熱拡散率とレーザーフラッシュ法
12,848 Views
•
•
개요
Source: Elise S.D. Buki, Danielle N. Beatty, and Taylor D. Sparks, Department of Materials Science and Engineering, The University of Utah, Salt Lake City, UT
The laser flash method (LFA) is a technique used to measure thermal diffusivity, a material specific property. Thermal diffusivity (α) is the ratio of how much heat is conducted relative to how much heat is stored in a material. It is related to thermal conductivity (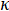 ), how much heat is transferred through a material due to a temperature gradient, by the following relationship:
), how much heat is transferred through a material due to a temperature gradient, by the following relationship:
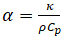 (Equation 1)
(Equation 1)
where ⍴ is the density of the material and Cp is the specific heat capacity of the material at the given temperature of interest. Both thermal diffusivity and thermal conductivity are important material properties used to assess how materials transfer heat (thermal energy) and react to changes in temperature. Thermal diffusivity measurements are obtained most commonly by the thermal or laser flash method. In this technique a sample is heated by pulsing it with a laser or xenon flash on one side but not the other, thus inducing a temperature gradient. This temperature gradient results in heat propagating through the sample towards the opposite side, heating the sample as it goes. On the opposite side an infrared detector reads and reports the temperature change with respect to time in the form of a thermogram. An estimate of the thermal diffusivity is obtained after these results are compared and fit to theoretical predictions using a least squares model.
The laser flash method is the only method that is supported by multiple standards (ASTM, BS, JIS R) and is the most widely used method for determining thermal diffusivity.
Principles
In the laser flash method, a sample with flat, parallel top and bottom surfaces is placed in a controlled atmosphere (air, oxygen, argon, nitrogen etc) inside a sealed furnace. Samples are often thin discs with diameter of 6mm to 25.4mm and thicknesses between 1mm and 4mm. A laser with power around 15 J/pulse provides an instantaneous energy pulse to the bottom face of the sample. An infrared detector lies above the top face of the sample; this detector registers the change in temperature with time of the top face of the sample after each laser pulse. Laser pulses and resulting temperature change data are recorded for set temperature measurement points, within the range of -120℃ to 2800℃, depending on the instrument. Between each measurement taken, the temperature of the sample is allowed to equilibrate. LFA can be run on powder, liquid, bulk, composite, layered, porous, and semi-transparent samples (some modifications may be necessary depending on sample type).
The resulting data is presented in the form of a thermogram and is compared to analytical, 1-dimensional heat transport models, which assume sample opacity, homogeneity, and minimal radial heat loss. These models also assume thermal properties and sample density remain constant within the temperature ranges measured. Experimental deviations from model assumptions often require correction calculations.
There are several mathematical models used for obtaining thermal diffusivity from results of the laser flash method. The original model (Park’s ideal model) involves solving a differential equation with boundary conditions that assume constant temperatures and that no heat escapes from the system during measurement. Both of these are false assumptions for real measurements. The Netzsch LFA 457 is often run using the Cowan model. This model corrects the ideal model; it takes energy and heat loss into consideration and gives more accurate fitting for many different material scans. This model is used here for an iron standard material.
Procedure
- Turn on the machine and wait for the warm-up process to end (approximately 2 hours).
- Fill up the detector compartment with liquid nitrogen using a small funnel until the nitrogen vapor can be seen coming from the detector. Let the liquid settle until there is no more vapor coming out and close the detector.
- Measure the thickness of your sample with a micrometer over several spots and calculate the average thickness and the standard deviation. The edges of the sample should be between 6mm and 25.4mm, with a flat geometry either round or rectangular. Additionally, the thickness of the sample should be uniform and between 1mm and 4mm. High thermal diffusivity samples work best with thicker samples. Here, we are using a standard iron disc sample.
- In order to maximize the absorbance of the sample and ensure uniform emissivity, spray a thin coating of graphite on the sample using colloidal graphite. Repeat three times allowing sample to dry between passes. Once done with the first side, carefully flip the sample and spray the other side.
- Once dry, place the sample in the bottom half of the small sample support and cover it with the top half of the sample support.
- Open the furnace by simultaneously pressing the safety button on the right side of the machine and the button on the front side of the machine labeled furnace with a down arrow. Rotate the detector around clockwise looking down in order to have more mobility around the furnace.
- The sample stage in the furnace has three locations designed to hold the samples. Put the sample support containing the sample in one of the three locations (take note of which one) then realign the detector and the furnace before closing the furnace. To do so, press the safety button and the labeled furnace with an up arrow.
- Before turning on the vacuum pump, make sure that the vent valve located to the right behind the detector is closed. Once closed, turn on the vacuum pump. Slowly open the vacuum valve and pump a vacuum until the pressure indicator light on the front side of the machine is stabilized to its lowest level. A vacuum is pulled to remove all air from the chamber before purging with inert gas.
- Open the regulator on the Argon cylinder and make sure the pressure is set between 5 psi and 10 psi. Close the vacuum valve, open the backfill valve then press the purge button to purge the sample space so there is no trapped gas from the sample.
- Repeat steps 8 and 9 three times to make sure that there is no air left in the chamber. This is to eliminate the chance of oxygen, nitrogen or other air constituents reacting with compounds present at the surface of the sample, particularly at elevated temperatures.
- The furnace should be left with a very slight positive pressure from the purge gas in order to ensure that air does not flow back into the furnace.
- Launch the machine's software from the desktop icon labeled "LFA 457". Select Service → Hardware Info → Switches then click the box to turn on the purge. This should turn on the Purge light on the front of the LFA-457.
- Open the vent valve while the purge light is on.
- Open a database or create a new one and enter all the necessary information, including all the necessary fields in the tabs General, Autosampler Position, Initial Conditions, Temperature Steps & Final Conditions.
- If the experiment takes longer than 8 hours, the detector will need to be filled up again. This might happen, especially if multiple samples are being run.
- Samples are then removed in a similar fashion to how they were inserted. The software automatically displays the results, here shown from an iron standard material.
Thermal diffusivity is an important property used to assess how a material transfers heat and reacts to changes in temperature. Thermal diffusivity, alpha, is the ratio of how much heat is conducted in a material relative to how much heat is stored. Similarly thermal conductivity, kappa, describes how much heat is transferred through a material due to a temperature gradient. Thermal diffusivity and thermal conductivity are related by the following equation where Roe is density and Cp is the specific heat capacity of the material. A material with a high thermal diffusivity, like a metal, is able to conduct thermal energy rapidly while a material with low thermal diffusivity, like plastic, is much slower. A material's thermal diffusivity is often measured using laser flash analysis or LFA. In this technique a sample is heated on one side by pulsing it with a laser inducing a temperature gradient which is then measured with respect to time. This video will introduce basics of how the laser flash method is used to measure thermal diffusivity. And then we'll demonstrate the technique in the laboratory using a standard sample.
First the laser flash method requires a sample with flat and parallel top and bottom surfaces and usually takes the shape of a thin disk. While a solid disk sample is the most straightforward sample the technique can be used on a powder, liquid, or even layered or porous samples. Once the sample is prepared it is suspended inside of a sealed furnace with a controlled atmosphere. A laser with power around 15 joules per pulse provides an instantaneous energy pulse to the bottom face of the sample. An infrared detector above the top face of the sample registers the change in temperature with time after each laser pulse. Between each pulse the sample is allowed to equilibrate. Laser pulses and the resulting temperature change data are recorded for set temperature measurement points.
The resulting data, called a thermogram, is a plot of the temperature change or measured signal with respect to time. An estimate of the thermal diffusivity is obtained after fitting to theoretical predictions using heat transport models which are usually incorporated into the system software. The most common model used is the Parks Ideal Model. This model involves solving a differential equation with boundary conditions that assume constant temperatures and that no heat escapes from the system during measurement. Both of these assumptions are false for nonideal measurements so this model is corrected using the Cowan Model which takes heat loss into consideration. Now that we've introduced the laser flash method let's take a look at how to run the measurement using a standard iron sample.
To begin turn on the laser flash instrument and allow it to warm up for about two hours. After the instrument has warmed up fill the detector compartment with liquid nitrogen using a small funnel. Let the liquid settle until there is no more vapor coming out. Then close the compartment. Now obtain your sample. Here we are using an iron standard disk. Measure the dimensions of the sample with calipers. It should be between six and 25.4 millimeters wide. The thickness should be uniform and between one and four millimeters. Calculate the average thickness of the sample as well as the standard deviation. To ensure uniform heating of the sample spray a thin coating of colloidal graphite on the surface. Repeat three times allowing the sample to dry between sprays then flip the sample over and spray the other side the same way.
Once dry place the sample in the bottom half of the small sample support, then cover it with the top half of the support. Open the furnace by simultaneously pressing the safety button on the right side of the machine and the button on the front side labeled furnace. Rotate the detector clockwise in order to have more mobility around the furnace. The sample stage within the furnace has three locations designed to hold the samples. Put the sample support containing the sample in one of the three locations taking note of which one it is. Then realign the detector and close the furnace by pressing the safety button simultaneously with the furnace button. Now evacuate the chamber before purging it with inert gas. First make sure that the vent valve is closed. Then turn on the vacuum pump and slowly open the vacuum valve to evacuate the chamber until the pressure indicator is stabilized. Next open the regulator on the Argon cylinder and set the pressure between five and 10 PSI. Then close the vacuum valve and open the backfill valve to fill the compartment with argon.
Close the backfill valve then slowly open the vacuum valve to evacuate the chamber again and allow the pressure to stabilize. Then close the vacuum valve and open the backfill valve again to refill with argon. Then close the backfill valve once again after the pressure stabilizes. Do this several more times to make sure that there is no air left in the chamber. This is to eliminate the chance of oxygen or nitrogen reacting with the compounds present on the surface of the sample at high temperature. Then turn on the purge and open the vent valve before turning on the controller. Now the furnace should be left with a very slight positive pressure from the purge gas in order to ensure that air does not flow into the furnace. Then launch the machine's software. The sample will be heated from 25 to 600 degree Celsius then will cool back to 25 degrees. Three pulses will be made at each temperature with measurements made every 50 degrees. Now adjust the purge flow rate on the flow gauge until the flow stabilizes, then launch the experiment. Periodically check the liquid nitrogen level in the detector and refill it as needed. Once the test is finished remove the sample from the furnace and sample holder.
Now let's take a look at the data. First we see two plots of measured signal versus time for a laser pulse on our iron standard sample. The one on the left is the response to a laser pulse at 48.2 degrees and the one on the right is the response to a laser pulse at 600 degrees. The blue trace shows the collected temperature data from the sample and the thin red line shows the calculated data from the Cowan Model. Both sets of data fit well to the model because it is a well-defined standard material. Generally experimentally calculated values match the Cowan Model best at high temperatures as shown by the greater deviation from the model trace for the laser pulses at low temperature versus high temperature. If we take a look at the calculated thermal diffusivity as compared to the temperature where each dot represents one laser pulse we can see that there is more noise at lower temperature but a better fit at higher temperature as expected.
It is essential to understand the thermal properties of a material when selecting an appropriate material for any application involving heat flow or temperature fluctuations. When looking at spacecraft for example, thermal protection tiles play an important role in successful atmospheric reentry. When entering the atmosphere a spacecraft is exposed to high temperatures and would melt, oxidize, or burn without a protective layer. Thermal tiles are typically made of pure silica glass fibers with tiny air-filled pores. These two components have low thermal conductivity and therefore minimize heat flux across the tiles. As electronic components are miniaturized the issue of heat dissipation in integrated circuits has become a key problem. Heating is generally caused by joule heating where the passage of electric current through a material produces heat like in the coils of this electric heater. These circuit components can generate hot spots thus materials must be selected that are able to dissipate heat and is why copper and silver have been traditionally selected. You have just watched JoVE's,
Introduction to Study in Thermal Diffusivity Via the Laser Flash Method. You should now understand why analyzing thermal diffusivity is essential to a wide range of engineering applications and how to measure the thermal diffusivity of a sample using the laser flash method. Thanks for watching.
Results
Figures 1, 2, and 3 show the data from an LFA run of an iron standard sample. Figures 1 and 2 show laser pulse vs time plots for two temperatures (48.2°C and 600°C); the blue trace shows the collected laser pulse from the iron sample and the thin red line shows the calculated pulse from the Cowan model. Both temperature pulses fit well to the model because this is a well-defined standard material. Generally, experimentally calculated values match the Cowan model best at high temperatures, as shown by the greater deviation from the model trace for the laser pulses at low temperatures (Figure 1) vs high temperatures (Figure 2). Low temperatures fit relatively well to the model for this standard material but deviate more than high temperature results because the lower set temperatures may not be reached in the time allowed for equilibration between each pulse. Each data point (red circle) in Figure 2 represents one laser pulse; the closer the data points fit the Cowan model, the better and more accurate the resulting thermal diffusivity values.

Figure 1: Laser signal vs time plot at 48.2 °C for an iron standard run in the LFA 457. The blue trace represents the signal from the laser hitting the sample. The thin red line represents the calculated pulse for the Cowan model.

Figure 2: Laser signal vs time plot at 600.6 °C for an iron standard run in the LFA 457. The blue trace represents the signal from the laser hitting the sample. The thin red line represents the calculated pulse for the Cowan model.

Figure 3: Thermal diffusivity (α) vs temperature plot for an iron standard disk, run in the LFA 457. Each red circle represents one laser pulse.
Applications and Summary
The laser flash method is a widely used technique for determination of thermal diffusivity which consists of radiating one side of a sample with thermal energy (from a laser source) and placing an IR detector on the other side to pick up the pulse. The wide range in temperature of different models enables measurement on various types of samples. The LFA requires relatively small samples. Other tools that measure thermal conductivity directly, rather than thermal diffusivity, include the Guarded Hot Plate, Heat Flow Meter and others. The Guarded Hot Plate system can hold relatively large square samples (300mm x 300mm) and requires careful calibration in order to calculate thermal flux necessary for thermal conductivity calculation. Neither of these tools can measure thermal diffusivity to high temperatures and typically operate below 250oC.
Thermal diffusivity is an important property that needs to be known when choosing the appropriate material for any applications involving heat flow or that are sensitive to heat fluctuations. For example, thermal conductivity, aong with diffusivity, also play an important role in insulation. When selecting a material to use for insulation, it is important to be able to measure and compare the thermal properties of different materials. These thermal properties are even more critical in aerospace. Thermal protection tiles play an important role in a spacecraft's successful atmospheric re-entry. When entering the atmosphere, a spacecraft is exposed to extremely high temperatures and would melt, oxidize, or burn without a protective layer. Thermal protection tiles are typically made of pure silica glass fibers with tiny air-filled pores. These two components have low thermal conductivity and therefore minimize heat flux across the tiles. The thermal conductivity of materials with a high porosity ( ) can be calculated with the following Maxwell's relation :
) can be calculated with the following Maxwell's relation :
 (Equation 2)
(Equation 2)
내레이션 대본
Thermal diffusivity is an important property used to assess how a material transfers heat and reacts to changes in temperature. Thermal diffusivity, alpha, is the ratio of how much heat is conducted in a material relative to how much heat is stored. Similarly thermal conductivity, kappa, describes how much heat is transferred through a material due to a temperature gradient. Thermal diffusivity and thermal conductivity are related by the following equation where Roe is density and Cp is the specific heat capacity of the material. A material with a high thermal diffusivity, like a metal, is able to conduct thermal energy rapidly while a material with low thermal diffusivity, like plastic, is much slower. A material’s thermal diffusivity is often measured using laser flash analysis or LFA. In this technique a sample is heated on one side by pulsing it with a laser inducing a temperature gradient which is then measured with respect to time. This video will introduce basics of how the laser flash method is used to measure thermal diffusivity. And then we’ll demonstrate the technique in the laboratory using a standard sample.
First the laser flash method requires a sample with flat and parallel top and bottom surfaces and usually takes the shape of a thin disk. While a solid disk sample is the most straightforward sample the technique can be used on a powder, liquid, or even layered or porous samples. Once the sample is prepared it is suspended inside of a sealed furnace with a controlled atmosphere. A laser with power around 15 joules per pulse provides an instantaneous energy pulse to the bottom face of the sample. An infrared detector above the top face of the sample registers the change in temperature with time after each laser pulse. Between each pulse the sample is allowed to equilibrate. Laser pulses and the resulting temperature change data are recorded for set temperature measurement points.
The resulting data, called a thermogram, is a plot of the temperature change or measured signal with respect to time. An estimate of the thermal diffusivity is obtained after fitting to theoretical predictions using heat transport models which are usually incorporated into the system software. The most common model used is the Parks Ideal Model. This model involves solving a differential equation with boundary conditions that assume constant temperatures and that no heat escapes from the system during measurement. Both of these assumptions are false for nonideal measurements so this model is corrected using the Cowan Model which takes heat loss into consideration. Now that we’ve introduced the laser flash method let’s take a look at how to run the measurement using a standard iron sample.
To begin turn on the laser flash instrument and allow it to warm up for about two hours. After the instrument has warmed up fill the detector compartment with liquid nitrogen using a small funnel. Let the liquid settle until there is no more vapor coming out. Then close the compartment. Now obtain your sample. Here we are using an iron standard disk. Measure the dimensions of the sample with calipers. It should be between six and 25.4 millimeters wide. The thickness should be uniform and between one and four millimeters. Calculate the average thickness of the sample as well as the standard deviation. To ensure uniform heating of the sample spray a thin coating of colloidal graphite on the surface. Repeat three times allowing the sample to dry between sprays then flip the sample over and spray the other side the same way.
Once dry place the sample in the bottom half of the small sample support, then cover it with the top half of the support. Open the furnace by simultaneously pressing the safety button on the right side of the machine and the button on the front side labeled furnace. Rotate the detector clockwise in order to have more mobility around the furnace. The sample stage within the furnace has three locations designed to hold the samples. Put the sample support containing the sample in one of the three locations taking note of which one it is. Then realign the detector and close the furnace by pressing the safety button simultaneously with the furnace button. Now evacuate the chamber before purging it with inert gas. First make sure that the vent valve is closed. Then turn on the vacuum pump and slowly open the vacuum valve to evacuate the chamber until the pressure indicator is stabilized. Next open the regulator on the Argon cylinder and set the pressure between five and 10 PSI. Then close the vacuum valve and open the backfill valve to fill the compartment with argon.
Close the backfill valve then slowly open the vacuum valve to evacuate the chamber again and allow the pressure to stabilize. Then close the vacuum valve and open the backfill valve again to refill with argon. Then close the backfill valve once again after the pressure stabilizes. Do this several more times to make sure that there is no air left in the chamber. This is to eliminate the chance of oxygen or nitrogen reacting with the compounds present on the surface of the sample at high temperature. Then turn on the purge and open the vent valve before turning on the controller. Now the furnace should be left with a very slight positive pressure from the purge gas in order to ensure that air does not flow into the furnace. Then launch the machine’s software. The sample will be heated from 25 to 600 degree Celsius then will cool back to 25 degrees. Three pulses will be made at each temperature with measurements made every 50 degrees. Now adjust the purge flow rate on the flow gauge until the flow stabilizes, then launch the experiment. Periodically check the liquid nitrogen level in the detector and refill it as needed. Once the test is finished remove the sample from the furnace and sample holder.
Now let’s take a look at the data. First we see two plots of measured signal versus time for a laser pulse on our iron standard sample. The one on the left is the response to a laser pulse at 48.2 degrees and the one on the right is the response to a laser pulse at 600 degrees. The blue trace shows the collected temperature data from the sample and the thin red line shows the calculated data from the Cowan Model. Both sets of data fit well to the model because it is a well-defined standard material. Generally experimentally calculated values match the Cowan Model best at high temperatures as shown by the greater deviation from the model trace for the laser pulses at low temperature versus high temperature. If we take a look at the calculated thermal diffusivity as compared to the temperature where each dot represents one laser pulse we can see that there is more noise at lower temperature but a better fit at higher temperature as expected.
It is essential to understand the thermal properties of a material when selecting an appropriate material for any application involving heat flow or temperature fluctuations. When looking at spacecraft for example, thermal protection tiles play an important role in successful atmospheric reentry. When entering the atmosphere a spacecraft is exposed to high temperatures and would melt, oxidize, or burn without a protective layer. Thermal tiles are typically made of pure silica glass fibers with tiny air-filled pores. These two components have low thermal conductivity and therefore minimize heat flux across the tiles. As electronic components are miniaturized the issue of heat dissipation in integrated circuits has become a key problem. Heating is generally caused by joule heating where the passage of electric current through a material produces heat like in the coils of this electric heater. These circuit components can generate hot spots thus materials must be selected that are able to dissipate heat and is why copper and silver have been traditionally selected. You have just watched JoVE’s,
Introduction to Study in Thermal Diffusivity Via the Laser Flash Method. You should now understand why analyzing thermal diffusivity is essential to a wide range of engineering applications and how to measure the thermal diffusivity of a sample using the laser flash method. Thanks for watching.
