an inverted two-photon laser-scanning microscope with a 25x/0.95 water objective or an equivalent setup.
- Place the dish with the embedded larvae onto the microscopic stage at the inverted microscope and position the desired region for ablation in the center of the field of view. The opercle can be easily identified with the help of the GFP fluorescence signal.
- Once the opercle is focused and centered in the field of view of the microscope, choose the z-stack settings (often 1-2 µm z-intervals are used) to image the entire breadth of the opercle. At the same time, choose the area in a specific z-plane to be ablated (usually in the posterior zone of the opercle). The two-photon laser ablation is only exerted in this plane.
NOTE: Higher resolution in the z dimension will lead to longer imaging times but will enable volumetric analyses of the acquired data later. - Image the opercle area of interest before ablation. For the GFP detection, use a white-light laser at 488 nm and a hybrid detector ranging from 495 nm to 550 nm or equivalent. Image the macrophages using 555 nm (white-light laser) excitation, with a mCherry detection range of 595 to 780 nm on a hybrid detector (or equivalent).
- Use the image format 512 x 512 for a good quality/time ratio (other formats can be used if higher resolution or alternatively higher speed are desired).
NOTE: A temperature-controlled incubator installed at the microscope can be used and adjusted, e.g., to 28 °C.
- Use the image format 512 x 512 for a good quality/time ratio (other formats can be used if higher resolution or alternatively higher speed are desired).
- Draw a circular region with a diameter of 25 µm (other shapes and sizes can be selected depending on the experiment) in a selected 2D plane, focusing the opercle using the ROI tool.
- To perform the ablation, apply ablation power (measured without objective) at 450 mW and expose the selected area to the two-photon laser at a scan speed of 400 Hz and a total duration of 10 s. During ablation, the area can be visualized using a hybrid detector in the range of 401-443 nm or equivalent.
- After ablation, confirm the ablation structurally by imaging again the same z-stack with the same settings as used prior to ablation. The ablated area does not contain fluorescent cells anymore.
5. Recovery of larvae and imaging to monitor immune cell recruitment
- If the imaging of immune cell recruitment needs to be performed with a delay, recover the larvae from the imaging and anesthesia. Remove the larvae carefully from the LMA using forceps or a dissection needle.
- First, remove a layer of agarose covering the larvae. Second, remove the agarose in direct contact with the larvae. Omit this step if immediate imaging of immune cell recruitment is taking place.
- Place the larvae back in a dish with pure E3 media and check their recovery. Recovery is evident by swimming of the larvae.
- At 4 or 6 hpl (hours post-lesion), image the opercle area again using the same settings at the same microscope to check for the effects of the ablation on macrophage recruitment. To do so, repeat the embedding (step 3) and imaging as performed before (step 4).
- After imaging, remove the larvae from the LMA. Sacrificing of the larvae can either be performed by incubation in ice water or with an overdose of MS-222 in E3, depending on the legal requirements. Specimens can then be used for follow-up studies or be disposed of.
6. Image analysis and statistical analysis
- Process the images using an image processing software such as Fiji32. Depending on the z-resolution of the acquired data, analyses can be performed in 2D or 3D.
- Quantify the macrophages manually by either including cells that are clearly separate, such as by discerning the main cell bodies, or alternatively quantifying cells with a nuclear label or by measuring the area in case cells are overlapping (next step).
- For quantification of the area, create a mask encompassing the macrophages. To do that, use the Image > Adjust > Threshold tool and create an ROI (Analyze > Tools > ROI Manager > Add) in Fiji. Exclude small particles in the background for quantification.
- Then, measure the area (Analyze > Measure). Consider whether areas of overlapping cellular fluorescence need to be included in the measurement.
NOTE: Here, the circular ablation area was omitted from the area measurements since recruited macrophages cannot be distinguished in this area.
- Then, measure the area (Analyze > Measure). Consider whether areas of overlapping cellular fluorescence need to be included in the measurement.
- Generate graphs plotting the measurement results using suitable software. To compare the data before and after ablation, perform an appropriate statistical test (here, a paired one-tailed t-test was performed).
NOTE: The figures can be prepared using photo editing and graphic design software.
Representative Results
Laser ablation was performed as indicated in the protocol above. The GFP signal of the osteoblasts in the ablated area disappeared instantaneously after ablation. To study the response of the osteoblast ablation in terms of the immune response, the presence of macrophages in 6 dpf larvae before and at 6 hpl was imaged. Before ablation, very few macrophages were observed in the opercle area10 (Figure 2). At 6 hpl, a strong accumulation of macrophages in the ablated opercle region and an increased number of macrophages in the field of view, including the opercle and the area surrounding the opercle, was detected10 (Figure 2).
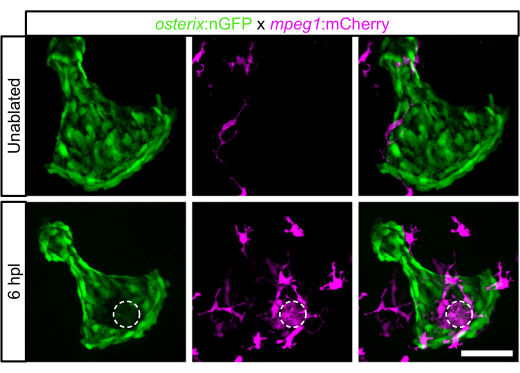
Figure 2: Macrophage recruitment at 6 hpl in 6 dpf zebrafish larvae. Opercle area without ablation and at 6 hpl. Opercular osteoblasts are labeled in green, macrophages in magenta, and the dashed circle indicates the laser-ablated area. Scale bar: 50 µm. Please click here to view a larger version of this figure.
Macrophage recruitment was also analyzed in 4 dpf larvae with much smaller opercles at 4 hpl observing similar results (Figure 3). Quantification of the macrophage number (outside the immediate laser ablation site) showed an increase within the investigated time frame (4.00 ± 2.37 macrophages before ablation versus 7.00 ± 2.76 macrophages at 4 hpl) (Figure 3B). Likewise, macrophage area (measured in µm2, 785.8 µm2 ± 723.1 µm2 before ablation versus 1553 µm2 ± 869.9 µm2 at 4 hpl) increased (Figure 3C). As macrophages are sometimes difficult to tell apart, measuring the macrophage area instead of the macrophage number is recommended, as was done here.
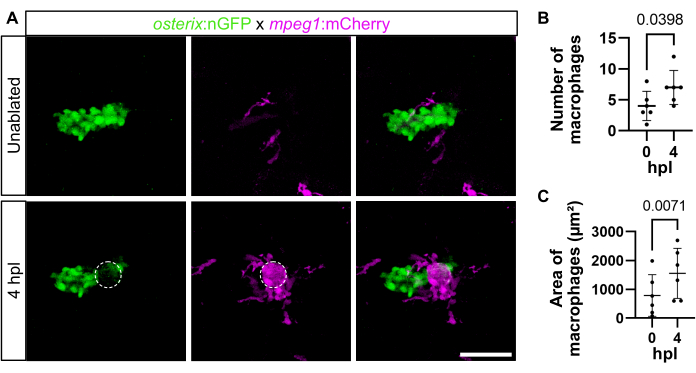
Figure 3: Macrophage recruitment at 4 hpl in 4 dpf zebrafish larvae. (A) Opercle area without ablation and at 4 hpl. Opercular osteoblasts are labeled in green, macrophages in magenta, and the dashed circle indicates the laser-ablated area. Scale bar: 50 µm. (B) Quantification of the number of macrophages in the opercle area directly before (0 hpl) and after ablation (4 hpl). (C) Quantification of macrophage area (site of laser ablation excluded). Paired one-tailed t-tests. Each dot represents the data of one larva, and the mean ± SD is shown, n = 6. Please click here to view a larger version of this figure.
Discussion
Laser ablation has been applied in various disciplines of biological research. In particular, it has been useful as a method to study tissue regeneration10,11,12. For example, the recruitment and phenotype changes of innate immune cells were recently analyzed over time using UV laser or two-photon laser ablation assays, along with the recovery of osteoblasts at the laser ablation site10. Live imaging of immune cell recruitment was carried out up to 12 h post-lesion (hpl); however, longer imaging periods can be envisioned with appropriate adjustments in anesthesia and approval from the respective authorities. In Geurtzen et al., analyses were carried out up to several days post-laser ablation and helped to define the inflammatory milieu at the ablation site, as well as to establish the supportive role of macrophages in bone regeneration10. Many other applications, such as studying the role of reactive oxygen species release33 upon cell death in bone tissue, are conceivable.
One of the advantages of using two-photon laser-mediated cell ablation is the possibility of combining it with confocal imaging. The software used here (see Table of Materials) can be set to a mode (Live Data Mode) in which imaging and ablation occur consecutively, speeding up the process. Another advantage of the technology is the accuracy of the ablation. The size and shape of the ablation can be easily adjusted in the software in a very precise manner. In the experimental setting presented here, a circular area with a diameter of 25 µm was chosen for ablation. However, other ablation experiments in Drosophila employed 25 µm linear ablations in order to reduce tissue tension in the embryonic epithelium by cutting neighbour-neighbour cellular junctions34. This accuracy allows for the ablation of a specific and controlled number of cells, single cells, or even cell membranes.
Notably, the type of lesion performed with a two-photon laser does not resemble injuries and cell death that naturally occur, which needs to be kept in mind in regeneration research but also other disciplines. In particular, laser ablations create very focused lesions by completely destroying (burning) the cells in the region of interest20 with minimal damage in surrounding tissues, while mechanical or other types of injuries tend to affect several tissue types and layers at a broader scale. This can lead to manifold consequences involving different types of cell death (e.g., necrosis, apoptosis, autophagy)35 and different regenerative outcomes. Moreover, reactions to laser ablation might differ in cellular and molecular terms. For example, it was shown that although cells surrounding the laser lesion site might lose plasma membrane integrity, they can survive the damage and restore integrity36. Laser ablation also leads to areas of the damaged extracellular matrix, which may result from "thermal explosions" in the very center of the applied laser beam and also strong heat exposure in the directly neighboring cells10,37. Nevertheless, cells surrounding laser ablation sites show an increased number of apoptotic bodies, as shown for laser-ablated heart tissue38, and an immune response is triggered, as shown here. In addition to the described differences, another limitation of laser-assisted ablation assays might be the depth at which the injury can be performed and the limited size of specimens. Structures hidden in thick specimens may not be appropriately ablated, and imaging will be difficult. This, however, will depend very much on the microscope and laser used, as well as the objective and embedding.
Embedding the larvae in a way so that the opercle is placed in close proximity to the glass bottom of the dish is essential in case an inverted setup is used, as in the presented protocol. Moreover, it is very important to test the temperature of the LMA before applying it to cover the larvae (step 3.4) since hot agarose will damage the larvae. To avoid that, take the LMA aliquot from the thermoblock early, e.g., while removing the leftover E3 media from the glass-bottom dish, and test the temperature, such as on the skin of the wrist. The LMA is supposed to be warm but not hot.
Once the larvae are covered with LMA, it is important to quickly put them into the correct position before the LMA starts to solidify. The lateral position is the easiest to achieve; in the presented study, the desired position was lateral with a slight tilt to the ventral side. In case the LMA solidifies before the larvae are positioned correctly, it is possible to remove the LMA (like in step 5.1) and repeat the embedding. Likewise, it is important that the area that is to be imaged (and laser ablated) is as close as possible to the glass bottom of the dish for maximum image quality and cell ablation, although this will also depend on the working distance of the objective.
It is possible to embed more than one larvae in the same dish for imaging. This will save time and make better use of the LMA. The number of larvae that can be embedded will depend on the experience of the experimenter, but 3-5 larvae are recommended.
Laser ablation can also be performed with upright microscopic setups employing dipping lenses. In this case, it is important to embed the larvae with a slightly ventral view (from above) to enable good imaging quality and laser ablation success of the opercle. In addition, only a thin layer of LMA should cover the larvae to allow good excitation and detection of fluorescent signals.
In this protocol, a two-photon laser-mediated ablation setup for osteoblasts is described as a methodology to perform lesions in developing bones, specifically in the opercle. This method can be adapted to ablate cells in other bones of developing zebrafish. Moreover, it is generally a useful approach to study the regeneration of injured tissues11,12. The ease of performing ablations and tissue cuts of different sizes and shapes allows its use in different experimental setups and research fields15,17,18,19,21,34. Altogether, two-photon laser-mediated ablation is a very adaptable method – for different tissues, organisms, and even in vitro settings – and it represents an important tool in biological research.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by the German Research Foundation (DFG) Transregio 67 (project 387653785), the DFG SPP 2084 µBone (project KN 1102/2-1) to FK. This work was supported by the Light Microscopy Facility (DFG project 413875620), a Core Facility of the CMCB Technology Platform at TU Dresden. The work at the TU Dresden was co-financed with tax revenues based on the budget agreed by the Saxon Parliament ('Landtag').
Materials
| Calcium chloride dihydrate, CaCl2·H2O | Carl Roth | 5239.1 | |
| Cell Culture Dish, PS, 100/20 mm | Greiner Bio-one | 664160 | |
| Dumont #55 Foceps | FST | 11295-51 | Tip shape straight, 11 cm, 0.05 x 0.02 mm |
| Falcon 6-well plate | Corning | 353502 | |
| Glass-bottom microwell dish | MatTek | P35G-1.5-14-C | 35 mm Dish, No. 1.5 Coverslip, 14 mm Glass Diameter, Uncoated |
| Insight X3 multiphoton laser | Spectra-Physics | ||
| Leica Application Suite | LAS X, Leica Microsystems | ||
| Low melting agarose | Biozyme | 840101 | Biozym Plaque Agarose |
| Magnesium sulfate heptahydrate, MgSO4·7H2O | Sigma-Aldrich | M5921 | |
| Mating cages | many varieties, e.g. Tecniplast | ||
| Methyleneblue | Carl Roth | A514.1 | |
| MS-222 | SIGMA Aldrich | A5040 | |
| Potassium Chloride, KCl | PanReac AppliChem | 131494 | |
| Sodium chloride, NaCl | Carl Roth | 3957.1 | |
| SP8 FALCON | Leica Microsystems | Equipped with a Insight X3 multiphoton laser and Leica Application Suite software | |
| Stainless Steel Dissect Needle | Bochem | 12010 | 140 mm |
| Stereo Microscope System SZX16 | Olympus | Equipped with a LED illumination base SZX2-ILLTQ | |
| Thermostatic Cabinets TS – WTW | xylem | TS 608/2-i | For incubation (embryo) |
| Transfer pipette, 3.5 mL | SARSTEDT | 861171 | 155 x 15 mm, LD-PE, transparent |
| Zeiss SteREO Discovery.V12 version 4.7.1.0. | Zeiss | Equipped with Axiocam MRm camera and AxioVision sofware |
Referências
- Marques, I. J., Lupi, E., Mercader, N. Model systems for regeneration: Zebrafish. Dev. 146 (18), dev167692 (2019).
- Dietrich, K., Fiedler, I. A. K., Kurzyukova, A. Skeletal biology and disease modeling in zebrafish. J Bone Miner Res. 36 (3), 436-458 (2021).
- Geurtzen, K., Knopf, F. Adult zebrafish injury models to study the effects of prednisolone in regenerating bone tissue. J Vis Exp. (140), e58429 (2018).
- Sousa, S., Valerio, F., Jacinto, A. A new zebrafish bone crush injury model. Biol Open. 1 (9), 915-921 (2012).
- González-Rosa, J. M., Mercader, N. Cryoinjury as a myocardial infarction model for the study of cardiac regeneration in the zebrafish. Nat Protoc. 7 (4), 782-788 (2012).
- Chassot, B., Pury, D., Jaźwińska, A. Zebrafish fin regeneration after cryoinjury-induced tissue damage. Biol Open. 5 (6), 819-828 (2016).
- Singh, S. P., Holdway, J. E., Poss, K. D. Regeneration of amputated zebrafish fin rays from de novo osteoblasts. Dev Cell. 22 (4), 879-886 (2012).
- Curado, S., et al. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn. 236 (4), 1025-1035 (2007).
- Ando, K., Shibata, E., Hans, S., Brand, M., Kawakami, A. Osteoblast production by reserved progenitor cells in zebrafish bone regeneration and maintenance. Dev Cell. 43 (5), 643-650.e3 (2017).
- Geurtzen, K., López-Delgado, A. C., Duseja, A., Kurzyukova, A., Knopf, F. Laser-mediated osteoblast ablation triggers a pro-osteogenic inflammatory response regulated by reactive oxygen species and glucocorticoid signaling in zebrafish. Dev. 149 (8), 1998030 (2022).
- Volpe, B. A., Fotino, T. H., Steiner, A. B. Confocal microscope-based laser ablation and regeneration assay in zebrafish interneuromast cells. J Vis Exp. (159), e60966 (2020).
- Johnson, C. S., Holzemer, N. F., Wingert, R. A. Laser ablation of the zebrafish pronephros to study renal epithelial regeneration. J Vis Exp. (54), e2845 (2011).
- Goldstein, A. M., Fishman, M. C. Notochord regulates cardiac lineage in zebrafish embryos. Dev Biol. 201 (2), 247-252 (1998).
- Zhang, J., Jeradi, S., Strähle, U., Akimenko, M. A. Laser ablation of the sonic hedgehog-a-expressing cells during fin regeneration affects ray branching morphogenesis. Dev Biol. 365 (2), 424-433 (2012).
- Marshall, A. R., et al. Two-photon cell and tissue level laser ablation methods to study morphogenetic biomechanics. Methods Mol Biol. 2438, 217-230 (2022).
- Angelo, J. R., Tremblay, K. D. Laser-mediated cell ablation during post-implantation mouse development. Dev Dyn. 242 (10), 1202-1209 (2013).
- Lee, J., Lee, S., Truong, V. G., et al. Laser ablation of pancreatic cancer using a cylindrical light diffuser. Lasers Med Sci. 37 (6), 2615-2621 (2022).
- Luther, E., Mansour, S., Echeverry, N., et al. Laser ablation for cerebral metastases. Neurosurg Clin N Am. 31 (4), 537-547 (2020).
- Smutny, M., Behrndt, M., Campinho, P., Ruprecht, V., Heisenberg, C. -. P. UV laser ablation to measure cell and tissue-generated forces in the zebrafish embryo in vivo and ex vivo. Methods Mol Biol. 1189, 219-235 (2015).
- Vogel, A., Venugopalan, V. Mechanisms of pulsed laser ablation of biological tissues. Chem Rev. 103 (2), 577-644 (2003).
- Wu, F. Heat-based tumor ablation: Role of the immune response. Adv Exp Med Biol. 880, 131-153 (2016).
- Renvoizé, C., Biola, A., Pallardy, M., Bréard, J. Apoptosis: Identification of dying cells. Cell Biol Toxicol. 14 (2), 111-120 (1998).
- Julier, Z., Park, A. J., Briquez, P. S., Martino, M. M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 53, 13-28 (2017).
- Petrie, T. A., Strand, N. S., Tsung-Yang, C., Rabinowitz, J. S., Moon, R. T. Macrophages modulate adult zebrafish tail fin regeneration. Dev. 141 (13), 2581-2591 (2014).
- Geurtzen, K., Vernet, A., Freidin, A. Immune suppressive and bone inhibitory effects of prednisolone in growing and regenerating zebrafish tissues. J Bone Miner Res. 32 (12), 2476-2488 (2017).
- Fleischhauer, L., López-Delgado, A. C., Geurtzen, K., Knopf, F. Glucocorticoid effects in the regenerating fin reflect tissue homeostasis disturbances in zebrafish by affecting Wnt signaling. Front Endocrinol. (Lausanne). 14, 1122351 (2023).
- Westerfield, M. . The Zebrafish Book: A Guide for the laboratory use of zebrafish (Danio rerio). , (2002).
- Brand, M., Granato, M., Nüsslein-Volhard, C. Keeping and raising zebrafish. Zebrafish: A Practical Approach. , (2002).
- Spoorendonk, K. M., Peterson-Maduro, J., Renn, J. Retinoic acid and Cyp26b1 are critical regulators of osteogenesis in the axial skeleton. Dev. 135 (22), 3765-3774 (2008).
- Ellett, F., Pase, L., Hayman, J. W., Andrianopoulos, A. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood. 117 (4), e49-e56 (2011).
- JoVE Science Education Database. Zebrafish Breeding and Embryo Handling. Biology II: Mouse, Zebrafish, and Chick. , (2023).
- Schindelin, J., Arganda-Carreras, I., Frise, E. Fiji: An open-source platform for biological-image analysis. Nat Methods. 9 (7), 676-682 (2012).
- Niethammer, P., Grabher, C., Look, A. T., Mitchison, T. J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 459 (7249), 996-999 (2009).
- Blanchard, G. B., Scarpa, E., Muresan, L., Sanson, B. Mechanical stress combines with planar polarised patterning during metaphase to orient embryonic epithelial cell divisions. Dev. 151 (10), dev202862 (2024).
- Park, W., Wei, S., Kim, B. -. S., et al. Diversity and complexity of cell death: A historical review. Exp Mol Med. 55 (8), 1573-1594 (2023).
- O’Connor, J., Akbar, F. B., Hutson, M. S., Page-McCaw, A. Zones of cellular damage around pulsed-laser wounds. PLoS One. 16 (9), e0253032 (2021).
- Griebel, M., Vasan, A., Chen, C., Eyckmans, J. Fibroblast clearance of damaged tissue following laser ablation in engineered microtissues. APL Bioeng. 7 (1), 16112 (2023).
- Matrone, G., Taylor, J. M., Wilson, K. S. Laser-targeted ablation of the zebrafish embryonic ventricle: a novel model of cardiac injury and repair. Int J Cardiol. 168 (4), 3913-3919 (2013).

.
