Measuring Glucose Uptake in Drosophila Models of TDP-43 Proteinopathy
概要
Glucose uptake is increased in Drosophila motor neurons affected by TAR DNA binding protein (TDP-43) proteinopathy, as indicated by a FRET-based, genetically encoded glucose sensor.
Abstract
Amyotrophic lateral sclerosis is a neurodegenerative disorder causing progressive muscle weakness and death within 2-5 years following diagnosis. Clinical manifestations include weight loss, dyslipidemia, and hypermetabolism; however, it remains unclear how these relate to motor neuron degeneration. Using a Drosophila model of TDP-43 proteinopathy that recapitulates several features of ALS including cytoplasmic inclusions, locomotor dysfunction, and reduced lifespan, we recently identified broad ranging metabolic deficits. Among these, glycolysis was found to be upregulated and genetic interaction experiments provided evidence for a compensatory neuroprotective mechanism. Indeed, despite upregulation of phosphofructokinase, the rate limiting enzyme in glycolysis, an increase in glycolysis using dietary and genetic manipulations was shown to mitigate locomotor dysfunction and increased lifespan in fly models of TDP-43 proteinopathy. To further investigate the effect on TDP-43 proteinopathy on glycolytic flux in motor neurons, a previously reported genetically encoded, FRET-based sensor, FLII12Pglu-700µδ6, was used. This sensor is comprised of a bacterial glucose-sensing domain and cyan and yellow fluorescent proteins as the FRET pair. Upon glucose binding, the sensor undergoes a conformational change allowing FRET to occur. Using FLII12Pglu-700µδ6, glucose uptake was found to be significantly increased in motor neurons expressing TDP-43G298S, an ALS causing variant. Here, we show how to measure glucose uptake, ex vivo, in larval ventral nerve cord preparations expressing the glucose sensor FLII12Pglu-700µδ6 in the context of TDP-43 proteinopathy. This approach can be used to measure glucose uptake and assess glycolytic flux in different cell types or in the context of various mutations causing ALS and related neurodegenerative disorders.
Introduction
Amyotrophic Lateral Sclerosis (ALS) is a progressive neurodegenerative disorder that is currently incurable. ALS affects upper and lower motor neurons leading to loss of motor coordination, irreversible paralysis, respiratory failure, and eventual death within 2-5 years of diagnosis1. ALS is associated with metabolic defects such as weight loss, dyslipidemia, and hypermetabolism (reviewed in2); however, it remains unclear how these alterations in metabolism relate to motor neuron degeneration. A common denominator in ALS and related neurodegenerative diseases is TDP-43, a nucleic acid binding protein involved in several steps of RNA processing3,4,5. Although mutations in TDP-43 only affect 3%-5% of patients, wild-type TDP-43 protein is found within cytoplasmic aggregates in >97% of ALS cases (reviewed in6). This pathology was modeled in Drosophila by overexpression of human wildtype or mutant TDP-43 (G298S) in motor neurons, which recapitulates multiple aspects of ALS, including cytoplasmic inclusions, locomotor dysfunction and reduced lifespan7,8. Using these models, it was recently reported that TDP-43 proteinopathy causes a significant increase in pyruvate levels and phosphofructokinase (PFK) mRNA, the rate limiting enzyme of glycolysis9. Similar increases in PFK transcripts were found in patient-derived motor neurons and spinal cords, suggesting that glycolysis is upregulated in the context of TDP-43 proteinopathy. Interestingly, further increase in glycolysis using dietary and genetic manipulations mitigated several ALS phenotypes such as locomotor dysfunction and increased lifespan in fly models of TDP-43 proteinopathy, consistent with a compensatory, neuroprotective mechanism in degenerating motor neurons.
To further probe changes in glycolysis and measure glucose uptake in Drosophila models of TDP-43 proteinopathy, a previously reported genetically encoded FRET-based sensor FLII12Pglu-700µδ610 was expressed in motor neurons specifically using the UAS-GAL4 expression system. The FLII12Pglu-700µδ6 glucose sensor uses resonance energy transfer between two variants of green fluorescent protein, cyan and yellow fluorescent proteins (CFP and YFP) to detect glucose at the cellular level. It consists of a bacterial glucose binding domain from the E. coli MglB gene fused to CFP and YFP at opposite ends of the molecule. When bound to a glucose molecule, the sensor undergoes a conformational change bringing CFP and YFP closer together and allowing FRET to occur, which can then be used to quantify intracellular glucose levels10,11,12 (Figure 1). Here, we show how the FLII12Pglu-700µδ6 sensor can be used to determine changes in glucose uptake caused by TDP-43 proteinopathy in motor neurons. The experiments described here show that overexpression of an ALS-associated mutant, TDP-43G298S, in motor neurons causes a significant increase in glucose uptake compared to controls. This approach can be used in other types of ALS (e.g., SOD1, C9orf72, etc.) and/or other cell types (e.g., glia, muscles) to determine changes in glucose uptake associated with neurodegeneration.
Protocol
The UAS FLII12Pglu-700µδ6 transgenic flies were reported in Volkenhoff et al.10 and kindly provided by Dr. S. Schirmeier. The UAS TDP-43G298S transgenic lines were kindly provided by Dr. T. Iwatsubo13. Recombinant Drosophila lines harboring both UAS FLII12Pglu-700µδ6 and UAS TDP-43 transgenes were generated in the Zarnescu laboratory using standard genetic approaches and reported in Manzo et al.9. D42 GAL4 was used to drive the expression of the glucose sensor alone or together with TDP-43G298S in motor neurons. All fly lines are maintained on molasses cornmeal media, at 25 °C in 12 h dark:light cycle.
1. Drosophila Ventral Nerve Cord (VNC) dissections
- Make silicone elastomer dissection dishes.
- Pour silicone elastomer components into a 50 mL tube as indicated in the manufacturer's protocol and stir well.
- Fill 35 mm tissue culture dishes with approximately 2 mL of the elastomer mixture. Use a syringe to remove any bubbles. Cover the dishes and place them on a level surface in a 60 °C oven overnight to cure.
- Dissect Drosophila larvae to expose the VNC while maintaining tissue viability.
- Collect a wandering third instar larva, rinse with ddH2O, and then place it in a drop of HL3-buffer (70 mM NaCl, 5 mM KCl, 20 mM MgCl2, 10 mM NaHCO3, 115 mM sucrose, 5 mM trehalose, 5 mM HEPES; pH 7.1) on an elastomer lined dish.
- Using a pair of forceps (#5 or 55 forceps) under a dissecting microscope, pin the anterior and posterior ends of the larva dorsal side up, carefully stretching the larva lengthwise with insect pins (Minutein Pins).
- Make an incision just above the posterior pin using a pair of angled iris scissors. Make a vertical cut starting from the incision toward the anterior end of the larva.
- Add a few drops of HL-3 buffer if needed. Remove the trachea and the rest of the floating organs without disturbing the CNS. Pin the flaps stretching the body wall to expose the CNS while keeping the neuromuscular system (VNCs, axons, and neuromuscular junctions) intact.
2. Image acquisition
- Optimize the imaging parameters.
- Turn on the microscope and lasers before starting dissections. Images must be acquired using an upright confocal microscope with a 40x water immersion lens. Use a 405 nm laser to excite CFP and acquire the images in both the CFP (465-499 nm) and the FRET (535-695 nm) detection channels.
- Optimize acquisition parameters such as scan speed (6), average (2), objective (40x), zoom (1x), pinhole size (1 AU), and spatial resolution (512 x 512). Adjust the gain such that the signal is in the optimal dynamic range. The same parameters must be used for all genotypes.
- Image motor neurons within the VNC.
- Place the silicone dish with the dissected sample under the lens. Lower the lens so that it comes in contact with trehalose-sucrose HL3 buffer (see 1.2.1). It is critical to ensure that the lens is completely submerged in the buffer. Add more buffer if required.
- Use the CFP and FRET channels to manually select an optical section that consists of at least 6 motor neurons in focus, located along the VNC midline. The motor neurons are identified based on the expression of the glucose sensor and position along the anterior-posterior axis.
- Acquire images every 10 s for 10 min. These images represent the baseline.
- Stimulate with glucose supplemented HL-3.
- Remove the trehalose-sucrose containing HL3-buffer using a Pasteur pipette and replace it with 5 mM glucose supplemented HL-3 (70 mM NaCl, 5 mM KCl, 20 mM MgCl2, 10 mM NaHCO3, 115 mM sucrose, 5 mM glucose, 5 mM HEPES, pH 7.1). This step needs to be performed with great care, to avoid the movement of the VNC as much as possible.
- Acquire images every 10 s for another 10 min. These images represent the stimulation phase.
- Save the images as .czi files or any file type supported by the imaging software with a file name including date, genetic background, experimental condition (baseline or stimulation), and channels used.
- Reuse the parameters to image all the genotypes (Figure 2A).
3. Image processing and ROI selection
- Open the czi files in Fiji/ImageJ and set Color Mode: Grayscale, choose View Stack with: Hyperstack, and select Split Channels into Separate Windows.
- Process images using the drift correction plugins: turboreg and stackreg. Each channel is corrected separately by selecting Stackreg under Plugins, and then choose Transformation: Translation.
- Manually select the Regions of interest (ROIs) to extract the mean gray value of each channel.
- Choose the ROIs by tracing all motor neurons that remain in focus over the entire time series. Use the ROI manager to save each motor neuron outline.
- Use the Multi-measure function to measure the mean gray value in each ROI at each time point. Copy the ROIs traced from the first channel to the second channel for the pre-stimulation image.
- Repeat this process for the post-stimulation images in each channel using the same cells/ROIs selected for the pre-stimulation image.
4. Data analysis
- Calculate the FRET/CFP ratio, using the mean gray values for the FRET and CFP channels. Changes in the FRET/CFP ratios reflect alterations in the intracellular glucose concentration.
- Plot FRET/CFP ratios for each ROI and time point versus time. Within every VNC some of the ROIs will exhibit a drop in FRET/CFP ratios following stimulation.
NOTE: This is interpreted as an inability of some neurons to uptake glucose, possibly due to stress caused by dissections and are therefore eliminated from the analysis. The remaining FRET/CFP ratios for each ROI and time point are averaged to generate a single FRET/CFP ratio per time point per genotype (glucose sensor alone and glucose sensor with TDP-43G298S). These single FRET/CFP ratios are used for all subsequent normalizations. 31 ROIs were analyzed for Glucose Sensor controls and 24 ROIs were analyzed for TDP-43G298S.
- Plot FRET/CFP ratios for each ROI and time point versus time. Within every VNC some of the ROIs will exhibit a drop in FRET/CFP ratios following stimulation.
- Baseline normalization: Calculate the average of FRET/CFP ratios for the first 10 min (baseline), and then normalize individual FRET/CFP ratios for each time point of the entire experiment to the 10 min baseline average (see Figure 2B).
- Timepoint normalization: Normalize TDP-43G298S FRET/CFP ratios to FRET/CFP ratios from glucose sensor alone at each time point (see Figure 3A).
- Bar graphs: For baseline versus stimulation comparisons in the form of bar graphs, use FRET/CFP ratios from 5-10 min (baseline) and 15-20 min (stimulation) for each genotype. Normalize stimulation ratio to the baseline ratio (see Figure 3B).
5. Statistical analyses
- Evaluate the normalized data sets using Mann-Whitney correction (for baseline and timepoint normalizations) or Kruskall-Walis test (for bar graph representation of stimulation versus baseline FRET/CFP ratios).
Representative Results
Image acquisition of the glucose sensor in the ventral nerve cord (VNC), ex vivo
To determine differences in glucose uptake in a Drosophila model of ALS based on TDP-43, a genetically encoded FRET-based glucose sensor was used. The sensor comprised CFP and YFP fused to the glucose binding domain from the E. coli MglB gene. Glucose binding elicits a conformational change, which can be detected by fluorescence resonance energy transfer (FRET) between the CFP and YFP 10,11,12. When glucose is not bound to the sensor, CFP excitation causes CFP emission only, and when glucose binds, the YFP signal can be detected due to FRET occurring between CFP and YFP (Figure 1A). The D42 GAL4 driver was used to express the sensor alone or together with the ALS-associated mutant TDP-43G298S in motor neurons via the bipartite UAS-GAL4 expression system14. Third instar larvae were dissected in HL3 buffer without glucose and pinned to expose the neuromuscular system, including the VNC still connected to the neuromuscular junctions via motor neuron axons. The VNC was imaged using a 40x water immersion lens with a spatial resolution of 512 x 512 pixels (see Protocol and Figure 1B). CFP and FRET signals were acquired in the 465-499 nm and 535-695 nm, respectively, following CFP excitation with the 405 nm laser. The slice to be imaged was chosen based on the plane where the VNC was visible with a minimum of six motor neurons in focus along the midline. The selected plane was imaged for 10 min, capturing an image every 10 s to determine the baseline fluorescence. The czi files were saved as: genotype_sample#_baseline. After the 10 min time course was completed for baseline conditions, the buffer was replaced with glucose supplemented HL3. The image was refocused to find the same plane that was imaged under baseline conditions. The same image settings were used for all samples. The VNC was then imaged again for 10 min acquiring a new image every 10 s and saved as: genotype_sample#_post stimulation for further analyses.
Image analysis
A significant challenge with live imaging experiments is the drift that VNCs undergo during the 20 min imaging interval. To address this issue, a drift correction plugin, Stackreg was used in FIJI prior to image analysis and ROI selection (see Protocol and Figure 2A). 6-8 individual cells/ROIs were selected from each VNC and outlined using the FRET/YFP channel. Selections were copied to the CFP channel, and the mean gray values were measured using the multi-measure function. Quantifications were performed by first averaging the FRET/CFP ratios per time point, and then calculating the average for the entire 10 min per genotype. These averages were then used to normalize the FRET/CFP ratios at each time point and plotted over time to generate a first evaluation of the data (Figure 2B). Using this normalization approach, it was found that upon stimulation, control motor neurons expressing glucose sensor alone, exhibit a small but significant increase in glucose uptake (3.9%, Pvalue < 0.0001) while motor neurons expressing TDP-43G298S show a significantly higher glucose uptake (16.76%, Pvalue < 0.0001). These data are consistent with TDP-43 proteinopathy causing increased glucose uptake in motor neurons, as previously shown in reference9.
Data analyses – measurement of glucose uptake in motor neurons in the Drosophila VNC
To gain further insights into differences between controls and TDP-43G298S mutant motor neurons, each FRET/CFP ratio was normalized to the control at each time point (Figure 3A). This allows for a comparison in glucose uptake between ALS motor neurons expressing TDP-43G298S and control motor neurons expressing the glucose sensor alone in real-time. Under baseline conditions, there was no difference between the genotypes. However, upon stimulation with glucose, a rapid (7.31% after 1 min of glucose application) and significant increase (12.76% after 10 min, Pvalue < 0.0001) in glucose uptake was observed in TDP-43G298S when compared to the control.
Bar graphs offer an alternative approach to illustrating differences between genotypes (TDP-43G298S versus controls) before and after glucose stimulation (Figure 3B). This was done by normalizing the FRET/CFP ratio for both conditions to the baseline of the respective genotypes and plotting the average ratio for the last 5 min of baseline and stimulation imaging, respectively. Upon stimulation, neurons expressing glucose sensor alone show a small but significant increase in FRET/CFP ratio (3.83% ± 0.08%, Pvalue < 0.0001) compared to the baseline. Whereas neurons expressing TDP-43G298S showed a significant increase in FRET/CFP ratio upon stimulation (14.73% ± 0.08%, Pvalue < 0.0001) when compared to the baseline. The net difference in FRET/CFP ratios between motor neurons expressing TDP-43G298S and glucose sensor alone is 10.9% (Pvalue = 0.005). This analysis also shows that the ratios stabilize around the last 5 min of each time-course imaging, so this time frame was chosen for the graph. This analysis provides a high-level overview of the results, including statistically significant differences between genotypes and treatments in one graph.
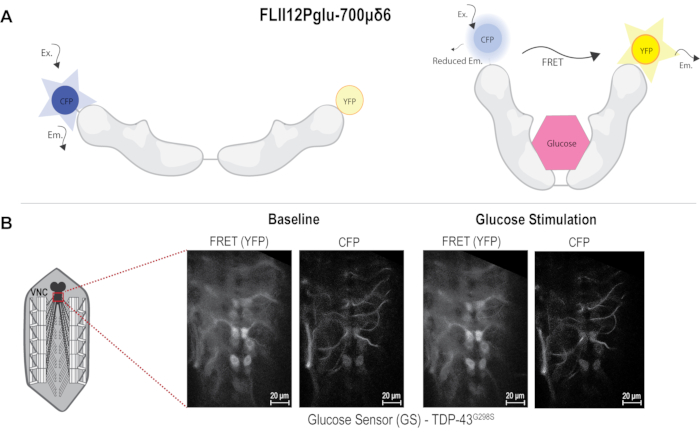
Figure 1: FRET sensor and image acquisition. (A) FRET-based Glucose sensor diagram used to measure glucose uptake in motor neurons. Glucose binding causes FRET to occur. (B) Diagram of neuromuscular junction (NMJ) dissection showing area imaged in the VNC. Images to the left showing TDP-43G298S before and after glucose stimulation in both FRET (YFP) and CFP channels. Scale bars: 20 µm. Magnification: 40x. Please click here to view a larger version of this figure.
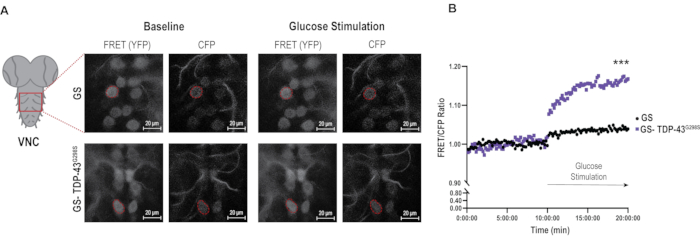
Figure 2: Image analysis of glucose sensor in the context of TDP-43 mutant. (A) Representative images of FRET and CFP signals in glucose sensor controls and TDP-43G298S samples under baseline and stimulation conditions. A representative motor neuron is outlined in each image as an example of an ROI. Scale bars: 20 µm. Magnification: 40x. (B) The average ratios of FRET/CFP in glucose sensor and TDP-43 mutant motor neurons over 20 min of imaging. Ratios were normalized to the average baseline ratio for each genotype (see baseline normalization in the Protocol). Values shown are the average of 25-30 motor neurons (ROI) from 5 VNCs per genotype. Mann-Whitney was used to evaluate statistical significance. *** = Pvalue < 0.001. Please click here to view a larger version of this figure.
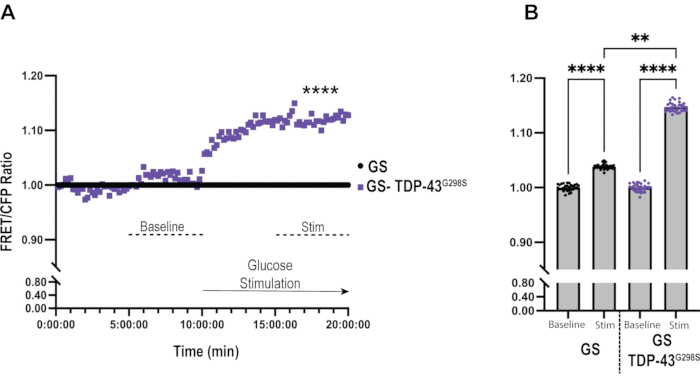
Figure 3: Data analysis of glucose sensor in the context of TDP-43G298S. (A) FRET/CFP ratios of TDP-43G298S are normalized to Glucose Sensor control at each time point. Mann-Whitney was used to evaluate statistical significance. **** = Pvalue < 0.0001. (B) Bar graph representation of glucose uptake in Glucose Sensor control and TDP-43G298S shows a significant increase in glucose uptake in control motor neurons upon stimulation and in the context of TDP-43G298S compared to controls. Kruskall-Walis was used to evaluate statistical significance. ** = Pvalue < 0.01, **** = Pvalue < 0.0001. Please click here to view a larger version of this figure.
Discussion
The technique described in detail here can be applied to measure glucose uptake in a specific cell type of interest in live Drosophila using FLII12Pglu-700µδ6, a FRET based sensor which can detect changes in glucose levels to a millimolar range10,11,12. This sensor has been previously used in conjunction with the UAS-GAL4 system to target its expression to specific cell types, including neurons9,10. In this study, D42 GAL4 was used to express FLII12Pglu-700µδ6 on its own or together with disease associated TDP-43G298S to demonstrate how this approach can be used to evaluate glucose uptake in ALS motor neurons. Furthermore, this method can be applied broadly to image a multitude of fluorescent sensors in the larval VNC.
Critical steps in the protocol include maximizing the signal-to-noise ratio. To this end, image acquisition parameters were set such that the fluorescence is not oversaturated, and the full range of signal intensity can be quantified. Imaging parameters such as gain, scanning speed, pinhole size, and zoom remained unchanged throughout the study. The experiments were performed over several days; however, in each experiment both the genotypes, i.e., controls and TDP-43G298S were imaged together to account for variability. Imaging Drosophila larval CNS using a water immersion lens could give rise to optic plane changes over time. Care should be taken to avoid drastic changes in optic plane by using sufficient buffer. Samples with changes in focus were discarded.
Although the conditions and parameters are maintained unchanged throughout the experiments, this technique requires a change in buffer during imaging. After 10 min of imaging the glucose free buffer must be replaced with glucose supplemented HL-3 buffer, which might change the optic focal plane. The lens must be re-focused to the same focal plane so that the same set of cells are imaged before and after glucose supplementation. A subset of motor neurons exhibited a drop in FRET/CFP ratios following stimulation. This indicates that some neurons do not uptake glucose, possibly due to stress caused by dissection and were eliminated from the analysis.
ROI selection, image processing and quantification were performed using Fiji/ImageJ. While ROI is selected manually in the first image of the series, it is critical to correct minor drift in time lapse imaging to keep ROI invariable throughout the imaging series. An open-sourced drift correction plugin, Stackreg, was used to account for this issue.
Availability of the right excitation laser is a major limitation to this technique. Although 436 nm excitation laser is ideal for this glucose sensor, we have shown that the sensor can be used with the 405 nm excitation laser, which is more widely available. Another limitation to this technique is that it can be used to measure glucose dynamics but not absolute glucose concentrations. A potential alternative strategy to measure absolute glucose levels is iGlucoSnFR-TS, a lifetime glucose sensor, which can be calibrated to measure absolute glucose concentrations15.
Glucose is a major source of energy in the brain and is required for physiological functions. Defects in glucose metabolism have been associated with several pathological conditions (reviewed in16). Drosophila is a powerful genetic model used to study neurodegenerative disorders17. However, there are limited techniques available to directly measure glucose levels in Drosophila. This FRET-based glucose sensor provides a direct measure of intracellular changes in glucose levels. Despite some experimental challenges and limitations this sensor can be successfully used to study glucose dynamics in specific cell types (e.g., motor neurons, glia, muscles) in various disease models, including multiple types of ALS and related neurodegenerative disorders.
開示
The authors have nothing to disclose.
Acknowledgements
We thank Stefanie Schirmeier and Takeshi Iwatsubo for providing Drosophila strains. We also thank Patricia Jansma for assisting with imaging in the Marley Imaging Core at the University of Arizona. This work was funded by National Institutes of Health NIH NS091299, NS115514 (to DCZ), HHMI Gilliam Fellowship (to EM) and the Undergraduate Biology Research Program (to HB).
Materials
| 35 mm tissue culture dishes | Sigma Aldrich | CLS430165 | |
| 40X water immersion lens | Zeiss | 440090 | dippable, N.A. 0.8 |
| dissection scissors | Roboz | RS-5618 | |
| Dumont #5 forceps | VWR | 100189-236 | |
| Dumont #55 forceps | VWR | 100189-244 | |
| Minutien pins | Fine Science tools | 26002-10 | used for dissections |
| SYLGARD 184 Silicone Elastomer Kit | Dow | 1317318 | |
| Zeiss LSM880 NLO upright multiphoton/confocal microscope | Zeiss | N/A |
参考文献
- Ingre, C., Roos, P. M., Piehl, F., Kamel, F., Fang, F. Risk factors for amyotrophic lateral sclerosis. Clinical Epidemiology. 7, 181-193 (2015).
- Dupuis, L., Pradat, P. F., Ludolph, A. C., Loeffler, J. P. Energy metabolism in amyotrophic lateral sclerosis. The Lancet Neurology. 10 (1), 75-82 (2011).
- Buratti, E., Baralle, F. E. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. The Journal of Biological Chemistry. 276 (39), 36337-36343 (2001).
- Polymenidou, M., et al. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nature Neuroscience. 14 (4), 459-468 (2011).
- Tollervey, J. R., et al. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nature Neuroscience. 14 (4), 452-458 (2011).
- Ling, S. C., Polymenidou, M., Cleveland, D. W. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron. 79 (3), 416-438 (2013).
- Estes, P. S., et al. Wild-type and A315T mutant TDP-43 exert differential neurotoxicity in a Drosophila model of ALS. Human Molecular Genetics. 20 (12), 2308-2321 (2011).
- Estes, P. S., et al. Motor neurons and glia exhibit specific individualized responses to TDP-43 expression in a Drosophila model of amyotrophic lateral sclerosis. Disease Models & Mechanisms. 6 (3), 721-733 (2013).
- Manzo, E., et al. Glycolysis upregulation is neuroprotective as a compensatory mechanism in ALS. eLife. 8, 45114 (2019).
- Volkenhoff, A., Hirrlinger, J., Kappel, J. M., Klambt, C., Schirmeier, S. Live imaging using a FRET glucose sensor reveals glucose delivery to all cell types in the Drosophila brain. Journal of Insect Physiology. 106, 55-64 (2018).
- Fehr, M., Lalonde, S., Lager, I., Wolff, M. W., Frommer, W. B. In vivo imaging of the dynamics of glucose uptake in the cytosol of COS-7 cells by fluorescent nanosensors. The Journal of Biological Chemistry. 278 (21), 19127-19133 (2003).
- Takanaga, H., Chaudhuri, B., Frommer, W. B. GLUT1 and GLUT9 as major contributors to glucose influx in HepG2 cells identified by a high sensitivity intramolecular FRET glucose sensor. Biochimedica et Biophysica Acta. 1778 (4), 1091-1099 (2008).
- Ihara, R., et al. RNA binding mediates neurotoxicity in the transgenic Drosophila model of TDP-43 proteinopathy. Human Molecular Genetics. 22 (22), 4474-4484 (2013).
- Brand, A. H., Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118 (2), 401-415 (1993).
- Diaz-Garcia, C. M., et al. Quantitative in vivo imaging of neuronal glucose concentrations with a genetically encoded fluorescence lifetime sensor. Journal of Neuroscience Research. 97 (8), 946-960 (2019).
- Mergenthaler, P., Lindauer, U., Dienel, G. A., Meisel, A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends in Neurosciences. 36 (10), 587-597 (2013).
- Lu, B., Vogel, H. Drosophila models of neurodegenerative diseases. Annual Review of Pathology. 4, 315-342 (2009).

