Directed Assembly of Elastin-like Proteins into defined Supramolecular Structures and Cargo Encapsulation In Vitro
Summary
At the interface of organic and aqueous solvents, tailored amphiphilic elastin-like proteins assemble into complex supramolecular structures such as vesicles, fibers and coacervates triggered by environmental parameters. The described assembly protocols yield Protein Membrane-Based Compartments (PMBCs) with tunable properties, enabling the encapsulation of various cargo.
Abstract
Tailored proteinaceous building blocks are versatile candidates for the assembly of supramolecular structures such as minimal cells, drug delivery vehicles and enzyme scaffolds. Due to their biocompatibility and tunability on the genetic level, Elastin-like proteins (ELP) are ideal building blocks for biotechnological and biomedical applications. Nevertheless, the assembly of protein based supramolecular structures with distinct physiochemical properties and good encapsulation potential remains challenging.
Here we provide two efficient protocols for guided self-assembly of amphiphilic ELPs into supramolecular protein architectures such as spherical coacervates, fibers and stable vesicles. The presented assembly protocols generate Protein Membrane-Based Compartments (PMBCs) based on ELPs with adaptable physicochemical properties. PMBCs demonstrate phase separation behavior and reveal method dependent membrane fusion and are able to encapsulate chemically diverse fluorescent cargo molecules. The resulting PMBCs have a high application potential as a drug formulation and delivery platform, artificial cell, and compartmentalized reaction space.
Introduction
The assembly of supramolecular structures for biotechnological applications is becoming increasingly important1,2,3,4,5. For the assembly of functional architectures such as coacervates, vesicles, and fibers with desired physicochemical properties it is important to understand and control the physicochemical and conformational properties of the components. Due to the molecular precision of molecules found in nature, building blocks for supramolecular structures are increasingly based on lipids, nucleic acids or proteins. Compared to synthetic polymers, proteinaceous building blocks allow for precise control over emergent supramolecular structures6 on the genetic level. The primary amino acid (aa) sequence of the individual protein building blocks intrinsically encodes the information for their assembly potential from the molecular up to the macroscopic level as well as the three dimensional shape and physical properties of the final supramolecular structure7.
Reported methods for the assembly of different supramolecular structures often involve amphiphilic proteins such as temperature sensitive elastin-like proteins (ELP)5,8,9, recombinant oleosin10 and artificial protein amphiphiles11. Temperature triggered methods have led to the assembly of micelles4,10,12, fibers13, sheets14 and vesicles9,15,16. Methods involving organic solvents have been applied for the formation of dynamic protein based vesicles8,11,14. So far, applied protocols for vesicle formation often lack assembly control over micrometer sized assemblies16,17 or have limited assembly yield5. In addition, some reported ELP based vesicles have impaired encapsulation potential12 or limited stability over time9. Addressing these drawbacks, the presented protocols enable the self-assembly of micrometer and sub micrometer sized supramolecular structures with distinct physiochemical properties, good encapsulation potential and long-time stability. Tailored amphiphilic ELPs assemble into supramolecular structures, spanning the range from spherical coacervates and highly ordered twisted fiber bundles to unilamellar vesicles depending on the applied protocol and associated environmental conditions. Large vesicular Protein Membrane-Based Compartments (PMBC) reveal all main phenotypes such as membrane fusion and phase separation behaviour analogous to liposomes. PMBCs efficiently encapsulate chemically diverse fluorescent cargo molecules which can be monitored using simple epifluorescence microscopy. The repetitive ELP domains used in this study are attractive building blocks for protein based supramolecular architectures18. The ELP pentapeptide repeat unit (VPGVG) is known to tolerate different aa besides proline at the fourth position (valine, V), while preserving its structural and functional properties19. The design of amphiphilic ELPs containing distinctive hydrophilic and hydrophobic domains was realized by inserting aa guest residues (X) in the VPGXG repeat with distinct hydrophobicity, polarity, and charge20. Amphiphilic ELP domains where equipped with hydrophobic phenylalanine (F) or isoleucine (I) while the hydrophilic domain contained charged glutamic acid (E) or arginine (R) as guest residues. A list of eligible amphiphilic ELP constructs and corresponding aa sequences can be found in the supplementary information and references8,21. All building blocks where equipped either with small fluorescent dyes or fluorescent proteins for visualization via fluorescence microscopy. mEGFP and other fluorescent proteins were N-terminally fused to the hydrophilic domains of the ELP amphiphiles . Organic dyes were conjugated via copper-free strain promoted alkyne–azide cycloaddition (SPAAC) to a co-translationally introduced unnatural amino acid (UAA). The co-translational incorporation of the UAA para-azidophenylalanine (pAzF)22 permits the N-terminal modification of the hydrophilic ELP domain. In this way the green fluorescent dye BDP-FL-PEG4-DBCO (BDP) or any small fluorescent molecule with a strained cyclooctyne can be used as fluorescent probe. Successful incorporation of the UAA pAzF and cycloaddition of the dye via SPAAC can be easily confirmed via LC-MS/MS due to efficient ionization of the corresponding tryptic peptides8. This small organic dye was applied to broaden the solvent choice for assembly protocols, since fluorescent proteins are incompatible with most organic solvents. The two most efficient assembly protocols for supramolecular structures developed in our lab are described below. The THF swelling method is only compatible with organic dye modified amphiphilic ELP. In contrast, the 1-butanol (BuOH) extrusion method is compatible with many proteins as fluorescent probe e.g. mEGFP, since the described method fully preserves the fluorescence of these fusion proteins. In addition, the encapsulation of small molecules and vesicular fusion behavior works best by employing the BuOH extrusion method.
Protocol
1. Design and cloning of amphiphilic elastin-like proteins (ELPs)
- Clone and design the constructs as described elsewhere8,20. Plasmids are available upon request.
2. Protein expression, purification and preparation
- Expression of F20E20-mEGFP and F20E20-mCherry
- Inoculate main expression culture from overnight pre-culture to an OD600 of 0.3. Incubate at 37 °C, 200 rpm in sterile 400 mL LB medium supplemented with appropriated antibiotics in a 2 L flask.
- Prepare IPTG stock solution (1 M) for induction of the expression culture in ultrapure water.
- When OD600 0.5–0.8 is reached, add IPTG to expression culture to a final concentration of 1 mM and reduce incubation temperature to 20 °C. Allow expression at 20 °C for approximately 20 h at 200 rpm.
- Expression of amphiphilic ELP containing UAA pAzF
- Inoculate main expression culture from overnight E. coli pre-culture containing the two plasmids pEVOL pAzF and e.g. pET28-NMBL-(TAG)R40F20-his to an OD600 of 0.3 (see supplementary information for amino acid sequences). Incubate at 37 °C, 200 rpm in sterile 400 mL LB medium supplemented with kanamycin and chloramphenicol in a 2 L flask.
- Prepare 100 mM pAzF stock solution in ultrapure water. For 10 mL of pAzF stock solution, weigh 206.2 mg pAzF and resuspend it in 8 mL of ultrapure water. To dissolve the pAzF raise the pH of the solution with 3 M NaOH and mix vigorously. When pAzF is dissolved, carefully lower the pH to 10.5 and add ultrapure water to a final volume of 10 mL. Use a sterile filter (0.22 µm) and aliquot the solution in 2 mL reaction tubes.
- Prepare 1 M IPTG stock solution in ultrapure water and 20% arabinose stock solution in ultrapure water.
- When OD600 0.5–0.8 is reached, add pAzF to the expression culture to a final concentration of 2 mM. Incubate culture for 10 min, 37 °C, 200 rpm to allow for pAzF uptake.
- Induce expression of target protein and expression of the necessary tRNA/t-RNA synthetase via simultaneous addition of IPTG (1 mM) and arabinose (2%) and reduce incubation temperature to 20 °C.
- Allow expression at 20 °C for approximately 20 h at 200 rpm. Harvest expression culture by centrifugation at 4 °C, 4000 x g, 40 min.
- Cell lysis and protein purification
- Resuspend the E. coli pellet in lysis buffer (10 mL per liter of culture; 50 mM Tris-HCl pH 8, 500 mM NaCl, 4 M urea, 0.25% Triton X-100) containing lysozyme (0.1 mg/mL) and PMSF (0.1 mM). Incubate for 30 min on ice and freeze and thaw twice afterwards by submerging the sample in liquid nitrogen.
- Sonicate the suspension (30%, 15 times, 30 s: 10 s break) and clear the lysate via centrifugation (4 °C, 10,000 x g for 40 min).
- Purify protein using affinity chromatography (e.g. on a 1 mL nickel column using a FPLC system connected to a fraction collector; see Table of Materials). Elute the protein with elution buffer (50 mM Tris-HCl, 500 mM NaCl, 4 M urea, 250–500 mM imidazole) and store at 4 °C until further processing.
- Analyze the purification efficiency via SDS-PAGE.
3. Dye-modification of ELPs via SPAAC
- Roughly estimate the concentration of the ELP solution. A280 absorption for concentration evaluation is not valuable since pAzF-R40F20 sequence is lacking amino acids absorbing in the UV range. Therefore, a previously lyophilized and weighted ELP amphiphile can be used as a reference for SDS PAGE band comparison. Through comparison of the summed gray value intesity of SDS PAGE bands from ELP solutions with known concentrations and your sample the rough concentration of your sample can be estimated.
- Add 1 µL of fluorescent dye BDP-FL-PEG4-DBCO (10 mM stock solution; 20 µM final concentration) to 500 µL of ELP solution (~20 µM). Incubate the reaction for about 10 h at 15 °C, while shaking and protected from light.
- For further use, dialyze the reaction to remove excessive BDP.
- Equilibrate a dialysis membrane (e.g. 12 kDa cutoff) in ultrapure water for 10 min. Cut the dialysis membrane into the correct size to be placed on top of the opening of an reaction tube containing the clicked ELP solution. To fix the dialysis membrane in the opening, place a reaction tube lid with punched out core on the opening, thus closing the tube.
- Place the reaction tube upside down in the chosen buffer. Exchange the buffer (2–5 L) twice after dialysis for at least 3 h every time. Remove any air bubbles trapped between the dialysis membrane and the buffer to ensure successful dialysis.
4. THF swelling protocol
- Dialyze homogenous ELP solution against phosphate or tris buffer (10 mM) with stable pH 7.5 to remove salts and remaining compounds from his-tag purification.
- Prepare the lyophilizer and cool down to starting temperature for freeze-drying.
- Aliquot the dialyzed protein solution in 1.5 mL reaction tubes (50–500 µL per tube) and shock freeze in liquid nitrogen. To avoid unwanted mixing of different protein solutions during freeze-drying, caps with a small hole can be put on top of the reaction tube to seal it partially.
- Take the frozen protein samples out of the liquid nitrogen and immediately place them in the lyophilizer to start freeze-drying. Freeze-drying is finished when the sample is completely dry (approximately 24–48 h). Subsequently, ventilate lyophilized amphiphilic ELPs with dry N2, then immediately close the reaction tube lids to avoid contact with air moisture.
- Add pure THF to the lyophilized samples (ELP, 5–10 µM) and place the solution in a water bath sonicator containing ice water for 15 min to allow for swelling of the ELP in THF.
- Preheat a thermocycler to 30–60 °C for vesicle formation or up to 90 °C for fiber formation and prepare new reaction tubes containing either ultrapure water or buffer (50 mM NaH2PO4/Na2HPO4, 50 mM NaCl, pH 5–13). Spherical coacervates assemble predominantly at 20 °C within pH 9–13. Vesicle formation is favored at 50–60 °C between pH 7 and 9. Fiber formation is predominently induced above 60 °C between pH 5 and 12.
- After the sonication step, place the ELP/THF solution as well as the prepared ultrapure water or buffer solution in the thermocycler and heat up to the desired temperature for 5 min. When temperature is reached the preheated ELP/THF solution should be carefully stratified on top of the preheated ultrapure water or buffer solution. A clear separation of the two phases with a distinct interface should be visible.
- Place the mixture in the thermocycler again and incubate for 20 min to allow for vesicle or fiber formation at the interface. Afterwards, let the samples cool down to room temperature for 10 min before analysis via fluorescence microscopy or dialysis.
- Dialyze solution containing the supramolecular structures against ultrapure water or buffer (50 mM NaH2PO4/Na2HPO4, 50 mM NaCl, pH 7–10).
5. BuOH extrusion protocol
- Prepare a 1–50 µM ELP solution in ultrapure water or buffer (50 mM PB pH 7.5, 100 mM NaCl, may contain up to 4 M urea). The concentration of the amphiphilic ELP F20R20-mEGFP and F20R20-mCherry solution can be determined using the molar extinction coefficients (F20R20-mEGFP A280 = 22015 M-1 cm-1 and F20R20-mCherry A280 = 34380 M-1 cm-1) (see supplementary information for aa sequences).
- Add 10%–20% (v/v) 1-butanol and immediately mix the solution by pipetting up and down or drawing it up through a syringe multiple times. A common 100 µL pipette or Hamilton syringe equipped with a 0.25 x 25 mm needle can be applied. The turbidity of the solution during mixing should increase, indicating vesicle formation. 1-octanol 5%–15% (v/v) can also be used for vesicle extrusion instead of 1-butanol.
- In order to achieve a narrow size distribution, extrude vesicles using a mini extruder through a membrane with a pore size of 1 µm at room temperature. The membrane size used for extrusion determines the upper size cutoff of the vesicles.
- Dialyze the vesicles as described above (step 3.3) to remove residual 1-butanol.
6. Dye encapsulation with the BuOH extrusion protocol
- Mix approximately 40 µL ELP solution in 10 mM Tris-HCl, pH 8 with 1 µL Dextran Texas Red (0.0025 mg/mL final concentration).
- Add 10 µL of BuOH to the solution and extrude 5–10 times through a syringe equipped with a 0.25 x 25 mm needle.
7. Analysis of supramolecular structures using fluorescence microscopy
- Place a reinforcement ring on a glass slide and firmly press the adhesive side to the glass.
- Add 5 µL of the sample to the inside of the reinforcement ring and place a cover slip on top.
- Seal the sample with nail polish at the edges of the cover slip to avoid evaporation of the sample during analysis.
- Carry out fluorescence microscopy as previously described8.
Representative Results
Protocol development for vesicle production
Figure 1 outlines the two different vesicle preparation methods. The THF swelling method on the left side is composed of three successive steps and results in different supramolecular assemblies of the ELP depending on the temperature. In Figure 1A epifluorescence microscopy images show vesicles assembled from BDP-R20F20 and fibrillary structures assembled from BDP-R40F20. The BuOH method, illustrated on the right side exclusively leads to the formation of ELP vesicles, yielding about two orders of magnitude more vesicles compared to the THF swelling method. The schematic illustration shows the preparation process of BuOH vesicles. For vesicle preparation in Figure 1B BDP-R40F20 was mixed with 10-15% (v/v) BuOH and vesicles were prepared via extrusion of the mixture.
Guiding supramolecular self-assembly into different structures
Figure 2 shows a schematic illustration and epifluorescence images of different supramolecular structures assembled from BDP-R40F20 via the THF swelling protocol. In this case lyophilized BDP-R40F20 was used for the different assembly protocols. The pH of the buffer and the temperature of the assembly process were adjusted to form either coacervates, fibrils or vesicles. The coacervates depicted in Figure 2A are 1–2 µm in diameter and were assembled from BDP-R40F20 at 20 °C and pH 13. Adjustment of the assembly temperature to 90 °C results in the formation of nanofiber bundles (Figure 2B) at pH 4–13 tested with BDP-R40F20. Stable vesicles could be assembled from the ELP at a temperature of 50 °C and pH 7 (Figure 2C). Small mistakes at one of the crucial steps in the assembly protocol can lead to the formation of aggregates depicted in Figure 2D.
Encapsulation of different cargo
Figure 3 shows the encapsulation of different cargo into the vesicle lumen of vesicles assembled from F20R20-mEGFP via the BuOH extrusion method. For the encapsulation of the positively charged dye Atto Rho13 in Figure 3A, the dye was mixed with the aqueous ELP solution before addition of (15% v/v) BuOH and syringe extrusion of the mixture. The confocal microscopy images show the vesicles formed from F20R20-mEGFP in the green channel, the red dye AttoRho13 in the red channel and the resulting merged channel shows the successful encapsulation inside the vesicle lumen.
The polysaccharide Dextran Red 3000 was successfully encapsulated using the BuOH extrusion method as described above. Images recorded in green channel depict the vesicles formed from F20R20-mEGFP while red channel shows the polysaccharide cargo. Merged green and red image in Figure 3B confirm the successful Dextran Red 3000 encapsulation in to the vesicle lumen.
Membrane component compatibility and phase separation of mixed BuOH vesicles before/after extrusion
Figure 4 shows the phase separation and fusion behavior of ELP amphiphiles upon mixing of single PMBC building blocks versus assembled PMBC populations. Mixing amphiphilic ELP building blocks (F20R20-mEGFP and F20R20-mCherry) prior to PMBC assembly leads to homogenously distributed molecules within the assembled PMBC membrane. The homogenous distribution of the fluorophores and associated ELP amphiphiles is evident upon merging the red and green channel of the respective fluorescence images. By mixing vesicle populations assembled from either F20R20-mEGFP or F20R20-mCherry clearly visible membrane patches of red or green fluorescence are evident immediately after mixing. This indicates that PMBC fusion events of differently labeled PMBCs occur and that these fusing membranes and their constituents stay phase separated for at least 20 min. A similar phase behavior is known from lipid rafts, within lipid membranes23.
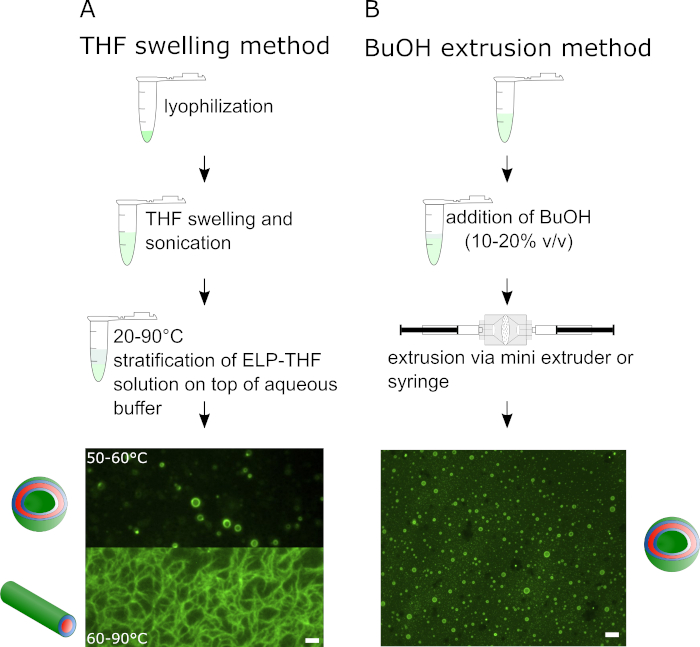
Figure 1: Illustration of the THF swelling method and the BuOH extrusion method for the guided self-assembly of amphiphilic ELPs into supramolecular structures such as vesicles or fibers. Schematic workflow and representative epifluorescence images of (A) the THF swelling method with BDP-R20F20 and BDP-R40F20 resulting in different supramolecular structures depending on temperature and pH and (B) the BuOH extrusion method exclusively yielding vesicles from BDP-R40F20 (scale bar 2 µm). This figure has been modified from Schreiber et al. 20198. Please click here to view a larger version of this figure.
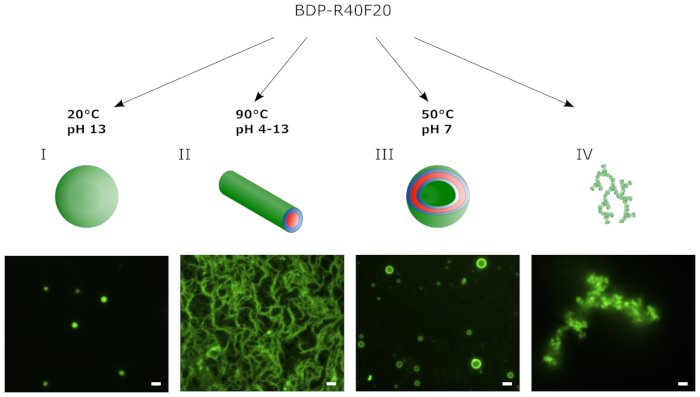
Figure 2: By applying the THF swelling method BDP-R40F20 self-assembles into different supramolecular structures. The environmental conditions applied during the assembly protocol (e.g. temperature or pH) determine the predominate supramolecular structure formed. Representative supramolecular structures at the respective conditions during the assembly were monitored via epifluorescence microscopy and range from (A) coacervates and (B) fibrils to (C) stable vesicles. (D) Failure in the assembly of defined structures during the THF swelling protocol leads to the formation of unspecific aggregates (scale bar 2 µm). This figure has been modified from8. Please click here to view a larger version of this figure.
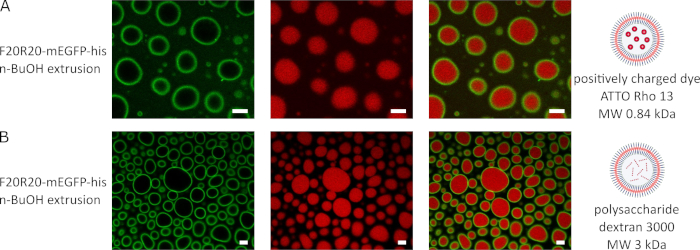
Figure 3: Different cargos can be encapsulated within ELP vesicles using the BuOH extrusion method. (A) shows representative confocal images of F20R20-mEGFP vesicles with encapsulated positively charged dye AttoRho13 and (B) the encapsulation of the polysaccharide dextran red (scale bar 5 µm). Please click here to view a larger version of this figure.
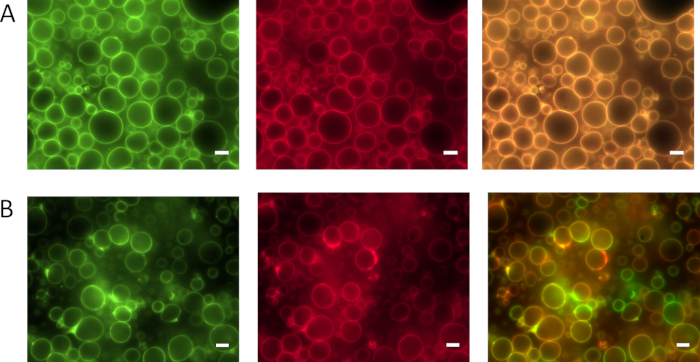
Figure 4: Membrane component compatibility and fusion behavior of vesicle membranes assembled from F20R20 via BuOH extrusion method. (A) Mixing of fluorescent F20R20-mEGFP and F20R20-mCherry protein solution prior to syringe-extrusion leads to PMBC membranes with homogenously distributed amphiphilic proteins visible in green channel (left image), red channel (middle image), and merged channel (right image). (B) PMBCs assembled from either F20R20-mEGFP or F20R20-mCherry and mixed subsequently via syringe extrusion lead to separated ELP amphiphile patches within the PMBC membranes. The separated ELP amphiphiles within the membrane are visible after PMBC fusion for at least 20 min in green channel (left image), red channel (middle image), and the merged channel (right image). Scale bars correspond to 5 µm. This figure has been modified from Schreiber et al. 20198. Please click here to view a larger version of this figure.
Discussion
A fault while following the described protocols for the assembly of defined supramolecular structures mainly leads either to the formation of unspecific aggregates (Figure 2, IV) or to homogeneously distributed ELP-amphiphiles. Critical steps of the protocol are discussed below:
For high expression yield of the amphiphilic ELP, a relatively low temperature of 20°C is optimal. For successful affinity based purification of the amphiphilic ELP an urea concentration of 4 M in the lysis buffer was proven to best solubilize the amphiphilic ELP and increase the protein yield in the soluble elution fraction. If lower urea concentrations in the lysis buffer are desired, affinity purification must be tested for the individual constructs. 2 M urea worked as well for some constructs, especially for those where the His-tag was fused to the hydrophilic domain and therefore still able to bind the resin. An additional purification step after His-tag purification via size exclusion chromatography can increase the vesicle yield as well.
In case of applying the THF-swelling protocol, the amphiphilic ELP needs to be labeled with a fluorescent organic dye for visualization. Importantly for the BDP labeling of the amphiphilic ELP (see supplementary information for amino acid sequences containing UAA pAzF) via SPAAC is the absence of any reductant such as TCEP, DTT nor β-mercaptoethanol in all purification buffers. This is necessary to avoid the well reported azide to amine reduction of pAzF prior to the SPAAC reaction24.
The exact reaction stoichiometry of dye to amphiphilic ELP (e.g. pAzF-R40F20) is not crucial since it is not necessary to label every single ELP molecule for simple vesicle visualization via epifluorescence microscopy. Therefore, the correlation of a reference SDS gel band and the corresponding weighted lyophilized sample is only necessary once for each protein construct. However, if close to 100% labeling yield is desired a ratio of 1:1 equivalents dye to ELP molecules is sufficient. Very similar amphiphilic ELPs were analyzed in our lab to be fully labeled at an equimolar addition of BDP (data not yet published).
For vesicle preparation using the THF swelling method, the most critical steps are the swelling of lyophilized amphiphilic ELP and subsequent stratification of this solution on top of the aqueous buffer phase. Therefore, the freshly lyophilized amphiphilic ELP should be as anhydrous as possible, which can be achieved by ventilation of the lyophilizer with dry nitrogen gas and immediate closure of the reaction tube lids. If available, septum sealed dry THF should be used to increase the vesicle yield, but THF p.a. (>99.5%) without septum works as well. The stratification step upon swelling the amphiphilic ELP in dry THF should be executed very carefully. Successful stratification of the two temperature-controlled solutions leads to a clearly visible phase boundary between organic and aqueous phase. The initial stratification step should be conducted slowly even though elevated temperatures lead to thermal induced mixing of these phases. Emergent turbidity of the solution is due to light scattering of formed vesicles, fibers or coacervates. In control samples lacking the protein, no turbidity appears though small sized structures (up to 200 nm) are reported for the THF water-interface25. The THF stratification step is the most critical and failure prone step of the swelling protocol. After the incubation step the supramolecular structures can be dialyzed against buffer or ultrapure water. Preferentially the same aqueous solution should be used which was applied for initial assembly in order to maintain the osmolarity and prevent swelling or shrinking of the assembled vesicles. After dialysis, the vesicles, fibers and coacervates are usually stable for at least one week. Depending on the environmental parameters during assembly often a small proportion of other supramolecular structures besides the main structure are present if the THF swelling method is applied8. The described THF method increases the vesicle assembly yield by one order of magnitude while the BuOH extrusion improves the yield by three orders of magnitude compared to our previously published in vitro method5.
The BuOH extrusion method is applied to obtain exclusively stable vesicular structures with high reproducibility, circumventing fibers and spherical coacervates. This method is less error prone and compatible with fluorescent proteins. Therefore F20R20-mEGFP or F20R20-mCherry can be applied as well as BDP-R40F20 or BDP-E20F20. The only critical step is the rapid mixing of the aqueous protein solution after addition of 10%–20% v/v BuOH. The F20R20-mEGFP or F20R20-mCherry concentration should be around 1–15 µM. By applying BuOH extrusion method vesicles can be assembled in ultrapure water or buffer containing up to 5 M NaCl or 4 M urea and pH ranging from 5 to 8. Extruded PMBCs in 20% v/v BuOH can be stored for at least 6 months at 4°C while preserving their vesicular structure. To narrow the vesicle size distribution, they can be extruded using a mini extruder through a membrane of 0.2-1 µm pore size. This pore extrusion can be done directly after BuOH addition to the amphiphilic ELP or after vesicle assembly. If PMBCs are too concentrated for imaging, assembled vesicles in BuOH can be diluted through rapid mixing using aqueous buffer containing 10%–20% v/v BuOH.
The major limitation of the BuOH extrusion method is that PMBC dialysis against aqueous buffers often results in poor vesicle yield. Further, the presence of residual BuOH within the membrane space cannot be excluded since simple fatty acids were able to incorporate into the PMBC membrane21. Therefore, PMBC membranes might be to some extent be composed of protein and alkanol moieties.
Encapsulation of chemically diverse cargo molecules works best using the BuOH extrusion method. Further, DMSO as solvent for the stock solution of the dye to be captured increases the dye encapsulation efficiency. For delicate cargo to be encapsulated, 5%–10% v/v 1-octanol can be used for PMBC assembly and has been proven to be better compatible, when compared to BuOH, with functional encapsulated enzymes such as DNA-ligase or TEV protease21,26. However, due to the shorter chain length of n-butanol it can be dialyzed against aqueous buffer in contrast to 1-octanol, which is not able to permeate the applied dialysis-membrane. Another method limitation is that the applied temperatures and pH values needed to control the desired suprastructure formation can affect enzyme activity. In future work, affinity purification or size exclusion purification should be established to separate non-encapsulated versus encapsulated molecules without deteriorating vesicle membrane integrity.
In contrast to film rehydration methods16,17 the herein described protocols enable the assembly of vesicles sizes greater than 600 nm. This allows monitoring of real time fusion events through simple epifluorescence microscopy and the observation of membrane phase separation8. Compared to temperature triggered vesicular assembly of amphiphilic ELP9 the protocols described here yield PMBC with a long time stability of up to 6 month. However, the main disadvantage is the need of organic solvent for structure formation. Even though BuOH fully preserves the integrity and function of fluorescent proteins27 (data not shown), the activity of encapsulated enzymes might be restricted by residual organic solvent and must be tested individually. However, catalytic reactions involving DNA- ligase, TEV-protease and lipase have been successfully conducted within the luminal space of the vesicles, assembled by 1-octanol or BuOH extrusion26,21. Additionally, even though THF dialysis after assembly is very unproblematic and vesicle integrity is preserved, the BuOH removal frequently results in loss of vesicle integrity due to unknown reasons.
The described protocols enable researchers to assemble micrometer and sub micrometer sized supramolecular structures with distinct physicochemical properties, good encapsulation properties, and long time stability. These supramolecular structures can be applied for the design of minimal cells26 or artificial cell research21, enzyme encapsulation, or drug formulation. The presented functional PMBCs are further promising candidates for drug delivery, since their building blocks are not immunogenic28, exhibit dynamic fusion behavior, and allow for diverse cargo encapsulation.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors thank the BMBF for financial support and the Center for Biological Systems Analysis (ZBSA) for providing the research facility. We are grateful to P. G. Schultz, TSRI, La Jolla, California, USA for providing the plasmid pEVOL-pAzF. We thank the staff of the Life Imaging Center (LIC) in the Center for Biological Systems Analysis (ZBSA) of the Albert-Ludwigs-University Freiburg for help with their confocal microscopy resources, and the excellent support in image recording.
Materials
| 1 µm and 0.2 µm Steril Filter | VWR | ||
| 1,4-Dithiothreitol | Merck | ||
| 1-butanol. >99.5% p.a. | Roth | ||
| 2log DNA ladder | NEB | ||
| 2-Mercaptoethanol | Roth | ||
| 50 mL Falcon tubes | VWR | ||
| 79249 Alkyne Mega Stokes dye | Sigma Aldrich | ||
| Acetic acid glacial | VWR | ||
| Acetonitrile, anhydrous, 99.8% | Sigma-Aldrich | ||
| Ampicillin sodium-salt, 99% | Roth | ||
| BDP-FL-PEG4-DBCO | Jena Bioscience | ||
| Biofuge | Heraeus | ||
| Bottle Top Filter with PES membrane (45 µm, 22 µm) | Thermo Scientific | ||
| Brillant Blue G250 (Coomassie) | Roth | ||
| BspQI | NEB | ||
| Camera DS Qi1 | Nikon | ||
| Centrifuge 5417r | Eppendorf | ||
| Centrifuge 5810r | Eppendorf | ||
| CF-400-Cu square mesh copper grid | EMS | ||
| Chloramphenicol | Roth | ||
| CompactStar CS 4 | VWR | ||
| Dextran, Texas Red, 3000 MW, neutral | Life Technologies | ||
| Digital sonifier | Branson | ||
| Dimethylsulfoxide (DMSO) | Applichem | ||
| Dnase I | Applichem | ||
| EarI | NEB | ||
| EcoRI-HF | NEB | ||
| Environmental shaker incubator ES-20 | Biosan | ||
| Ethanol absolute | Roth | ||
| Ethidium bromide solution | Roth | ||
| Filter supports | Avanti | ||
| Glass plates | Bio-Rad | ||
| Glycerol Proteomics Grade | Amresco | ||
| Glycin | Applichem | ||
| H4-Azido-Phe-OH | Bachhem | ||
| Heat plate MR HeiTec | Heidolph | ||
| HindIII | NEB | ||
| HisTrap FF crude column | GE Life Sciences | Nickel column | |
| Hydrochloride acid fuming, 37%, p.a. | Merck | ||
| Illuminator ix 20 | INTAS | ||
| Illuminator LAS-4000 | Fujifilm | ||
| Imidazole | Merck | ||
| Immersions oil for microscopy | Merck | ||
| Incubators shakers Unimax 1010 | Heidolph | ||
| Inkubator 1000 | Heidolph | ||
| IPTG, >99% | Roth | ||
| Kanamycinsulfate | Roth | ||
| L(+)-Arabinose | Roth | ||
| Laboratory scales Extend ed2202s/224s-OCE | Sartorius | ||
| LB-Medium | Roth | ||
| Lyophilizer Alpha 2-4 LSC | Christ | ||
| Lysozyme, 20000 U/mg | Roth | ||
| Microscope CM 100 | Philips | ||
| Microscope Eclipse TS 100 | Nikon | ||
| Microscopy cover glasses (15 x 15 mm) | VWR | ||
| Microscopy slides | VWR | ||
| Microwave | Studio | ||
| Mini-Extruder Set | Avanti Polar Lipids | ||
| NaCl, >99.5%, p.a. | Roth | ||
| Natriumhydroxid pellets | Roth | ||
| Ni-NTA Agarose, PerfectPro | 5 Prime | ||
| Nucleopore Track-Etch Membrane | Avanti | ||
| PH meter 766 calimatic | Knick | ||
| Phenylmethylsulfonylflourid (PMSF) | Roth | ||
| Polypropylene Columns (1 mL) | Qiagen | ||
| PowerPac basic | BioRad | ||
| Propanol-2-ol | Emplura | ||
| Protein ladder 10-250 kDa | NEB | ||
| Recirculating cooler F12 | Julabo | ||
| Reinforcement rings | Herma | ||
| SacI HF | NEB | ||
| SDS Pellets | Roth | ||
| Sodiumdihydrogen phosphate dihydrate, NaH2PO4 | VWR | ||
| Sterile syringe filter 0.2 mm Cellulose Acetate | VWR | ||
| T4 DNA Ligase | NEB | ||
| TEMED | Roth | ||
| TexasRed Dextran-Conjugate | MolecularProbes | ||
| Thermomix comfort | Eppendorf | ||
| THF, >99.5% p.a. | Acros | ||
| Triton X 100 | Roth | ||
| Trypton/Pepton from Casein | Roth | ||
| Ultrasonic cleaner | VWR | ||
| Urea p.a. | Roth | ||
| Vacuum pump 2.5 | Vacuubrand | ||
| XbaI | NEB | ||
| XhoI | NEB | ||
| ZelluTrans regenerated cellulose tubular membrane (12.0 S/ 3.5 S/ 1.0 V) | Roth |
Referências
- Elzoghby, A. O., Samy, W. M., Elgindy, N. A. Protein-based nanocarriers as promising drug and gene delivery systems. Journal of Controlled Release. 161 (1), 38-49 (2012).
- Jang, Y., Champion, J. A. Self-Assembled Materials Made from Functional Recombinant Proteins. Accounts of Chemical Research. 49 (10), 2188-2198 (2016).
- Timmermans, S. B. P. E., van Hest, J. C. M. Self-assembled nanoreactors based on peptides and proteins. Current Opinion in Colloid & Interface Science. 35, 26-35 (2018).
- Dreher, M. R., et al. Temperature Triggered Self-Assembly of Polypeptides into Multivalent Spherical Micelles. Journal of the American Chemical Society. 130 (2), 687-694 (2008).
- Huber, M. C., et al. Designer amphiphilic proteins as building blocks for the intracellular formation of organelle-like compartments. Nature Materials. 14 (1), 125-132 (2014).
- Matsuurua, K. Rational design of self-assembled proteins and peptides for nano- and micro-sized architectures. RSC Advances. 4 (6), 2942-2953 (2013).
- Rocklin, G. J., et al. Global analysis of protein folding using massively parallel design, synthesis, and testing. Science. 357 (6347), 168-175 (2017).
- Schreiber, A., Stühn, L. G., Huber, M. C., Geissinger, S. E., Rao, A., Schiller, S. M. Self-Assembly Toolbox of Tailored Supramolecular Architectures Based on an Amphiphilic Protein Library. Small. 15 (30), 1900163 (2019).
- Jang, Y., Hsieh, M. -. C., Dautel, D., Guo, S., Grover, M. A., Champion, J. A. Understanding the Coacervate-to-Vesicle Transition of Globular Fusion Proteins to Engineer Protein Vesicle Size and Membrane Heterogeneity. Biomacromolecules. 20 (9), 3494-3503 (2019).
- Vargo, K. B., Sood, N., Moeller, T. D., Heiney, P. A., Hammer, D. A. Spherical micelles assembled from variants of recombinant oleosin. Langmuir: the ACS journal of surfaces and colloids. 30 (38), 11292-11300 (2014).
- Bellomo, E. G., Wyrsta, M. D., Pakstis, L., Pochan, D. J., Deming, T. J. Stimuli-responsive polypeptide vesicles by conformation-specific assembly. Nature Materials. 3 (4), 244-248 (2004).
- Martín, L., Castro, E., Ribeiro, A., Alonso, M., Rodríguez-Cabello, J. C. Temperature-Triggered Self-Assembly of Elastin-Like Block Co-Recombinamers:The Controlled Formation of Micelles and Vesicles in an Aqueous Medium. Biomacromolecules. 13 (2), 293-298 (2012).
- Li, Y., Rodriguez-Cabello, J. C., Aparicio, C. Intrafibrillar Mineralization of Self-Assembled Elastin-Like Recombinamer Fibrils. ACS Applied Materials & Interfaces. , (2017).
- Vargo, K. B., Parthasarathy, R., Hammer, D. A. Self-assembly of tunable protein suprastructures from recombinant oleosin. Proceedings of the National Academy of Sciences of the United States of America. 109 (29), 11657-11662 (2012).
- Park, W. M., Champion, J. A. Thermally Triggered Self-Assembly of Folded Proteins into Vesicles. Journal of the American Chemical Society. 136 (52), 17906-17909 (2014).
- Vogele, K., et al. Towards synthetic cells using peptide-based reaction compartments. Nature Communications. 9 (1), 3862 (2018).
- Vogele, K., et al. In Vesiculo Synthesis of Peptide Membrane Precursors for Autonomous Vesicle Growth. Journal of Visualized Experiments. (148), e59831 (2019).
- Huber, M. C., et al. Designer amphiphilic proteins as building blocks for the intracellular formation of organelle-like compartments. Nature Materials. 14 (1), 125-132 (2015).
- Urry, D. W., et al. Elastin: a representative ideal protein elastomer. Philosophical Transactions of the Royal Society B: Biological Sciences. 357 (1418), 169-184 (2002).
- Huber, M. C., Schreiber, A., Wild, W., Benz, K., Schiller, S. M. Introducing a combinatorial DNA-toolbox platform constituting defined protein-based biohybrid-materials. Biomaterials. 35 (31), 8767-8779 (2014).
- Schreiber, A., Huber, M. C., Schiller, S. M. Prebiotic Protocell Model Based on Dynamic Protein Membranes Accommodating Anabolic Reactions. Langmuir. 35 (29), 9593-9610 (2019).
- Chin, J. W., Santoro, S. W., Martin, A. B., King, D. S., Wang, L., Schultz, P. G. Addition of p-Azido-l-phenylalanine to the Genetic Code of Escherichia coli. Journal of the American Chemical Society. 124 (31), 9026-9027 (2002).
- Sonnino, S., Prinetti, A. Membrane domains and the “lipid raft” concept. Current Medicinal Chemistry. 20 (1), 4-21 (2013).
- Bräse, S., Gil, C., Knepper, K., Zimmermann, V. Organische Azide – explodierende Vielfalt bei einer einzigartigen Substanzklasse. Angewandte Chemie. 117 (33), 5320-5374 (2005).
- Li, Z., et al. Large-Scale Structures in Tetrahydrofuran–Water Mixture with a Trace Amount of Antioxidant Butylhydroxytoluene (BHT). The Journal of Physical Chemistry B. 115 (24), 7887-7895 (2011).
- Huber, M. C., Schreiber, A., Schiller, S. M. Minimalist Protocell Design: A Molecular System Based Solely on Proteins that Form Dynamic Vesicular Membranes Embedding Enzymatic Functions. ChemBioChem. 20 (20), 2618-2632 (2019).
- Raghunathan, G., et al. A comparative study on the stability and structure of two different green fluorescent proteins in organic co-solvent systems. Biotechnology and Bioprocess Engineering. 18 (2), 342-349 (2013).
- Sallach, R. E., et al. Long-term biostability of self-assembling protein polymers in the absence of covalent crosslinking. Biomaterials. 31 (4), 779-791 (2010).

