Obtainment of Macrophages from Human Monocytes to Assess Leishmania braziliensis Infection Rate and Innate Host Immune Response
Summary
This protocol describes the process for obtaining human macrophages from monocytes for infection with Leishmania braziliensis. It also allows researchers to evaluate infection rate and parasite viability, ROS production by fluorescence microscopy, and the production of inflammatory mediators in culture supernatants to investigate macrophage response to infection.
Abstract
Macrophages are multifunctional cells essential to the immune system function, and the primary host cell in Leishmania braziliensis (Lb) infection. These cells are specialized in microorganism recognition and phagocytosis, but also activate other immune cells and present antigens, as well as promote inflammation and tissue repair. Here, we describe a protocol to obtain mononuclear cells from peripheral blood (PBMC) of healthy donors to separate monocytes that then differentiate into macrophages. These cells can then be infected in vitro at different Lb concentrations to evaluate the ability to control infection, as well as evaluate host cell immune response, which can be measured by several methods. PBMCs were first isolated by centrifuging with Ficoll-Hypaque gradient and then plated to allow monocytes to adhere to culture plates; non-adherent cells were removed by washing. Next, adherent cells were cultured with macrophage-colony stimulating factor (M-CSF) for 7 days to induce macrophage differentiation. We suggest plating 2 x 106 cells per well on 24-well plates in order to obtain 2 x 105 macrophages. Fully differentiated macrophages can then be infected with Lb for 4 or 24 hours. This protocol results in a significant percentage of infected cells, which can be assessed by optical or fluorescence microscopy. In addition to infection index, parasite load can be measured by counting the numbers of parasites inside each cell. Further molecular and functional assays can also be performed in culture supernatants or within the macrophages themselves, which allows this protocol to be applied in a variety of contexts and also adapted to other intracellular parasite species.
Introduction
The intracellular protozoan parasite of the genus Leishmania is the causative agent of a neglected disease complex known as leishmaniasis1. These tropical diseases have a wide range of clinical manifestations that can range from skin lesions to complications arising from the visceral form of the disease, which can be fatal if not treated. Cutaneous leishmaniasis (CL) is the most frequent form of leishmaniasis and is characterized by a single or few ulcerated skin lesions with exacerbated chronic inflammation2. The development of disease is dependent on the Leishmania species, in addition to a combination of factors associated with host immune response, which both define clinical outcomes3,4. Leishmania braziliensis is the main species that causes CL in Brazil, with cases reported throughout all states of the country5. The immune response against L. braziliensis is considered protective, since it restricts the parasite to the inoculation site, and involves several immune cell types, such as macrophages, neutrophils e lymphocytes4,6,7.
Macrophages are multifunctional cells essential for the immune system, since they are specialized in the detection and phagocytosis of microorganisms, and can present antigens and activate other cell types. Macrophages are able to regulate processes from inflammation to tissue repair and the maintenance of homeostasis8,9. These cells play an essential role in the early immune response against intracellular parasites, such as Leishmania, being important for their elimination10,11,12.
During L. braziliensis infection, macrophages can respond through different mechanisms to eliminate the parasite, such as the production of reactive oxygen species (ROS) and inflammatory mediators13,14. Immune responses can be guided by the production of proinflammatory or anti-inflammatory cytokines, which contribute to an exacerbated inflammatory state or tissue repair processes6,15,16. The plasticity of macrophages is fundamental to the immunopathogenesis of CL, as well as to parasite-host interaction, and these cells are considered crucial to the elucidation of disease mechanisms and to the development of new therapeutic approaches.
As CL is a complex disease, investigations require researchers to explore cell types that mimic those found in humans. The immune responses observed in different experimental models can vary and produce results that do not reflect the immune response observed in naturally infected humans. Thus, the protocol presented herein was designed to enable the study of human macrophages and their immune responses during CL caused by L. braziliensis.
Protocol
The Institutional Review Board for Ethics in Human Research at the Gonçalo Moniz Institute (Oswaldo Cruz Foundation-IGM-FIOCRUZ, Salvador, Bahia-Brazil), approved this study (protocol number: CAAE 95996618.8.0000.0040).
1. Isolation of human PBMCs
- Ensure that the blood samples, 1.077 g/mL density gradient (e.g., Ficoll-Histopaque), and saline solution are at room temperature.
- Dilute blood samples with saline solution at 1:1 ratio.
- Transfer 10-12 mL of density gradient to 50 mL tubes.
- Carefully overlay up to 40 mL of the diluted blood sample on top of density gradient. Separate the blood and density gradient layers.
- First centrifugation: centrifuge tubes containing blood and density gradient layers at 400 x g for 30 min at 24 ºC.
NOTE: Switch-off the brake before starting the centrifuge. - Remove the plasma above the PBMC ring with a pipette (the buffy coat layer is located between the plasma and density gradient layers; below that are red blood cells/granulocytes pellet).
- Transfer the cloud-like PBMC layer (buffy coat layer) with a pipette to a 15 mL tube and fill with cold saline (kept on ice or at 4 °C).
- Second centrifugation: centrifuge tubes at 300 x g for 10 min at 4 °C with the brake switched-on.
- Discard the supernatant and resuspend the pellet by filling the tube with cold saline.
- Third centrifugation: centrifuge tubes at 250 x g for 10 min at 4 °C with the brake switched-on.
- Discard the supernatant and resuspend the pellet, filling the tube with cold saline.
- Fourth centrifugation: centrifuge the tube at 200 x g for 10 min at 4 °C with the brake switched-on.
- Discard the supernatant and resuspend the pellet with 1 mL of cold RPMI medium.
- Count cells to determine the number of cells obtained.
2. Differentiation into human macrophages
NOTE: For a 24-well plate, calculate the amount of total cells needed to plate 2 x 106 cells per well, which will yield 2 x 105 macrophages. This yield is based on an average of 10% monocytes in human blood. Alternatively, monocytes can also be released by non-enzymatic methods and then counted for plating.
- On a 24-well plate, place 13 mm round glass coverslips at the bottom of each well. Plate the equivalent of 2×106 cells in 1 mL of RPMI incomplete per well.
NOTE: Limit the number of cells in each well, since overestimated amounts can hinder cell adhesion and compromise culturing. In addition, all coverslips must be clean and sterile. - Incubate plates for 30 min at 37 ºC under 5% CO2 for cell adhesion.
- Remove the supernatants and wash once with 0.9% saline at room temperature to remove any non-adherent cells.
- After washing, remove the saline and add 250 µL of supplemented RPMI medium at room temperature (10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin and 50 ng/mL M-CSF) to each well.
- Incubate the cells for 7 days at 37 ºC under 5% CO2.
- Add 125 µL of supplemented RPMI medium to each well every two days. At the end of cell differentiation, the final volume will be 500 µL per well.
- To analyze cell viability, perform another culture in parallel on a 96-well plate (2 x 105 per well).
- After differentiation into macrophages (7 days), add 20 µL of AlamarBlue reagent.
- After 7 hours of incubation, read plates on a spectrophotometerat wavelengths of 570 nm and 600 nm.
3. Leishmania culture and infection
NOTE: L. braziliensis promastigotes from two different strains (MHOM/BR/01/BA788 and MHOM/BR88/BA-3456) were used in this assay.
- Count Lb parasites and calculate the volume to obtain 5 x 105/mL parasites.
- Prepare supplemented Schneider's Insect medium (10% FBS, 2 mM L-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin).
- Incubate parasites in a total volume of 5 mL in a 24 ºC incubator until the stationary phase is reached (4 to 6 days).
NOTE: Count parasites every day to assess growth. - After reaching the stationary phase, centrifuge Leishmania cultures at 100 x g for 10 min at 4 ºC to remove any dead parasites (precipitated at the bottom of the tube).
- Transfer the supernatant to a new tube and centrifuge at 1,800 x g for 10 min at 4 ºC to recover viable parasites. Discard the supernatant.
- Resuspend the pellet with 1 mL of supplemented RPMI medium to count parasites.
- Calculate the volume of parasites to obtain a 10:1 parasite:cell ratio. Transfer parasites to culture plates containing macrophages previously cultured in supplemented RPMI medium (~300 µL/well) at room temperature.
- Remove the supernatant from each well containing differentiated macrophages.
NOTE: As soon as possible, replace the medium in each well to avoid cells spending extended periods without medium. We suggest removing and replacing medium in three wells at a time. - Transfer the calculated amount of Lb to each well containing differentiated macrophages.
- Infect cells for 4 or 24 hours at 37 ºC under 5% CO2.
- After 4 hours, wash macrophages 3 times with saline at room temperature to remove any non-internalized parasites.
- For the 24-hour infection period, add 300 µL of supplemented RPMI medium to each well after washing, and then reincubate for another 20 hours at 37 °C under 5% CO2.
- Remove the supernatant to measure inflammatory mediators using an enzyme-linked immunosorbent assay (ELISA) following the manufacturer's instructions.
- Centrifuge the collected supernatant at 1,800 x g for 10 min at room temperature. Transfer the supernatant to a new tube. This procedure is performed to remove any non-internalized parasites. Supernatants can be frozen and kept at -80oC until the time of future analysis.
NOTE: Cells may be used to assess infection rate, parasite viability or ROS production.
- Centrifuge the collected supernatant at 1,800 x g for 10 min at room temperature. Transfer the supernatant to a new tube. This procedure is performed to remove any non-internalized parasites. Supernatants can be frozen and kept at -80oC until the time of future analysis.
4. Evaluation of infection
- Quantification of infection rate
- Add 300 µL of methanol to each well after removing the supernatant. Allow 15 min to fix cells adhered to coverslips.
- Remove the coverslips from the wells and place on a support to soak in the cell staining solution (e.g., Quick Panoptic 2) for 1 min.
- Submerge coverslips twice in cell staining solution (e.g., Quick Panoptic 3) and wait until dried.
- Place 15 µL of mounting medium (e.g., Entellan) on slides, and then place coverslips over the medium.
NOTE: All macrophages attached to coverslips should be in contact with the mounting medium. - Count 100 cells at random under an optical microscope using an 100x objective to quantify infection rate and number of internalized amastigotes.
- Parasite viability
- After 4 hours of infection, wash cells with saline at room temperature 3 times.
- Add 300 µL of supplemented Schneider's Insect medium.
- Incubate in a 24 ºC incubator.
- Quantify parasite growth after 48, 72, 96 and 120 hours.
5. Evaluation of ROS production by fluorescence microscopy
- After the infection period, remove the supernatant and wash cells with 500 µL of saline.
- Add 300 µL of the fluorogenic probe reagent (e.g., CellROX Green Reagent at 5 µM) to each well.
- Incubate cells for 30 min at 37 ºC and 5% CO2.
- Wash cells 3x with 500 µL of phosphate buffered saline (PBS).
- Fix cells with 300 µL of 3.7% formaldehyde and let sit for 15 min.
NOTE: Measure the florescence signal within 24 hours. - Add 5 µL of DAPI staining agent (e.g., DAPI ProLong Gold antifade) for cell staining. Place the coverslip with the surface containing macrophages in direct contact with the DAPI staining agent.
- Analyze the florescence signal by fluorescence microscopy at an excitation wavelength of 485/520 nm.
- Calculate the Corrected Total Cell Fluorescence (CTCF) from 30 cells for each coverslip using ImageJ.
CTCF = Integrated Density (Area of a selected cell x Mean fluorescence of background readings)
6. Statistical analysis
- Use the Mann-Whitney test to compare two groups with unpaired samples. Perform the statistical analyses using GraphPad Prism 7.0. Consider differences statistically significant when p < 0.05.
Representative Results
The comprehension of parasites and host cells interaction is crucial to elucidate mechanisms involved in the pathogenesis of several diseases. Although cultured human cells are less used due to limitations of cell culture compared to cell lineages, the protocol presented herein shows a robust and reproducible differentiation of human macrophages. This protocol enables the analysis of several aspects of the immune response and cell biology, from the production of inflammatory mediators up to the susceptibility of an infectious agent in human macrophages.
The first evidence that cellular differentiation is taking place is macrophage morphology (Figure 1A). On the plating day, cells are rounded and small when compared to the morphology after seven days of culture. The cellular spreading is observed when cultures are treated with macrophage colony-stimulating factor (M-CSF). In the absence of M-CSF, cell differentiation takes more time and results in an heterogenous population of macrophage-like cells (data not shown). After 7 days of differentiation, macrophages were incubated with the Alamarblue reagent for 24 hours until reading. This method allows the quantification of the cellular capacity to reduce resazurin to resorufin, thus differentiating viable from dead cells. The "ctrl" group refers to macrophages cultured for 7 days in supplemented medium, while the "dead" group refers to macrophages submitted to osmotic lysis during differentiation, which serves as a control for the technique. Once the differentiation in complete (for seven days), the macrophages derived from human monocytes remain viable and prompt to further assays that can last up to 24 hours after differentiation (Figure 1B) or few days (data not shown).
The first moments of interaction between Leishmania and a phagocyte is marked by close contact that will culminate in phagocytosis and internalization of the parasite. To understand the process of infection will help to explain the mechanisms involved in parasite killing or susceptibility to a certain pathogen. Based on the results, the first four hours of infection present the highest infection rate of L. braziliensis (both BA788 and BA3456 tested strains). After 24 hours of infection, there is a reduction in the infection rate of both strains, but we found statistical significance only for BA788 (Figure 2A, D). Considering longer periods of infection, no internalized parasites were found inside the cells after 72 hours (data not shown), suggesting that human macrophages are able to control L. braziliensis infection in vitro. The infection rate is measured by the count of 100 cells and, among those, the infected ones. This estimates the percentage of infection, which can vary due to the immune response of the cell donor, the amount of Leishmania parasites in the stationary phase and also due to the experimenter bias. Figure 2H shows representative images of a low infection rate (left) and a higher rate (right) by optical microscope.
Another data that can be assessed in cultured human macrophages is the parasite load, which is important to indicate the ability of macrophages to control the infection. It is measured by the average of internalized parasites in each cell and, after different time points, it is possible to determine whether the number of parasites has increased or reduced (Figure 2B, E). Finally, results of parasite viability further assemble information about the infection control. It is measured by the count of viable parasites in the culture after replacing RPMI to Schneider medium. Regarding L. braziliensis infection, we have already tested different time points, such as 48 h, 72 h, 96 h and 120 h to quantify viable parasites. The results indicate that 72 hours is recommended to evaluate parasite viability in these conditions (Figure 2G).
Based on our protocol, it is also possible to measure the inflammatory response against L. braziliensis infection in culture supernatant as soon as 4 hours after the infection. We were able to detect IL-6, TNF-α and LTB4 (Figure 3A-C), but IL-10 and IL-1β production was below the detection level (data not shown).
Another important aspect of the immune response against L. braziliensis is the production of reactive oxygen-derived species (ROS) by macrophages. This is one of the main mechanisms for parasite killing. The protocol presented herein show that ROS production from macrophages is significantly increased after 4 hours of infection (Figure 4A). The quantification of ROS based on corrected total cell fluorescence (CTCF) allowed the detection of almost twice the ROS production between infected and uninfected macrophages (Figure 4B).
Together, the results show that macrophage derived from humanmonocytes allows the study of several aspects of the immune response against L. braziliensis infection in vitro. Thus, this protocol enables research groups to further explore the role of human macrophages in leishmaniasis, minimizing the bias of lineage or murine cell models.

Figure 1. Cell morphology during human macrophage differentiation in vitro. (A) Representative images of the morphology of adherent cells at first day of culture (left) and after seven days of differentiation. (B) Cell viability after culture for seven days for differentiation. Ctrl = macrophages in culture with supplemented medium; Dead = macrophages subjected to osmotic lysis; Objective 60x; n = 3. Please click here to view a larger version of this figure.
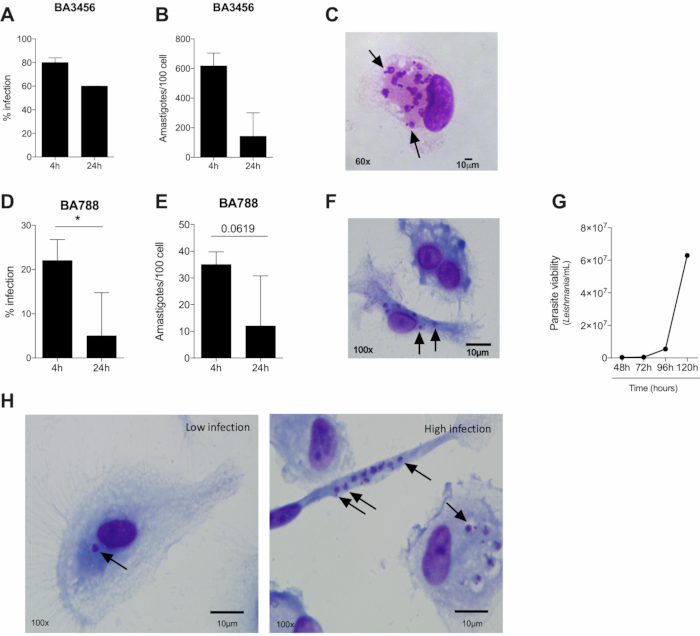
Figure 2. Several parameters of infection caused by two strains of Leishmania braziliensis can be assessed by optical microscopy. (A, D) Infection rate (B, E), parasite load (four and 24 hours of infection) and (C, F) representative images of macrophages infected by L. braziliensis from BA5456 (A-C) or BA788 (D-E) strains (four hours of infection). (G) Macrophage-derived viable L. braziliensis after four hours of infection with BA788 strain. (H) Representative images of human macrophages infected by L. braziliensis (BA788 strain) showing low (left) and high infection rate (right). Arrows = intracelular amastigotes; Scale bar 10 µm; *p < 0.05; (A,B) n = 3; (D,E) n = 6; (G) n = 6. Please click here to view a larger version of this figure.
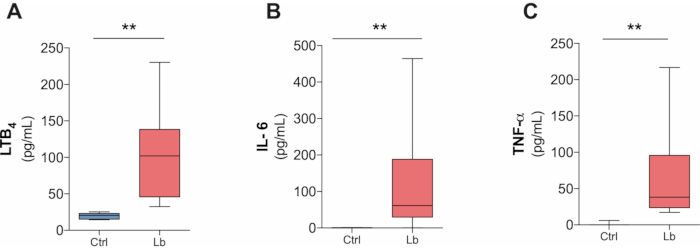
Figure 3. L. braziliensis-induced production of inflammatory mediators by human macrophages in four hours after infection. (A) LTB4, (B) IL-6 and (C) TNF-α production in culture supernatant after four hours infection by L. braziliensis (BA788 strain) measured by ELISA. **p < 0.01; n = 5. Please click here to view a larger version of this figure.
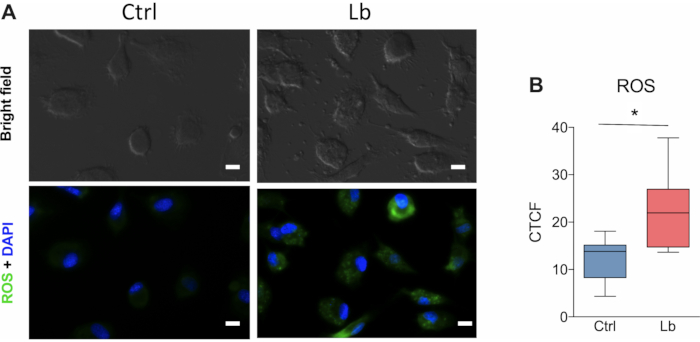
Figure 4. ROS production by human macrophages after in vitro infection with L. braziliensis. (A) Representative images of ROS fluorescent labelling in cultured human macrophages after four hours of infection by L. braziliensis (BA788 strain). (B) Quantification of ROS production based on the corrected total cell fluorescence (CTCF) using Image J. Green = ROS; Blue = nucleus; Scale bar 10 µm; * p < 0.05; n =6. Please click here to view a larger version of this figure.
Discussion
The protocol presented herein for human monocytes differentiation into macrophages followed by the infection with two strains of L. braziliensis allows the evaluation of several aspects of parasite-cell interaction. These tools can be crucial to elucidate unanswered questions about CL. With the establishment of this protocol, our group was able to uncover some aspects of the immune response of macrophages obtained from individuals with diabetes and CL14.
The differentiation process of monocytes into human macrophages is complex and requires attention from the first day of culture. The researcher must monitor the differentiation, checking the development of the culture by cellular morphology daily. Usually, the culture of monocytes for seven days with M-CSF containing medium is sufficient for complete differentiation. It is important to mention that cell morphology depends on the donor, thus several stages of cellular differentiation can be observed between donors. This can be overcome with an increase in the number of donors, which will allow the identification of outliers. Moreover, the use of M-CSF is crucial for fully differentiation into macrophages17; otherwise, it will result in a highly heterogeneous population of dendritic cells-like, macrophage-like cells and monocytes. Other growth factors or a combination of these has been used as tolls to further polarize macrophages into M1 or M2 profile17,18. Several studies have shown that macrophages cultured with M-CSF develop an M2 profile, while cells treated with GM-CSF exhibit an M1 profile19. However, macrophages cultured with M-CSF can polarize to the M1 profile after stimulation20. Based on the results, we were unable to determine a macrophage profile prior to infection, but hypothesize that this probably trended toward an M2 profile. On the other hand, after 4 hours of infection, macrophages produced high levels of pro-inflammatory cytokines and ROS, which is characteristic of a classic macrophage profile. Another relevant aspect about the infection of macrophages with Leishmania is the dispersion observed in the percentage of infected cells (infection rate). This is a marked feature of assays with human macrophages, also due to the responsiveness of each donor. To minimize this effect, the stationary-phase of Leishmania cultures should be confirmed and methods to purify metacyclic promastigotes can be considered21,22,23. In addition, our findings show that increased infection periods result in a decrease in the infection rate, since human macrophages seem to be able to control L. braziliensis.
The interaction of macrophages and Leishmania involves the production of mediators that are a combination of a protective response of the host cell to kill the parasite and escape mechanisms developed by each Leishmania species. Thus, to define the profile of mediators produced during the infection is essential to understand the pathogenesis of CL24. Based on our protocol, it is possible to measure pro-inflammatory mediators after 4 hours of infection with L. braziliensis. With this method, we were also able to show this inflammatory response of macrophages from individuals with diabetes after infection in vitro with L. braziliensis14. The possibility to evaluate different inflammatory mediators is crucial to better understand CL as a chronic inflammatory skin disease24,25,26.
The production of mediators involved in parasite killing also play a significant role in the disease development and outcome. It has already been described that ROS production is one of the most efficient mechanism to control L. braziliensis infection13,14. The protocol presented herein allow the evaluating of ROS production within the macrophages infected by L. braziliensis using fluorescence microscope. ROS production seems to be key to define susceptibility to the infection14,25. Unlike other methods, the quantification of ROS through fluorescence microscopy has the advantage of locally identifying production inside the cell. Other methods only allow the indirect quantification of ROS production from an entire cell population, without considering that cells can respond differently to the same stimulus13,25.
In summary, the results show that the protocol described herein enables studies that aim to explore the interaction between macrophages and L. braziliensis, assessing aspects such as the infection rate, parasite killing and production of mediators. This allows to extrapolate the findings and to correlate it with naturally occurring mechanisms.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) under Grant number PET0009/2016 and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES) under Finance Code 001.
Materials
| AlamarBlue Cell Viability Reagent | Invitrogen | DAL1100 | |
| Cell Culture Flask 25 cm² | SPL | 70125 | |
| CellROX Green Reagent | Invitrogen | C10444 | |
| Coverslip circles 13 mm | Perfecta | 10210013CE | |
| DAPI (4',6-diamidino-2-phenylindole) | ThermoFisher | D1306 | |
| Disposable support for blood collection | BD Vacutainer | 364815 | |
| Eclipse blood collection needle 21 g x 1.25 in | BD Vacutainer | 368607 | |
| Entellan | Sigma Aldrich | 107961 | |
| Falcon Conical Tubes, 15 mL | Sigma Aldrich | CLS430791-500EA | |
| Falcon Conical Tubes, 50 mL | StemCell Technologies | 100-0090 | |
| Fetal Bovine Serum | Gibco | A4766801 | |
| Formaldehyde 3.7% | Merck | 252549 | |
| Glass slide 25,4×76,2mm | Perfecta | 0200 | |
| Histopaque | Sigma Aldrich | 10771 | |
| Human IL-6 ELISA Kit | RD | DY206 | |
| Human M-CSF Recombinant Protein | PeproTech | 300-25 | |
| Human TNF-a ELISA Kit | RD | DY210 | |
| Leukotriene B4 ELISA Kit | Cayman | 520111 | |
| Methanol | Merck | MX0482 | |
| Penilicin-Sreptomycin-Glutamine (100x) | ThermoFisher | 10378-016 | |
| Phosphate Buffered Saline pH 7.2 (10x) | Gibco | 70013032 | |
| Plasma tube, 158 USP units of sodium heparin (spray coated) | BD Vacutainer | 367874 | |
| Quick H&E Staining Kit (Hematoxylin and Eosin) | abcam | ab245880 | |
| RPMI 1640 Medium | Gibco | 11875093 | |
| Schneider's Insect Medium | Sigma Aldrich | S0146 | |
| Tissue Culture 24-wells Plate | TPP | Z707791-126EA | |
| Trypan Blue | Gibco | 15250061 |
Referências
- Desjeux, P. Leishmaniasis: current situation and new perspectives. Comparative Immunology, Microbiology and Infectious Diseases. 27 (5), 305-318 (2004).
- Burza, S., Croft, S. L., Boelaert, M. Leishmaniasis. The Lancet. 392 (10151), 951-970 (2018).
- Arenas, R., Torres-Guerrero, E., Quintanilla-Cedillo, M. R., Ruiz-Esmenjaud, J. Leishmaniasis: A review. F1000Research. 6, 1-15 (2017).
- Scott, P., Novais, F. O. Cutaneous leishmaniasis: Immune responses in protection and pathogenesis. Nature Reviews Immunology. 16 (9), 581-592 (2016).
- Bittencourt, A. L., Barral, A. Evaluation of the histopathological classifications of American cutaneous and mucocutaneous leishmaniasis. Memórias do Instituto Oswaldo Cruz. 86 (1), 51-56 (1991).
- Scorza, B. M., Carvalho, E. M., Wilson, M. E. Cutaneous manifestations of human and murine leishmaniasis. International Journal of Molecular Sciences. 18 (6), (2017).
- Carvalho, L. P., Passos, S., Schriefer, A., Carvalho, E. M. Protective and pathologic immune responses in human tegumentary leishmaniasis. Frontiers in Immunology. 3, 1-9 (2012).
- Wood, W., Martin, P. Macrophage Functions in Tissue Patterning and Disease: New Insights from the Fly. Developmental Cell. 40 (3), 221-233 (2017).
- Okabe, Y., Medzhitov, R. Tissue biology perspective on macrophages. Nature Immunology. 17 (1), 9-17 (2016).
- Ribeiro-Gomes, F. L., et al. Macrophage Interactions with Neutrophils Regulate Leishmania major Infection. The Journal of Immunology. 172 (7), 4454-4462 (2004).
- Liu, D., Uzonna, J. E. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Frontiers in Cellular and Infection Microbiology. 2, 1-8 (2012).
- Giudice, A., et al. Macrophages participate in host protection and the disease pathology associated with Leishmania braziliensis infection. BMC Infectious Diseases. 12, (2012).
- Morato, C. I., et al. Essential role of leukotriene B4 on Leishmania (Viannia) braziliensis killing by human macrophages. Microbes and Infection. , (2014).
- Bonyek-Silva, I., et al. Unbalanced Production of LTB 4/PGE 2 Driven by Diabetes Increases Susceptibility to Cutaneous Leishmaniasis. Emerging Microbes & Infections. , (2020).
- Tomiotto-Pellissier, F., et al. Macrophage Polarization in Leishmaniasis: Broadening Horizons. Frontiers in Immunology. 9, 2529 (2018).
- Anderson, C. F., Mosser, D. M. A novel phenotype for an activated macrophage: the type 2 activated macrophage. Journal of Leukocyte Biology. 72 (1), 101-106 (2002).
- Jin, X., Kruth, H. S. Culture of macrophage colony-stimulating factor differentiated human monocyte-derived macrophages. Journal of Visualized Experiments. 2016 (112), 6-11 (2016).
- Rios, F. J., Touyz, R. M., Montezano, A. C. Isolation and differentiation of human macrophages. Methods in Molecular Biology. 1527, 311-320 (2017).
- Hamilton, T. A., Zhao, C., Pavicic, P. G., Datta, S. Myeloid colony-stimulating factors as regulators of macrophage polarization. Frontiers in Immunology. 5, 1-6 (2014).
- Jaguin, M., Houlbert, N., Fardel, O., Lecureur, V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cellular Immunology. 281 (1), 51-61 (2013).
- Wozencraft, A. O., Blackwell, J. M. Increased infectivity of stationary-phase promastigotes of Leishmania donovani: Correlation with enhanced C3 binding capacity and CR3-mediated attachment to host macrophages. Immunology. 60 (4), 559-563 (1987).
- Sinha, R., et al. Genome plasticity in cultured Leishmania donovani: Comparison of early and late passages. Frontiers in Microbiology. 9, 1-20 (2018).
- Rebello, K. M., et al. Leishmania (Viannia) braziliensis: Influence of successive in vitro cultivation on the expression of promastigote proteinases. Experimental Parasitology. 126 (4), 570-576 (2010).
- Maspi, N., Abdoli, A., Ghaffarifar, F. P. r. o. -. Pro- and anti-inflammatory cytokines in cutaneous leishmaniasis: a review. Pathogens and Global Health. 110 (6), 247-260 (2016).
- Nunes, S., et al. Integrated analysis reveals that miR-193b, miR-671, and TREM-1 correlate with a good response to treatment of human Localized cutaneous leishmaniasis caused by Leishmania braziliensis. Frontiers in Immunology. 9, 1-13 (2018).
- De Moura, T. R., et al. Toward a Novel Experimental Model of Infection To Study American Cutaneous Leishmaniasis Caused by Leishmania braziliensis. Infection and Immunity. 73 (9), 5827-5834 (2005).
- Lima, F. R., Ferreira, L. D. M., Bonyek-silva, I., Santos, R. L., Tavares, N. M. Metformin promotes susceptibility to experimental Leishmania braziliensis infection. Memórias do Instituto Oswaldo Cruz. 115, 1-8 (2020).

.
