A Modified Inflammatory Pain Model to Study the Analgesic Effect in Mice
概要
Here, we present a modified inflammatory pain model with the both-hind-paw carrageenan injection to evaluate the analgesic effect.
Abstract
The hot plate test is widely used to evaluate analgesic effects on inflammatory pain in mice. A commonly used model of inflammatory pain was induced with an intraplantar injection of carrageenan in one hind paw. However, the findings from our laboratory showed that mice with a single-hind-paw injection of carrageenan lifted their paws to avoid thermal nociception during the hot plate test. Because of this response, previous injection method cannot accurately reflect the thermal pain threshold. Thus, we investigated a new method to avoid this issue. In the present study, we modified the previous method by injecting carrageenan into both hind paws to establish the model of inflammatory pain. The results demonstrated that both-hind-paw injection with carrageenan was sensitive and a better method to induce inflammatory pain when using the hot plate test than single-hind-paw injection. On the basis of these findings, we designed further experiments in which mice with either both-hind-paw or single-hind-paw injection of carrageenan were treated intragastrically with celecoxib (30 mg/kg). The results of the hot plate test showed that celecoxib augmented the thermal pain threshold in mice with both-hind-paw injection of carrageenan but not in mice with single-hind-paw injection of carrageenan. In summary, we developed a superior method to induce a model of inflammatory pain to evaluate analgesic effect.
Introduction
Pain is a public health problem worldwide. It is triggered by noxious thermal, mechanical, or chemical stimulation. It is difficult to perform pain studies in humans because of ethical concerns. Therefore, using animal pain models is a key approach to understanding pain mechanisms and research treatments1. The hot plate test has been widely used for the assessment of thermal nociception and hyperalgesia2. Currently, the model of inflammatory pain used for the hot plate test is induced with an intraplantar injection of carrageenan in one hind paw. For example, to investigate the antinociceptive effect of rotigotine-loaded microspheres, an animal model of inflammatory pain was prepared by a single-hind-paw injection3. To assess the effects of catechol-o-methyl-transferase inhibitors on thermal nociception in mice, carrageenan was administered into the plantar region of the right hind paw4. To investigate the analgesic role of the extract of Posidonia oceanica (L.) Delile, an inflammatory pain model was prepared in mice by intraplantar injection of carrageenan in the right hind paw5. However, the findings from our laboratory showed that a mouse with the single-hind-paw injection of carrageenan would lift its paw to avoid thermal nociception, which inaccurately extends the animal’s response to a thermal stimulus in the hot plate test. Thus, we hypothesized that the inflammatory pain model with the single-hind-paw injection of carrageenan could not accurately reflect the thermal pain threshold. Therefore, we optimized the method by injecting carrageenan into both hind paws to establish a modified inflammatory pain model.
The mice subjected to both-hind-paw injection of carrageenan had no way to avoid the thermal stimulus for both hind paws at the same time. The pain threshold was defined as the time from placing the mouse on the hot plate to the mouse licking either hind paw. The results confirmed that the modified inflammatory pain model is more suitable for evaluating the nociceptive temperature threshold. This may improve the efficacy of the hot plate test for the research and development of analgesic drugs.
Protocol
The experimental protocol was approved by the Institutional Animal Care and Use Committee at Yantai University (No. YTDX20220425). The procedures followed the National Institutes of Health Guidelines for the Use of Laboratory Animals.
1. Animals
- Use ICR female mice weighing 20-25 g for the experiments.
- Allow the animals to acclimate to the environment of the experimental room for 3 days before starting the experiments.
- Maintain the experimental room with a 12-h light-dark cycle, and provide the mice with free access to water and chow.
2. Preparation of carrageenan solution
- Weigh 0.1 g of carrageenan. Add 10 mL of sterilized normal saline to a tube.
- Add 0.1 g of carrageenan to the tube and vortex the tube for 30 s.
- Collect the 1% sterilized carrageenan solution.
- Prepare the carrageenan solution before the experiments.
3. Intraplantar injection of carrageenan
- Use a syringe (0.05 mL, 29G x ½ inch, 0.33 mm × 12.7 mm) to inject the 1% carrageenan solution.
- Shake the carrageenan solution before drawing it into the syringe. Make sure that there is no bubble in the syringe.
- Disinfect the injection site (hind paw) with 75% alcohol.
- Inject 30 µL6,7 of 1% carrageenan solution subcutaneously into the plantar surface of the hind paw. For the control group mice, inject 30 µL of normal saline.
- Insert the needle diagonally upwards into the hind paw foot pad. Maintain the stability of the syringe.
- After injection, withdraw the syringe slowly and press on the injection site for 2 s to prevent leakage.
4. Hot plate test
- Conduct the hot plate test 3 h after the intraplantar injection.
- Clean and disinfect the surface of the hot plate.
- Start the hot plate apparatus. Click the Temperature Mode button. Click CONSTANT to set the temperature to 55 ± 0.2 °C.
- Click Start experiment button and click REACH. Take a mouse from the accommodation container. Gently grasp its trunk. Place the mouse in the center of the hot plate. Click START.
- Observe and record the time to lick the left hind paw (for mice with single-hind-paw injection) or hind paw (for mice with both-hind-paw injection) in response to the heat stimulation. Press the pedal of the hot plate to see the time on the hot plate screen. Define pain threshold as the time from placing the mouse on the hot plate to licking the hind paw.
- Set a 60 s limit for the hot plate test4,8,9. Record a pain threshold of 60 s if the limit is reached.
NOTE: Clean the hot plate apparatus with 75% alcohol before placing the next mouse to avoid possible bias due to residual odor from the previous mouse.
5. Preparation of 1.5 mg/mL celecoxib suspension
- Take 1 capsule containing 200 mg celecoxib. Get the content of the capsule and grind it into a powder.
- Add 0.5% sodium carboxymethyl cellulose. The final volume is 13.3 mL. This is the store suspension of celecoxib.
- Take 1 mL of the store suspension celecoxib. Add 9.0 mL of 0.5% sodium carboxymethyl cellulose to obtain 1.5 mg/mL of celecoxib suspension.
- Administer 0.2 mL/10 g of the celecoxib suspension intragastrically to the mice.
6. Evaluating the modified inflammatory pain model
- Randomly divide ICR female mice into three groups: control, model, and celecoxib groups.
- Inject 30 µL of 1% carrageenan solution subcutaneously into the plantar surface of the hind paw (single-hind-paw or both-hind-paw). For the control group mice, inject 30 µL of normal saline.
- After the injection of carrageenan or normal saline, perform the treatment immediately. Intragastrically administer celecoxib at a dose of 30 mg/kg for the mice in the celecoxib group. For the mice in the control and model groups, intragastrically administer 0.5% sodium carboxymethyl cellulose.
- Conduct the hot plate test (section 2) at 3 h after the injection of carrageenan.
7. Statistical methods
- Present the experimental results as mean ± standard deviation. Conduct a normality distribution test with the Kolmogorov-Smirnov statistical goodness of fit test.
- Analyze the comparison between the two groups using an unpaired t-test. Perform multiple group comparisons using a one-way analysis of variance followed by Dunnett's test. Consider the value of p < 0.05 statistically significant.
Representative Results
The pain threshold in inflammatory pain models
The pain threshold of the inflammatory pain model with single-hind-paw injection of carrageenan was 32.23 ± 11.11 s, whereas the pain threshold of the control group was 27.73 ± 7.08 s. Compared with the control group, the pain threshold of the single-hind-paw injection model did not show a significant reduction (p > 0.05, Figure 1A). The pain threshold of the inflammatory pain model with both-hind-paw injection of carrageenan was 21.84 ± 5.63 s, whereas the pain threshold of the control group was 28.21 ± 8.61 s. Compared with that of the control group, the pain threshold of the both-hind-paw injection model decreased significantly (p < 0.05, Figure 1B).
The analgesic effect of celecoxib in different inflammatory pain models
In the inflammatory pain model with a single-hind-paw injection of carrageenan, the pain threshold of the celecoxib group (27.01 ± 10.98 s) did not show a significant increase when compared with that of the model group (29.77 ± 13.97 s, p > 0.05). Consistent with the results above, the pain threshold of the model group did not show a significant reduction when compared with that of the control group (30.94 ± 14.54 s), which indicated that single-hind-paw injection of carrageenan did not induce an inflammatory pain model (Figure 2A). In the inflammatory pain model with both-hind-paw injection of carrageenan, the pain threshold of the model group (15.61 ± 4.85 s) decreased significantly when compared with that of the control group (23.55 ± 3.96 s, p < 0.05). Compared with the model group, the pain threshold of the celecoxib group (20.29 ± 3.10 s) was significantly decreased (Figure 2B).
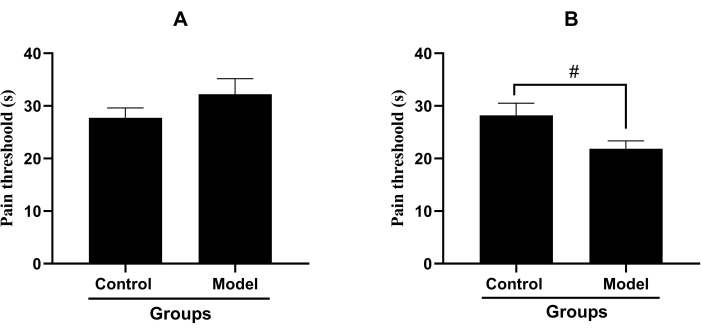
Figure 1: The pain thresholds in inflammatory pain models. (Mean ± standard deviation, n = 15). (A) The pain threshold of the inflammatory pain model with the single-hind-paw injection of carrageenan. (B) The pain threshold of the inflammatory pain model with both-hind-paw injection of carrageenan, (#P<0.05). Please click here to view a larger version of this figure.
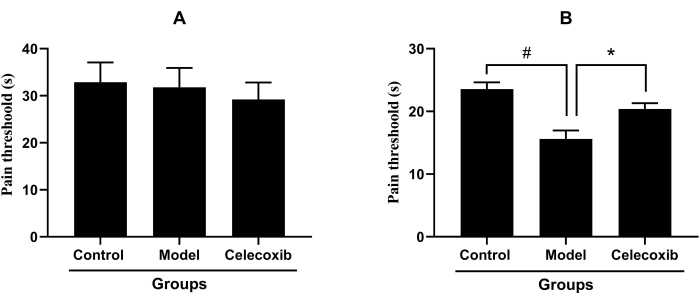
Figure 2: The analgesic effect of celecoxib in different inflammatory pain models (Mean ± standard deviation, n = 14). (A) The analgesic effect of celecoxib in the inflammatory pain model with the single-hind-paw injection of carrageenan. (B) The analgesic effect of celecoxib in the inflammatory pain model with both-hind-paw injection of carrageenan, ( #P<0.05, *P<0.05). Please click here to view a larger version of this figure.
Discussion
Pain is a protective response triggered by tissue damage or injury. However, it also has a negative impact on quality of life10. Therefore, the development of effective analgesics and exploration of pain mechanisms remain active areas of research. The methods for evaluating pain play a key role in the development of analgesic drugs. Single-hind-paw injection of carrageenan is a classic method for the hot plate test. The modified model induced by both-hind-paw injection of carrageenan we present here provides a reliable way to investigate the efficacy of potential therapeutic agents to relieve pain.
In the present study, we found that mice with the single-hind-paw injection of carrageenan did not show a decrease in pain threshold. However, in other studies, this method led to significant reductions in pain threshold in the hot plate test11,12. The discrepancy in results between the previous studies and this study may be explained by the different animal species used in the experiments13,14,15,16. Another possible explanation is that we observed the mice with the single-hind-paw injection of carrageenan often lift the injected paw or step lightly on the plate to avoid the thermal stimulus. Evaluation of the pain threshold requires observing the typical nociceptive response of licking or shaking the injected paw. We thus obtained a negative result, indicating that the pain threshold of the model with the single-hind-paw injection of carrageenan did not significantly decrease. Mice with both-hind-paw injection of carrageenan were not able to avoid the thermal stimulus by lifting the paw or stepping lightly on the plate. The pain threshold was considered as licking or shaking of either hind paw, reflecting mice's actual nociceptive response.
To verify the superiority of the modified inflammatory pain model, mice with single-hind-paw or both-hind-paw injections of carrageenan were treated with intragastric administration of celecoxib. Consistent with the hypothesis of this study, celecoxib exerted an analgesic effect in the modified inflammatory pain model, but it did not show any effects in the model with single-hind-paw injection of carrageenan. This finding demonstrated that the modified inflammatory pain model is reliable for screening potential analgesics.
There are limitations in this study. Many different mouse strains have been used in hot plate tests. Different mouse strains may exhibit different responses to thermal stimuli. The present experiment used only ICR mice to test the hypothesis. Therefore, whether the modified method accurately and consistently detects pain thresholds in the other mouse strains in the hot plate test remains to be determined. Additionally, we did not design a pilot experiment to evaluate the pain threshold of the mice and thus did not exclude any animals with a high pain threshold. However, this limitation may also be considered as an advantage of the present modified method. It not only simplifies the process of the analgesic test but also reflects the range of responses of mice in the hot plate test. Furthermore, the measurement of hind paw volume will help confirm the induction of inflammation and verify the analgesic effect of nonsteroidal anti-inflammatory drugs (NSAIDs). However, our experience demonstrated that it is not easy to measure the volume of the paw of mice. The paw of the mouse is too small to get an exact value. Therefore, the present study was not designed to investigate the change in the volume of the paw after inflammation.
In conclusion, following the steps outlined above, the inflammatory pain model with both-hind-paw injection of carrageenan allows the determination of the thermo-nociceptive thresholds of mice in a reliable manner. Furthermore, upon administration of analgesics, an increase in the pain threshold was statistically detected. The modified inflammatory pain model is useful for screening potential analgesics. In addition, this method can be used for pain biology research, new drug development, and clinical treatment, as well as to provide new ideas and solutions for pain management.
開示
The authors have nothing to disclose.
Acknowledgements
We thank Lesley McCollum, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn), for editing the English text of a draft of this manuscript.
Materials
| AL204 electronic balance | Yuheng Battery Co., Ltd | YH-400A | |
| Carboxymethylcellulose | Tianjin Chemical Co., Ltd | 20210326 | |
| Carrageenan | Sigma, MO, USA | 29H0715 | |
| Celecoxib | Pfizer Inc. | 8142838 | |
| Hot plate | UGO Ltd. Co., Italy | PB-200 | |
| ICR female mice | Jinan Pengyue Co., Ltd. | SCXK20220007 | |
| Normal saline | Kelun Pharmaceutical Co., Ltd | H51021157 |
参考文献
- Deuis, J. R., Dvorakova, L. S., Vetter, I. Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci. 10, 284 (2017).
- Menéndez, L., Lastra, A., Hidalgo, A., Baamonde, A. Unilateral hot plate test: a simple and sensitive method for detecting central and peripheral hyperalgesia in mice. J Neurosci Methods. 113 (1), 91-97 (2002).
- Li, T., et al. Rotigotine-loaded microspheres exerts the antinociceptive effect via central dopaminergic system. Eur J Pharmacol. 910, 174443 (2021).
- Kambur, O., et al. Inhibitors of catechol- O -methyltransferase sensitize mice to pain. Br J Pharmacol. 161 (7), 1553-1565 (2010).
- Micheli, L., et al. Efficacy of Posidonia oceanica extract against inflammatory pain: In vivo studies in mice. Mar Drugs. 19 (2), 48 (2021).
- McCarson, K. E. Models of inflammation: Carrageenan- or complete Freund’s adjuvant (CFA)-induced edema and hypersensitivity in the rat. Curr Protoc Pharmacol. 70, 5.4.1-5.4.9 (2015).
- Fehrenbacher, J. C., Vasko, M. R., Duarte, D. B. Models of Inflammation: Carrageenan- or Complete Freund’s Adjuvant (CFA)-Induced Edema and Hypersensitivity in the Rat. Curr Protoc Pharmacol. Chapter 5 (Unit 5.4), (2012).
- Asl, M. K., Nazariborun, A., Hosseini, M. Analgesic effect of the aqueous and ethanolic extracts of clove. Avicenna J Phytomed. 3 (2), 186-912 (2013).
- Wang, Y. Analgesic effects of glycoproteins from Panax ginseng root in mice. J Ethnopharmacol. 148 (3), 946-950 (2013).
- Zia, F. Z., et al. Are psychedelic medicines the reset for chronic pain? Preliminary findings and research needs. Neuropharmacology. 233, 109528 (2023).
- Sałat, K., Furgała, A., Sałat, R. Evaluation of cebranopadol, a dually acting nociceptin/orphanin FQ and opioid receptor agonist in mouse models of acute, tonic, and chemotherapy-induced neuropathic pain. Inflammopharmacology. 26 (2), 361-374 (2018).
- Wang, J., et al. Anti-inflammatory and analgesic actions of bufotenine through inhibiting lipid metabolism pathway. Biomed Pharmacother. 140, 111749 (2021).
- Gong, L., et al. Antinociceptive and anti-inflammatory potentials of Akebia saponin D. Eur J Pharmacol. 845, 85-90 (2019).
- Deng, J., et al. Comparison of analgesic activities of aconitine in different mice pain models. PloS One. 16 (4), e0249276 (2021).
- Penn, N. W. Potentiation of morphine analgesic action in mice by beta-carotene. Eur J Pharmacol. 284 (1-2), 191-193 (1995).
- Akkol, E. K., Güvenç, A., Yesilada, E. A comparative study on the antinociceptive and anti-inflammatory activities of five Juniperus taxa. J Ethnopharmacol. 125 (2), 330-336 (2009).

.
