Translational Rabbit Model of Chronic Cardiac Pacing
Summary
We present a minimally invasive leporine model of long-term cardiac pacing that can be utilized for artificial pacing and heart failure development in preclinical studies.
Abstract
Animal models of cardiac pacing are beneficial for testing novel devices, studying the pathophysiology of artificially paced heart rhythms, and studying arrhythmia-induced cardiomyopathies and subsequent heart failure. Currently, only a few such models are available, and they mostly require extensive resources. We report a new experimental cardiac pacing model in small mammals with the potential to study arrhythmia-induced heart failure.
In six New Zealand white rabbits (mean weight: 3.5 kg) under general inhalational anesthesia the jugular region was dissected and a single pacing lead was inserted via the right external jugular vein. Using fluoroscopic guidance, the lead was further advanced to the right ventricular apex, where it was stabilized using passive fixation. A cardiac pacemaker was then connected and buried in a subcutaneous pocket.
The pacemaker implantation was successful with good healing; the rabbit anatomy is favorable for the lead placement. During 6 months of follow-up with intermittent pacing, the mean sensed myocardial potential was 6.3 mV (min: 2.8 mV, max: 12 mV), and the mean lead impedance measured was 744 Ω (min: 370 Ω, max: 1014 Ω). The pacing threshold was initially 0.8 V ± 0.2 V and stayed stable during the follow-up.
This present study is the first to present successful transvenous cardiac pacing in a small-mammal model. Despite the size and tissue fragility, human-size instrumentation with adjustments can safely be used for chronic cardiac pacing, and thus, this innovative model is suitable for studying the development of arrhythmia-induced cardiomyopathy and consequent heart failure pathophysiology.
Introduction
In heart failure research and cardiac pacing development, translational models are frequently required for preclinical testing1. Furthermore, novel devices, materials, and lead refinements need to be tested for their potential complications before their clinical use. Thus, cardiac pacing models have a wide range of applications, including the analysis of artificially paced heart rhythms and the study of their pathophysiological effects on cardiac function2,3. Cardiac pacing- or tachycardia-induced cardiomyopathy experiments can utilize models of various animal sizes, with the development of heart failure within weeks of high-rate pacing1,3,4,5.
Previous studies have reported the use of large animal models — porcine, canine, and ovine — in such applications2,3,6. However, the availability of these models is limited, and they require extensive resources for animal surgery and handling. In contrast, the use of small mammals could address the abovementioned concerns and, consequently, serve as an optimal and affordable research model. However, cardiac pacing studies on small mammals have rarely been reported, and this could be due to their delicate anatomy, tissue fragility, and the higher rate of pacing required7,8,9,10,11,12.
Only surgical models of partially implanted pacing leads with external pacemakers11,12 or wireless microscopic pacing devices5,7,8,9 have been used in small-mammal pacemaker studies, but to our knowledge, the use of fully implanted, human-sized, transvenous pacemaker systems has not been reported to date. Previous evidence in leporine models shows that pacing at fast heart rates over weeks leads to myocardial depression11,12. This paper presents the first practically viable small-mammal model, demonstrating the successful implantation of a human-sized pacemaker in rabbits. The described methodology aims to present a clinically relevant model of cardiac pacing and can be closely translated to human studies of tachycardia- or pacing-induced cardiomyopathy and the consequent heart failure pathophysiology2,11,12.
Protocol
This experimental protocol was reviewed and approved by the Institutional Animal Expert Committee at the First Faculty of Medicine, Charles University, and performed at the University experimental laboratory, Department of Physiology, First Faculty of Medicine, Charles University in Prague, Czech Republic, in accordance with Act No. 246/1992 Coll. on the protection of animals against cruelty. All the animals were treated and cared for in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition, published by National Academies Press, 2011. All the procedures were performed according to standard veterinary conventions in the presence and under the guidance of a licensed veterinarian. Six New Zealand white rabbits were included in this series of experiments. Their mean body weight was 3.5 kg ± 1 kg on the day of pacemaker implantation. All the values are expressed as mean ± SEM and, if appropriate, by ranges of minimum and maximum measured values. A P value < 0.05 was considered significant. For successful mastering of the technique, basic skills in animal anesthesia and surgery are necessary; previous experience with cardiac pacing is advisable but not required.
1. Preoperative care
- Allow the rabbits to acclimate to their housing facility for at least 2 weeks and become comfortable with human touch and manipulation to facilitate the handling and management of the animals on the day of the surgery.
- Feed the animals hay and hay-based pelleted rabbit diet. Provide fresh water at regular intervals.
- Carry out a short daily check of their vitals (body temperature, respiratory rate) and overall condition, including optimal dietary intake and absence of distress.
2. Anesthesia, premedication, and monitoring
- After 30 min of fasting, administer premedication: buprenorphine (0.01-0.04 mg/kg IM), midazolam (0.3-0.6 mg/kg IM), medetomidine (0.03-0.06 mg/kg IM), and ketamine (5-10 mg/kg IM).
- Insert a cannula into the marginal ear vein for intravenous drug application. Collect blood samples using low-volume tubes (0.5 mL) for hematological and biochemical analyses.
- Shave the rabbit's skin using a shaver at the right jugular region on the neck — the surgical site — and on the limbs to attach the electrodes for ECG monitoring. Shave with care, as the skin of rabbits is easily susceptible to irritation and small tears are commonly seen.
- Place the animal on a heating pad to prevent hypothermia.
- Monitor the vital functions, including ECG, rectal body temperature, and oxygen saturation by a pulse oximeter, according to the anesthesia protocol.
- Place a mask over the animal's mouth and nose and secure it by a rubber seal around the animal's face. Use ointment to protect the animal's eyes from dryness.
- To achieve adequate sedation, provide the animals with isoflurane (mixed with oxygen) via the facial mask. Start with a 3.5% concentration and reduce as needed according to the animal's responses in terms of a suppressed corneal reflex and motoric pain response.
NOTE: To reduce the risk of lung injury during the anesthesia, spontaneous ventilation is recommended, but a neonatal manual or automatic ventilator must be kept ready in case of hypoventilation. - Prepare all sterile instrumentation.
- Position the animal on a fluoroscopy table. Wear full X-ray personal protective equipment.
3. Ventricular lead implantation
- Locate the external jugular vein and mark its position on the skin.
- Sterilize the entire region using povidone-iodine and proceed to cover the site of surgery with a sterile drape with a hole over the marked jugular area.
- Make an incision on the skin parallel over the marked jugular vein. Locate the external jugular vein and isolate a length of 1 cm from the adjacent fibrous tissue and the vascular bundle. Find the carotid artery for orientation and to prevent its injury.
- Create a pocket in the subcutaneous tissue to accommodate the pacemaker. Use scissors for blunt dissection to prevent excessive bleeding and tissue damage.
- Secure the vessel using a rubber tie on both ends of the isolated vessel segment and occlude the blood flow (Figure 1).
- Using the standard cut-down technique, make a cut of approximately 1/3 of the circumference of the vessel wall with a blade.
- Use a vessel pick to widely open the cut and introduce a single passive pacing lead into the lumen.
- Under fluoroscopic guidance, further advance its tip to the apex of the right ventricle (Figure 2). Pre-shape a stylet into a curve and use it to guide the lead to pass the tricuspid annulus. Ensure that the tip of the lead is unsupported by the stylet so that the lead remains flexible and atraumatic when touching the tissue.
- Test the pacing parameters. The ventricular lead sensed signal and impedance must be stable, and the pacing threshold should be low. There should be no fasciculation of the adjacent muscles (Figure 3).
- Secure the lead position by stitching it over a protective rubber sleeve to the underlying fibrous tissue and seal the vessel lumen around the lead using a silk tie (Figure 4).
4. Pacemaker implantation
- Connect the pacemaker to the pacing lead and secure the IS-1 connector using a screw. If the non-invasive pacing study function (see step 6) will be utilized during the follow-up, connect the pacemaker to the atrial channel socket.
- Bury the pacemaker and the extra length of the lead into the preformed subcutaneous pocket (Figure 5).
- Flush the pocket with povidone-iodine. Suture the skin wound using a monofilament thread.
- Set the desired pacing program and perform a final check of the pacing parameters (Figure 3).
5. Postoperative care
- Withdraw the anesthetics and observe the animal carefully until it regains adequate consciousness.
- Administer atipamezole (0.01-0.03 mg/kg IM) to revert the action of the medetomidine.
- After consciousness has been restored and optimal body temperature achieved, administer meloxicam (0.4-0.6 mg/kg) subcutaneously for pain relief. Add buprenorphine after 6-8 h if the pain relief is not adequate according to a valid pain assessment scale (e.g., Rabbit grimace scale).
- Administer metoclopramide (0.5-1 mg/kg IV) to prevent further gastrointestinal stasis and to stimulate gastric motility and continue 3x per day until adequate food intake and stool production are restored.
- Follow a wide-spectrum intravenous antibiotic regimen until the wounds have healed (enrofloxacin at 10-20 mg/kg 2x per day for 3-7 days).
- Transfer the animal to a comfortable and familiar environment and observe it until it regains sufficient consciousness. Do not return the rabbit to the company of other animals until it has fully recovered.
- Maintain meloxicam (0.4-0.6 mg/kg SC) administration daily for at least 5 days.
- Monitor and dress the wounds regularly to ensure safe and timely healing.
- When fully healed, approximately 14 days after the procedure, remove the non-absorbable skin sutures.
- Perform remote interrogation and check the pacing parameters regularly (i.e., pacing threshold, myocardial sensing, and lead impedance).
NOTE: The obtained values should follow a stable trend.
6. Pacing protocol and data collection
- Interrogate the pacemaker and set the backup pacing mode by selecting minimal base rate in the Parameters menu.
NOTE: Owing to the high heart rate and its high variability native to small animals, continuous artificial pacing can be achieved at a rate of 300-400 bpm, according to the specified requirements. Intermittent pacing can be achieved during each pacemaker interrogation (follow step 6.4 and Figure 6). - Record the pacing lead impedance continuously; in the pacemaker programmer Diagnostics menu, start data collection.
- Record the myocardial potential continuously and check it manually every week by interrogation of the pacemaker; in the pacemaker programmer Test menu under the Sensing tab, measure the unipolar and bipolar myocardial potential amplitudes.
- Assess the pacing threshold regularly (weekly) by interrogation. Use the non-invasive pacing study (select NIPS under Test menu) function to measure the pacing threshold with a sufficient pacing rate (Figure 6). Assess the pacing threshold for various stimulus durations (from 0.1 ms to 1.5 ms) and express it in volts. Use the intracardiac electrograms or surface ECG for the determination of the loss of capture when the pacing stimulus output becomes subthreshold.
- Perform all the procedures according to standard veterinary conventions, sacrifice the animal according to the institutional regulations at the completion of each study, and perform a necropsy. Explant the pacemaker and lead and examine them for inflammatory responses, biofilm formation, and fibrosis.
NOTE: A potassium overdose was given under deep anesthesia to euthanize the animals in this protocol.
Representative Results
A total of six animals were included in the study. In all the animals, the pacing lead was successfully implanted via the external jugular vein into the right ventricular apex (Supplemental Figure S1). The position was verified by fluoroscopy, and the lead was stitched to the adjacent tissues over a rubber sleeve. According to the X-ray imaging, the lead maintained its position over the whole pacing protocol period. The attached pacemaker was palpable in the lateral neck region, causing no obvious trouble to the animal. All the wounds healed fully and without local complications.
The lead tip was equipped with two titanium-platinum electrodes — a distal hemispherical ring, and a proximal cylindrical ring electrode — with an interelectrode distance of 25 mm (Figure 2). The leads were freely advanced into the apex and passively attached there by their silicon fixing tines. This allowed for unipolar pacing from the tip electrode and for bipolar pacing between both electrodes located in the right ventricle.
A representative ventricular myocardial potential sensed signal is shown in Figure 3, and the measured pacing parameters are given in detail in Table 1 and Figure 7. At the time of the procedure, the mean sensed myocardial potential was 5.6 V ± 0.8 mV (min: 2.8 mV, max: 8 mV), the lead impedance was 675 Ω ± 74 Ω (min: 468 Ω, max: 951 Ω), and the pacing threshold was 0.8 V ± 0.26 V (min: 0.2 V, max: 2.2 V), with the stimulus duration set to the standard 0.4 ms.
After follow-up of 3 months and 6 months with intermittent pacing, the mean sensed myocardial potential was 7.4 mV ± 1.2 mV (min: 4.0 mV, max: 12.0 mV) and 6.3 mV ± 1.0 mV (min: 4.2 mV, max: 10.3 mV), respectively. The mean lead impedance measured was 869 Ω ± 32 Ω (min: 760 Ω, max: 975 Ω) and 725 Ω ± 96 Ω (min: 370 Ω, max: 1014 Ω), respectively, and the pacing threshold changed to 1.2 V ± 0.3 V (min: 0.2 V, max: 2.2 V) and to 1.4 V ± 0.3 V (min: 0.5 V, max: 2.3 V), respectively. All the parameter changes were not statistically significant over this period (P > 0.05), and the bipolar and unipolar parameters followed comparable trends (Figure 7 and Table 1).
One case was terminated early due to partial lead penetration, which presented with an abrupt impedance drop observed on the second day after the implantation. Later, during the second month of follow-up, a gradual threshold increase was noticed, and pacing on high output caused muscular fasciculation. The animal remained asymptomatic, but during necropsy, the tip of the pacing lead was found to have penetrated through the myocardial inferior wall into the pericardium by a length of approximately 3 mm. No bleeding and no signs of infection were observed.
Before the procedure, on day 1 post procedure, and on day 7 post procedure, the mean white blood cell counts were 5.9 × 109/L, 7.37 × 109/L, and 7.42 × 109/L, respectively, the mean hemoglobin levels were 105 g/L, 113 g/L, and 110 g/L, respectively, and the mean platelet counts were 317 × 109/L, 274 × 109/L, and 219 × 109/L, respectively. The laboratory values did not demonstrate significant changes during the first week after the procedure (P > 0.05 for all). Under microscopic evaluation, the pacing lead silicon surface was covered by fibrous tissue (with an approximate thickness of 100 µm), but no cells were found (Figure 8).
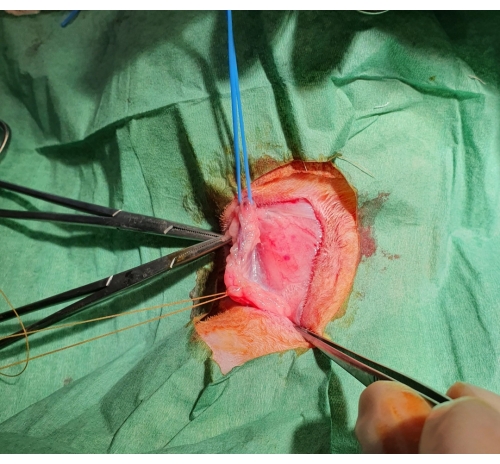
Figure 1: Surgical jugular vein dissection. After the skin is cut, a subcutaneous pocket is formed, and the jugular vein is exposed, ligated distally, and supported by a blue rubber band proximally. Please click here to view a larger version of this figure.
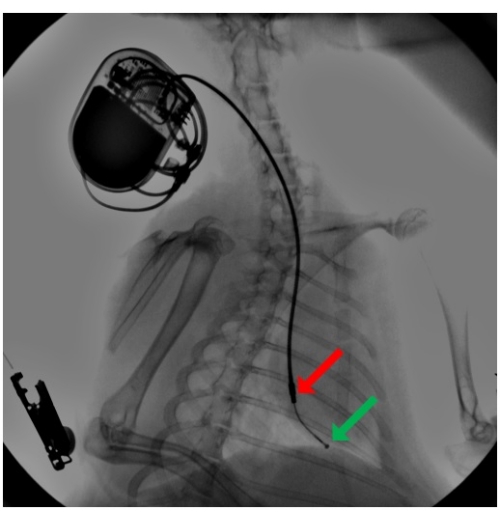
Figure 2: Fluoroscopy during pacemaker lead implantation. A pacing lead is introduced via the external jugular vein, and using a pre-shaped stylet, the lead advanced to the right ventricular apex, where it is secured by passive fixation by its silicon tines. An attached pacemaker is buried in a subcutaneous pocket in the neck region. The arrow point to the distal hemispherical ring (green) and the proximal cylindrical ring (red) electrodes. Please click here to view a larger version of this figure.
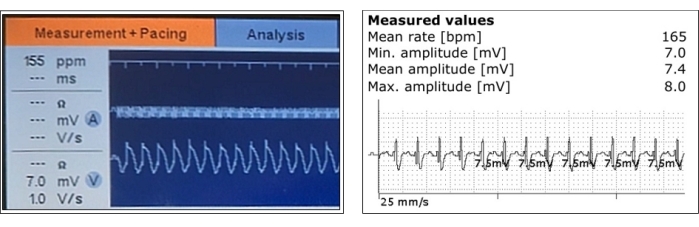
Figure 3: Representative measurements of sensed ventricular myocardial potentials. Ventricular sensing and its amplitude measurements are shown during the acute phase after pacing lead positioning (left) and after the pacemaker implantation (right). Please click here to view a larger version of this figure.
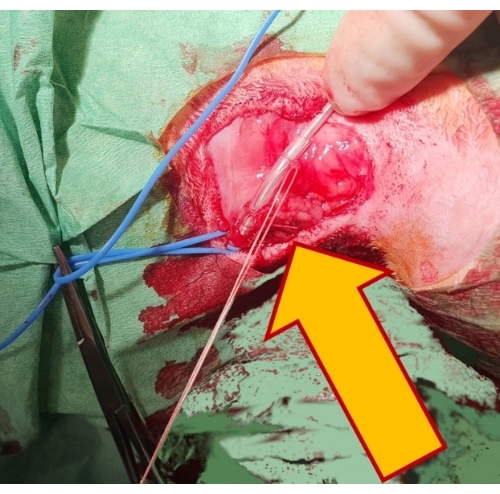
Figure 4: Securing the pacing lead. Fixing the lead by two non-absorbable sutures over a rubber sleeve (arrow) to the underlying tissue secures it in position and prevents its dislocation. Please click here to view a larger version of this figure.
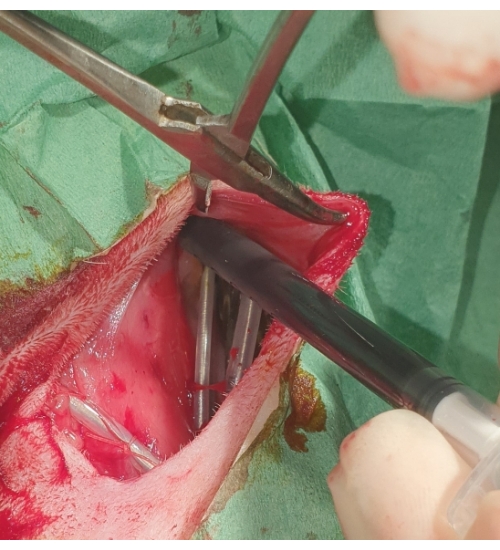
Figure 5: Placement of the pacemaker. The pacemaker is buried into the subcutaneous pocket and flushed with povidone-iodine. Please click here to view a larger version of this figure.
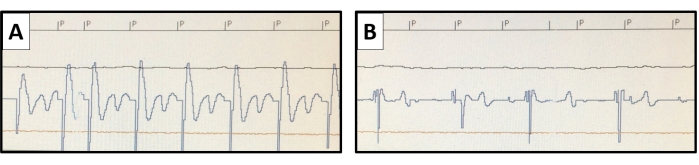
Figure 6: Pacing threshold measurement. Using the non-invasive pacing study function of the pacemaker, pacing at higher than the native heart rate is conducted. The pacing stimuli are marked with P. The pacing threshold is evaluated with varying stimulus outputs. (A) A representative example of the endocardial potential of ventricular capture is shown for an output of 0.8 V at 0.4 ms, (B) but a loss of capture is seen with the output reduced to 0.6 V at 0.4 ms. Please click here to view a larger version of this figure.
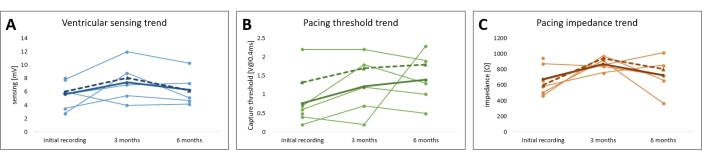
Figure 7: Follow-up of the leporine cardiac pacing model pacing parameters. The trends of the (A) pacing threshold, (B) pacing impedance, and (C) myocardial sensing for all the subjects are plotted. The average unipolar (full line) and bipolar values (dotted line) are shown in bold. Please click here to view a larger version of this figure.
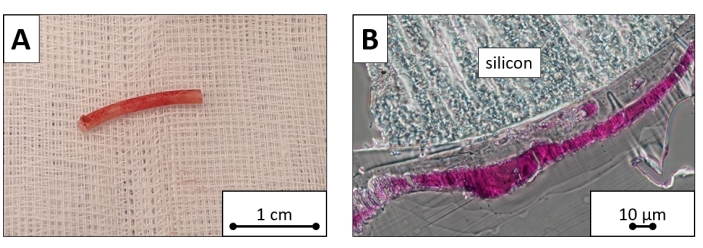
Figure 8: Explanted pacing lead sample. Ventricular portion cut of the explanted pacing lead. (A) The macroscopic image and (B) microscopic image dyed with toluidine blue reveal the silicon surface covered by a layer of fibrous tissue. Scale bars = (A )1 cm, (B) 10 µm. Please click here to view a larger version of this figure.
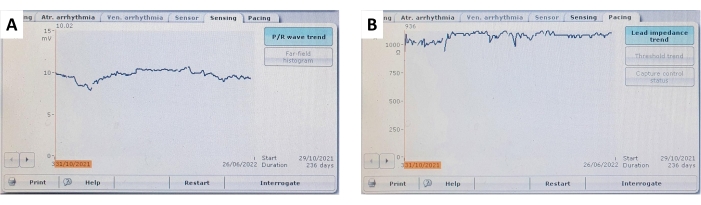
Figure 9: Ventricular sensing and lead impedance trends. A representative example of (A) continuous and stable ventricular myocardial sensing and (B) ventricular lead impedance trends over a follow-up of 236 days. Please click here to view a larger version of this figure.
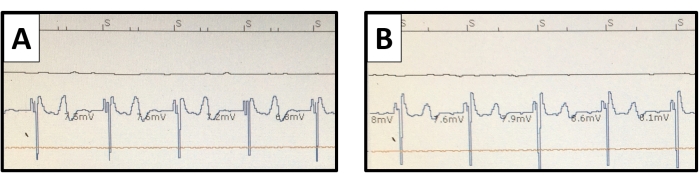
Figure 10: Ventricular endomyocardial electrograms. The pacemaker interrogation sensed ventricular potentials are pictured with (A) unipolar and (B) bipolar connections. The T wave potential is more distinct with the unipolar connection but does not cause oversensing. Please click here to view a larger version of this figure.
| Unipolar | periprocedural | follow-up | |||||||
| 3 months | 6 months | ||||||||
| Sensed myocardial potential [mV] | 5.6 ± 0.8 | 7.4 ± 1.2 | 6.3 ± 1.0 | ||||||
| Pacing threshold [V at 0.4 ms] | 0.8 ± 0.3 | 1.2 ± 0.3 | 1.4 ± 0.3 | ||||||
| Lead impedance [Ω] | 675 ± 74 | 869 ± 32 | 725 ± 96 | ||||||
Table 1: Follow-up of the leporine cardiac pacing model pacing parameters. The values of the sensed myocardial potential, pacing threshold, and lead impedance are expressed as mean ± SEM for 3 months and 6 months of follow-up.
Supplemental Figure S1: Schematic of the implanted human-sized transvenous pacing system in a rabbit. Please click here to download this File.
Discussion
Despite their specific constraints, small mammalian models offer advantages for clinical research13. With an established methodology, cardiac pacing models can provide an optimal platform for the simulation of a wide range of cardiovascular diseases and circulatory pathological states7,14 with significantly lower resource requirements compared to large animal models or clinical trials. This paper presents an innovative, minimally invasive model of long-lasting cardiac pacing in rabbits. By following this protocol, it is feasible to use a fully implanted, full-sized human pacemaker system, including a full-length pacing lead, in a small-mammal model.
At the time of pacemaker implantation, we were able to position the lead in a stable, optimal location in the apex of the right ventricle in all the animals. The invasively measured pacing parameters were within normal ranges, similar to the values common in large animal experiments or human medicine2,3. The measured mean myocardial potential of 6.5 mV ± 1.9 mV in the right rabbit ventricle is clearly recognized by a standard implantable pacemaker. The maximum measured pacing threshold was 2.5 V, with a stimulus duration of 0.4 ms, and the impedance remained within normal ranges during the follow-up. Overall, these represent optimal pacing parameters.
During follow-up, the pacing parameters were verified non-invasively by interrogating the implanted pacemaker, and these parameters are summarized in Figure 7, Figure 9, and Table 1. The ventricular sensing and lead impedance did not demonstrate any significant changes over 6 months. Despite an increasing trend in the pacing threshold averaged across all subjects, no significant changes were observed, allowing pacing to be conducted safely throughout the whole study. The small fluctuation in the pacing parameters can be attributed to local inflammatory responses or fibrosis and could be mitigated by utilizing steroid-eluting materials. For use in long-term pacing studies, the pacing parameters should be monitored and adjusted frequently.
The blood analysis did not suggest systemic inflammation or anemia during the first week after implantation. The trend of increased platelet counts before the procedure can be attributed to the acute stress caused by animal handling and sedation, as the values remained stable during the follow-up. A feared complication of pacemaker implantation is lead penetration. Especially with the fragility of small-mammal tissues, penetration should be suspected when the pacing parameters change abruptly, and it must be stressed that the lead should always be manipulated carefully into its proper position. An X-ray image can confirm lead penetration. An acute cardiac implantable electronic device (CIED)-associated bacterial infection is another potentially severe complication that contributes significantly to mortality and morbidity rates15. Hence, it is extremely important to study novel materials, pacing techniques, and lead refinements to reduce the rates of infection and extend the durability of the pacing systems. The presented methodology provides an appropriate animal model for such vital experimental research.
Ryu et al. induced cardiomyopathy with progressive heart failure using surgically implanted atrial pacing leads and an external pulse generator12. Similarly, Freeman et al. concluded that sustained ventricular pacing leads to myocardial depression in rabbits over 3-4 weeks11. Owing to small animals' high native heart rate, the pacemaker must be capable of pacing frequencies around 300-400 bpm to maintain a fully paced rhythm. As these higher pacing frequencies lead to progressive heart failure over weeks11,12, the presented leporine model is optimal for the development and investigation of the resultant cardiomyopathy. Considering their size, these small models are ideal for specific applications such as the evaluation of humoral or myocardial tissue changes11,16. Echocardiography can further be used to evaluate the dimensions and contractility of the leporine heart12,17. In comparison, larger animal models of heart failure have other advantages, such as the possibility for detailed invasive hemodynamic evaluation, including coronary circulation or pressure-volume assessments2.
The specific selection of the leporine model for pacing studies was based on its multiple advantages. Rabbits tolerate the procedure well, are one of the smallest mammals to demonstrate the capacity for receiving a human-sized pacemaker system, and require the deployment of fewer resources than other larger animals. Some authors18 believe that the physiology of small mammals may not reflect that of humans, yet we found that the pacing parameters observed in these small mammals are quite similar to those seen in humans or large animals1,2,3,19, meaning they can be readily utilized for translational research.
During lead placement and pacemaker implantation in this small-mammal model, we encountered similarities to previous experiments in large animal models, but the significant differences should be pointed out. Leporine tissues are fragile, and the vessel and ventricular walls are thin. Gentle manipulation is necessary during the whole procedure; the lead tip should always be unsupported by the stylet, and thus, flexible. Especially when passing through the tricuspid annulus and positioning the lead tip to the apex of the right ventricle, manipulation must be conducted with extreme care and under fluoroscopic guidance to avoid injury. Positioning the tip in other locations should also be possible. We have tested the right atrial appendage and ventricular outflow tract positions with optimal periprocedural parameters, but the lead stability may be limited, and the current data cannot support alternative pacing sites. The external jugular vein of the rabbit is appropriately sized for the insertion of a single pacing lead. If the implantation of multiple leads is intended, the use of a larger animal may be advised.
The lead fixation in the myocardial trabeculation was accomplished passively with silicon tines at the lead tip. Based on our experience, the use of active fixation by a helix screwed into the thin myocardial layer must be avoided to prevent tissue injury due to tamponade or chest bleeding. Despite the small size of the rabbit right ventricle, the pair of pacing electrodes spaced by 25 mm allowed for both unipolar and bipolar sensing and pacing configurations (Figure 10). This can offer versatility for cardiac pacing studies.
Due to the high native heart rate of small mammals18, continuous pacing can be achieved by custom programming of the implantable pacemaker. Alternatively, the method of simple in-house modification of a common human-certified pacing system can be used to obtain high-rate pacing frequencies, as described in detail previously2,20. The loss of capture was assessed using the non-invasive pacing study function, which is a unique approach that allows testing even in the condition of a high native heart rate. The reported pacing parameters were measured regularly. The implanted pacemaker was able to record the sensing of myocardial potentials and lead impedance automatically and continuously, but the pacing threshold had to be measured manually due to the high native heart rate. Therefore, if continuous pacing is required, frequent assessments are recommended to prevent loss of capture.
Gutruf et al. previously reported the use of highly miniaturized, wireless, battery-free pacemakers in small animal models7. Compared to their studies, the implantation of a human-sized pacemaker described here represents a different approach that provides the possibility for innovative lead testing, close translation to clinical research, and wider applications with generally available materials. Zhou et al. presented the development of a miniature cardiac pacemaker designed to be implanted percutaneously into the fetal heart to treat atrioventricular block. They reported the use of adult rabbit experiments to confirm the feasibility of such a device9. Others have previously reported the advantages of rabbit intubation for invasive procedures. Based on our experience, the approach of maintaining spontaneous breathing with an oro-nasal mask has more benefits for such short procedures as it minimizes the risk of complications caused by manipulation of the airways. Furthermore, pressure lung injuries can also be prevented.
Although the study protocol was prepared carefully and the total number of animals included was adequate, several limitations have to be pointed out. The small size of the rabbit right ventricle did not allow for multiple lead placements. Although we tried testing the positioning of the lead tip in the right ventricular outflow tract, we have limited knowledge about its stability and expect it to be rather limited. The pacing impedance trend showed a drop within the first week after the lead placement. This could be because of local inflammation and mild fibrosis, but shortly after, the lead impedance was restored, and a trend of stability was continually maintained. A single-chamber pacing system was used in this study. In future studies, advancing a pair of pacing leads through the unilateral jugular vein should also be investigated. Although this was not tested in this study, we believe a second lead could be introduced and stabilized in the right atrium.
In general, animal models of cardiac pacing have numerous applications in cardiovascular research. First, pacing at non-physiological high frequencies over several weeks leads to tachycardia-induced cardiomyopathy, as previously reported, and allows for the study of the pathophysiology and treatment of chronic heart failure2,3,11,12. Further, research on refined materials and technologies can utilize the presented leporine model, which could be suggested for medium-term pacing studies. To our knowledge, this study is the first to demonstrate the benefits of such a small mammalian model for complex cardiac pacing experiments21. In conclusion, with the described methodology, a human-sized pacing system can be successfully implanted in small mammals, despite the tissue fragility and delicate anatomy. After training, this technique is easily reproducible, and it provides a basis for models of paced tachycardia with broad applications in cardiovascular research.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors wish to gratefully acknowledge the advice and assistance of Maria Kim, Jana Bortelová, Alena Ehrlichová, Matěj Hrachovina, Leoš Tejkl, Jana Míšková, and Tereza Vavříková for their inspiration, work, and technical support. This work was funded by MH CZ-DRO (NNH, 00023884), IG200501 grant.
Materials
| Medication | |||
| atipamezole | Eurovet Animal Health, B.V. | Atipam | anesthetic |
| buprenorphine | Vetoquinol | Bupaq | analgetic |
| enrofloxacin | Krka | Enroxil | antibiotic |
| isoflurane | Baxter | Aerrane | anesthetic |
| ketamine hydrochloride | Richter Gedeon | Calypsol | anesthetic |
| medetomidine | Orion Corp. | Domitor | anesthetic |
| meloxicam | Cymedica | Melovem | analgetic |
| povidone iodine | Egis Praha | Betadine | disinfection |
| Silver Aluminium Aerosol | Henry Schein | 9003273 | tincture |
| Surgical materials | |||
| 2-0 Perma-Hand Silk | Ethicon | A185H | silk tie suture |
| 2-0 Vicryl | Ethicon | V323H | absorbable braided suture |
| 4-0 Monocryl | Ethicon | MCP494G | monofilament |
| BearHugger | 3M | BearHugger | heating pad |
| cauterizer | |||
| Metzenbaum scissors, lancet with #22 blade, DeBakey forceps, needle driver | basic surgical equipment | ||
| sterile drapes | |||
| Diagnostic devices | |||
| Acuson VF10-5 | Siemens Healthcare | sonographic vascular probe | |
| Acuson x300 | Siemens Healthcare | ultrasound system | |
| ESP C-arm | GE Healthcare | ESP | X-ray fluoro C-arm |
| Pacing devices | |||
| 400 | Medico | CAT400 | bipolar pacing lead |
| Effecta DR | Biotronic | 371199 | implantable pacemaker |
| ERA 3000 | Biotronic | 128828 | external pacemaker |
| ICS 3000 | Biotronic | 349528 | pacemaker programmer |
Referências
- Power, J. M., Tonkin, A. M. Large animal models of heart failure. Australian and New Zealand Journal of Medicine. 29 (3), 395-402 (2008).
- Hála, P., et al. Tachycardia-induced cardiomyopathy as a chronic heart failure model in swine. Journal of Visualized Experiments. (132), e57030 (2018).
- Powers, J. C., Recchia, F. Canine model of pacing-induced heart failure. Methods in Molecular Biology. 1816, 309-325 (2018).
- Whipple, G. H., Sheffield, L. T., Woodman, E. G., Theophilis, C., Friedman, S. Reversible congestive heart failure due to chronic rapid stimulation of the normal heart. Proceedings of the New England Cardiovascular Society. 20, 39-40 (1962).
- Laughner, J. I., et al. A fully implantable pacemaker for the mouse: From battery to wireless power. PLoS One. 8 (10), 76291 (2013).
- Yue-Chun, L., et al. Establishment of a canine model of cardiac memory using endocardial pacing via internal jugular vein. BMC Cardiovascular Disorders. 10, 30 (2010).
- Gutruf, P., et al. Wireless, battery-free, fully implantable multimodal and multisite pacemakers for applications in small animal models. Nature Communications. 10 (1), 5742 (2019).
- Zhou, L., et al. A percutaneously implantable fetal pacemaker. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2014, 4459-4463 (2014).
- Zhou, L., Chmait, R., Bar-Cohen, Y., Peck, R. A., Loeb, G. E. Percutaneously injectable fetal pacemaker: Electrodes, mechanical design and implantation. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2012, 6600-6603 (2012).
- Jordan, C. P., et al. Minimally invasive resynchronization pacemaker: A pediatric animal model. The Annals of Thoracic Surgery. 96 (6), 2210-2213 (2013).
- Freeman, G. L., Colston, J. T. Myocardial depression produced by sustained tachycardia in rabbits. American Journal of Physiology. 262, 63-67 (1992).
- Ryu, K. H., et al. Force-frequency relations in the failing rabbit heart and responses to adrenergic stimulation. Journal of Cardiac Failure. 3 (1), 27-39 (1997).
- Hulsmans, M., et al. A miniaturized, programmable pacemaker for long-term studies in the mouse. Circulation Research. 123 (11), 1208-1219 (2018).
- Nishida, K., Michael, G., Dobrev, D., Nattel, S. Animal models for atrial fibrillation: Clinical insights and scientific opportunities. Europace. 12 (2), 160-172 (2010).
- Clementy, N., et al. Pacemaker complications and costs: A nationwide economic study. Journal of Medical Economics. 22 (11), 1171-1178 (2019).
- Armoundas, A. A., et al. Cellular and molecular determinants of altered Ca2+ handling in the failing rabbit heart: primary defects in SR Ca2+ uptake and release mechanisms. American Journal of Physiology-Heart and Circulatory Physiology. 292 (3), 1607-1618 (2007).
- Giraldo, A., Talavera López, J., Brooks, G., Fernández-Del-Palacio, M. J. Transthoracic echocardiographic examination in the rabbit model. Journal of Visualized Experiments. (148), e59457 (2019).
- Spannbauer, A., et al. Large animal models of heart failure with reduced ejection fraction (HFrEF). Frontiers in Cardiovascular Medicine. 6, 117 (2019).
- Byrne, M. J., et al. An ovine model of tachycardia-induced degenerative dilated cardiomyopathy and heart failure with prolonged onset. Journal of Cardiac Failure. 8 (2), 108-115 (2002).
- Hála, P., et al. Increasing venoarterial extracorporeal membrane oxygenation flow puts higher demands on left ventricular work in a porcine model of chronic heart failure. Journal of Translational Medicine. 18 (1), 75 (2020).
- Riehle, C., Bauersachs, J. Small animal models of heart failure. Cardiovascular Research. 115 (13), 1838-1849 (2019).

