Optogenetic Phase Transition of TDP-43 in Spinal Motor Neurons of Zebrafish Larvae
概要
We describe a protocol to induce phase transition of TAR DNA-binding protein 43 (TDP-43) by light in the spinal motor neurons using zebrafish as a model.
Abstract
Abnormal protein aggregation and selective neuronal vulnerability are two major hallmarks of neurodegenerative diseases. Causal relationships between these features may be interrogated by controlling the phase transition of a disease-associated protein in a vulnerable cell type, although this experimental approach has been limited so far. Here, we describe a protocol to induce phase transition of the RNA/DNA-binding protein TDP-43 in spinal motor neurons of zebrafish larvae for modeling cytoplasmic aggregation of TDP-43 occurring in degenerating motor neurons in amyotrophic lateral sclerosis (ALS). We describe a bacterial artificial chromosome (BAC)-based genetic method to deliver an optogenetic TDP-43 variant selectively to spinal motor neurons of zebrafish. The high translucency of zebrafish larvae allows for the phase transition of the optogenetic TDP-43 in the spinal motor neurons by a simple external illumination using a light-emitting diode (LED) against unrestrained fish. We also present a basic workflow of live imaging of the zebrafish spinal motor neurons and image analysis with freely available Fiji/ImageJ software to characterize responses of the optogenetic TDP-43 to the light illumination. This protocol enables the characterization of TDP-43 phase transition and aggregate formation in an ALS-vulnerable cellular environment, which should facilitate an investigation of its cellular and behavioral consequences.
Introduction
Ribonucleoprotein (RNP) granules control a myriad of cellular activities in the nucleus and cytoplasm by assembling membrane-less partitions via liquid-liquid phase separation (LLPS), a phenomenon in which a homogeneous fluid demixes into two distinct liquid phases1,2. The dysregulated LLPS of RNA-binding proteins that normally function as RNP granule components promote abnormal phase transition, leading to protein aggregation. This process has been implicated in neurodevelopmental and neurodegenerative diseases3,4,5. The precise evaluation of a causal relationship between aberrant LLPS of RNA-binding proteins and disease pathogenesis is crucial for determining whether and how LLPS can be exploited as an effective therapeutic target. LLPS of RNA-binding proteins is relatively easy to study in vitro and in unicellular models but is difficult in multicellular organisms, especially in vertebrates. A critical requirement for analyzing such LLPS in individual cells within a tissue environment is to stably express a probe for the imaging and manipulation of LLPS in a disease-vulnerable cell type of interest.
Amyotrophic lateral sclerosis (ALS) is an ultimately fatal neurological disorder in which motor neurons of the brain and spinal cord are selectively and progressively lost due to degeneration. To date, mutations in more than 25 genes have been associated with the heritable (or familial) form of ALS, which accounts for 5%-10% of total ALS cases, and some of these ALS-causing genes encode RNA-binding proteins consisting of RNPs, such as hnRNPA1, TDP-43, and FUS6,7. Moreover, the sporadic form of ALS, which accounts for 90%-95% of total ALS cases, is characterized by the cytoplasmic aggregation of TDP-43 deposited in degenerating motor neurons. A major characteristic of these ALS-associated RNA-binding proteins is their intrinsically disordered regions (IDRs) or low-complexity domains that lack ordered three-dimensional structures and mediate weak protein-protein interactions with many different proteins that drive LLPS7,8. The fact that ALS-causing mutations often occur in the IDRs has led to the idea that aberrant LLPS and phase transition of these ALS-related proteins may underlie ALS pathogenesis9,10.
Recently, the optoDroplet method, a Cryptochrome 2-based optogenetic technique that allows the modulation of protein-protein interactions by light, was developed to induce phase transition of proteins with IDRs11. As this technique has been extended successfully to TDP-43, it has begun to uncover the mechanisms underlying pathological phase transition of TDP-43 and its associated cytotoxicity12,13,14,15. In this protocol, we outline a genetic method to deliver an optogenetic TDP-43 to ALS-vulnerable cell types, namely, spinal motor neurons in zebrafish using the BAC for the mnr2b/mnx2b gene encoding a homeodomain protein for motor neuron specification16,17. The high translucency of zebrafish larvae allows for simple, noninvasive light stimulation of the optogenetic TDP-43 that triggers its phase transition in the spinal motor neurons. We also present a basic workflow for the live imaging of the zebrafish spinal motor neurons and image analysis using the freely available Fiji/ImageJ software to characterize the responses of the optogenetic TDP-43 to the light stimulation. These methods allow for an investigation of TDP-43 phase transition in an ALS-vulnerable cellular environment and should help to explore its pathological consequences at cellular and behavioral levels.
Protocol
All fish work was conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the Institutional Animal Care and Use Committee (approval identification number 24-2) of the National Institute of Genetics (Japan), which has an Animal Welfare Assurance on file (assurance number A5561-01) at the Office of Laboratory Animal Welfare of the National Institutes of Health (NIH, USA).
1. Construction of BACs for expression of optogenetic TDP-43 gene from the mnr2b promoter
- BAC preparation
- Purchase a zebrafish BAC clone containing the zebrafish mnr2b locus (CH211-172N16, BACPAC Genomics). Purify the BAC DNA from a 5 mL overnight LB culture of DH10B Escherichia coli (E. coli) harboring CH211-172N16 as described in Warming et al.18.
- Transform CH211-172N16 into SW102 E. coli cells by electroporation, as described in Warming et al.18.
NOTE: Purification of the BAC DNA from the E. coli on the same day of electroporation usually gives a higher success rate of BAC transformation18. Otherwise, the purified BAC DNA is kept at -20 °C until use. - Introduce the iTol2-amp cassette for Tol2 transposon-mediated BAC transgenesis19 into the backbone of CH211-172N16 by electroporation, as described in Asakawa et al.20.
- Make a glycerol stock of the E. coli cell clones carrying CH211-172N16 with the iTol2-amp cassette integration (CH211-172N16-iTol2A) after confirming the homologous recombination-mediated integration by polymerase chain reaction (PCR) (Ex Taq) using the primer pair Tol2-L-out (5'-AAA GTA TCT GGC TAG AAT CTT ACT TGA-3') and pTARBAC-13371r (5'-TAG CGG CCG CAA ATT TAT TA-3') and the following conditions: a denaturation step at 98 °C for 1 min, followed by 25 cycles of denaturation at 95 °C for 10 s, primer annealing at 55 °C for 15 s, and primer extension at 72 °C for 1 min, amplifying a PCR product of 354 base pairs (bp).
- mnr2b-hs:opTDP-43h construction
- Construct a plasmid carrying the expression cassette for the human wild-type TDP-43/TARDBP (TDP-43h) that is tagged with mRFP1 and CRY2olig at the N- and C-termini, respectively (hereafter, opTDP-43h)14.
NOTE: The opTDP-43h fragment should be flanked with the zebrafish hsp70l gene promoter sequence (650 bp) and polyadenylation (polyA) signal sequence, followed by a kanamycin resistance gene (hsp70lp-opTDP-43h-polyA-Kan)14. - Amplify the hsp70l-opTDP-43h-polyA-Kan cassette with primers that anneal hsp70l and Kan and contain 45 bp sequences of the upstream and downstream of the initiator codons of the mnr2b gene by PCR (GXL DNA Polymerase) using the primer pair mnr2b-hspGFF-Forward (5'-tat cag cgc aat tac ctg caa ctc taa aca caa caa aag tgt tgc aGA ATT CAC TGG AGG CTT CCA GAA C-3') and Km-r (5'-ggt tct tca gct aaa agg gcg tcg atc ctg aag ttc ttt gac ttt tcc atC AAT TCA GAA GAA CTC GTC AAG AA-3') with the following conditions: a denaturation step at 98 °C for 1 min, followed by 30 cycles of denaturation at 95 °C for 10 s, primer annealing at 55 °C for 15 s, and primer extension at 68 °C for 30 s, amplifying a PCR product of ~5.7 bp.
- Separate the PCR products by agarose gel electrophoresis (100 V) and purify the hsp70lp-opTDP-43h-polyA-Kan DNA band with a DNA column. Adjust the concentration of the purified hsp70lp-opTDP-43h-polyA-Kan cassette to 50 ng/µL in Tris-EDTA buffer (TE) containing 10 mM Tris-HCl (pH 8.0) and 1 mM EDTA.
- Introduce the hsp70lp-opTDP-43h-polyA-Kan cassette into CH211-172N16-iTol2A by electroporation as described in Warming et al.18 and select ampicillin- and kanamycin-resistant transformants on LB agar plates. CH211-172N16-iTol2A carrying the hsp70lp-opTDP-43h-polyA-Kan cassette is designated as mnr2b-hs:opTDP-43h.
- Purify mnr2b-hs:opTDP-43h using a BAC purification kit and dissolve it at 250 ng/µL in TE after phenol/chloroform extraction.
- Construct a plasmid carrying the expression cassette for the human wild-type TDP-43/TARDBP (TDP-43h) that is tagged with mRFP1 and CRY2olig at the N- and C-termini, respectively (hereafter, opTDP-43h)14.
- mnr2b-hs:EGFP-TDP-43z construction
- Construct another plasmid carrying the zebrafish wild-type tardbp that is tagged with enhanced green fluorescent protein (EGFP) at its N-terminus (EGFP-TDP-43z) instead of opTDP-43h but is otherwise identical to the hsp70l-opTDP-43h-polyA-Kan construct (hsp70l-EGFP-TDP-43z-polyA-Kan)14. Use EGFP-TDP-43z as an internal control for the light stimulation of opTDP-43h.
- Construct CH211-172N16-iTol2A harboring the hsp70lp-EGFP-TDP-43z-polyA-Kan cassette in the mnr2b locus as described in 1.2.1-1.2.5. CH211-172N16-iTol2A carrying the hsp70lp-EGFP-TDP-43z-polyA-Kan cassette is designated as mnr2b-hs:EGFP-TDP-43z.
2. Tol2 transposon-mediated BAC transgenesis in zebrafish
- Prepare the injection solution containing 40 mM KCl, phenol red (10% v/v), 25 ng/µL of the mnr2b-hs:opTDP-43h DNA, and 25 ng/µL Tol2 transposase mRNA19.
- Inject 1 nL of the injection solution (a droplet with a diameter of approximately 123 µm calibrated in mineral oil) into the cytosol of wild-type zebrafish embryos at the one-cell stage. Screen the injected fish for the formation of red fluorescent protein (RFP)-positive aggregates in various embryonic tissues, including spinal motor neurons, under a fluorescence stereomicroscope at 2-3 days post-fertilization (dpf). Raise the RFP-positive fish to adulthood.
- Inject the mnr2b-hs:EGFP-TDP-43z DNA as described in 2.1 and 2.2. Raise EGFP-positive fish to adulthood.
- After a few months, put sexually matured injected fish and wild-type fish in pairs in standard 2 L mating cages to obtain F1 offspring. Screen F1 fish at 3 dpf for RFP (opTDP-43h) or EGFP (EGFP-TDP-43z) fluorescence in the spinal motor column using an epifluorescence microscope equipped with a Plan-Neofluar 5x/0.15 objective lens. Typically, one founder fish is identified from 10−20 injected fish (the germline transmission rate is 5%−10%).
- Isolate and compare multiple Tg[mnr2b-hs:opTDP-43h] and Tg[mnr2b-hs:EGFP-TDP-43z] inserts from different founder fish, as the intensity, but not the pattern, of opTDP-43h or EGFP-TDP-43z expression may vary between founder fish due to chromosomal position effects.
- Cross Tg[mnr2b-hs:opTDP-43h] and Tg[mnr2b-hs:EGFP-TDP-43z] fish lines to obtain offspring containing Tg[mnr2b-hs:opTDP-43h] Tg[mnr2b-hs:EGFP-TDP-43z] double-transgenic fish in a Mendelian ratio.
- Raise the fish in a plastic dish containing 30 mL of E3 buffer (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, 10-5% Methylene Blue) and add 0.003% (w/v) N-phenylthiourea at 8-10 h post-fertilization (hpf) to inhibit melanogenesis.
- Cover the plastic dish with aluminum foil after 30 hpf.
3. Preparation of LED for blue light illumination
- Turn on an LED panel by using the associated application installed on a tablet/phone. Put the probe of a spectrometer into an empty well of a 6 well dish and adjust the LED light to the wavelength peaking at ~456 nm through the application. Place the optical sensor of an optical power meter in the empty well and adjust the power of the LED light (~0.61 mW/cm2). The LED light setting can be saved and is retrievable in the application.
- Introduce the dish/LED panel setting to the incubator at 28 °C. Finish this step before imaging of fish starts at 48 hpf.
4. Imaging of zebrafish larvae expressing optogenetic TDP-43
- Select Tg[mnr2b-hs:opTDP-43h] Tg[mnr2b-hs:EGFP-TDP-43z] double-transgenic fish at least before 47 hpf, based on RFP (opTDP-43h) or EGFP (EGFP-TDP-43z) fluorescence in the spinal motor column using the epifluorescence microscope set-up described above.
- Dechorionate the Tg[mnr2b-hs:opTDP-43h] Tg[mnr2b-hs:EGFP-TDP-43z] double-transgenic fish.
- Preheat 1% low melting temperature agarose containing 250 µg/mL of ethyl 3-aminobenzoate methanesulfonate salt at 42 °C.
- Briefly anesthetize Tg[mnr2b-hs:opTDP-43h] Tg[mnr2b-hs:EGFP-TDP-43] double-transgenic fish at 48 hpf in E3 buffer containing the same concentration of Tricane.
- Put a drop of the preheated 1% low melting temperature agarose on the glass base dish at the room temperature. The diameter of the dome-shaped agarose drop on the glass dish is 8-10 mm.
- Using a Pasteur pipette, add the anesthetized fish to the low melting temperature agarose on the glass base dish, and then mix by pipetting a few times. Minimize the amount of the E3 buffer added to the agarose along with the fish.
- Maintain the fish on its side by using a syringe needle during the solidification of agarose (typically ~1 min) to ensure that the spinal cord is in an appropriate horizontal position. After the solidification, put a couple of drops of E3 buffer onto the dome-shaped agarose-mounted fish.
- Acquire serial confocal z-sections of the spinal cord by scanning with a confocal microscope equipped with a 20x water immersion objective lens with the numerical aperture 1.00, using a scan speed of 4.0 µs per pixel (12 bits per pixel), a step size of 1.0 µm per slice for the objective, and a combination of excitation/emission wavelengths: Channel 1) 473/510 nm for EGFP and Channel 2) 559/583 nm for mRFP1.
NOTE: The cloaca on the ventral side of the fish is included in the regions of interest (ROI) as a reference, which helps to identify and compare the spinal segments (levels 16-17) across the time points. - Remove the fish from the agarose by carefully cracking the agarose with a syringe needle as soon as the imaging is complete. Keep the amount of time the fish is embedded in the agarose as short as possible, although the agarose embedding for <30 min does not affect the viability of the fish.
5. Light stimulation of opTDP-43h-expressing fish by field illumination of a blue light-emitting diode (LED) light
- Add 7.5 mL of E3 buffer to the well and place the imaged Tg[mnr2b-hs:opTDP-43h] Tg[mnr2b-hs:EGFP-TDP-43z] double-transgenic fish into the well. Place the six-well dish on the LED panel by keeping the dish and LED panel 5 mm apart with a spacer (for example, with five slide glasses stacked).
- Turn on the blue LED light. Keep some of the Tg[mnr2b-hs:opTDP-43h] Tg[mnr2b-hs:EGFP-TDP-43z] double-transgenic fish in a separate six-well dish covered with aluminum foil when unilluminated control fish are necessary (i.e., in dark conditions)14.
- After the illumination (e.g., for 24 h at 72 hpf in Figure 3), image the spinal cord of the illuminated fish by repeating the steps 4.3 – 4.9.
6. Visualization of cytoplasmic relocation of optogenetic TDP-43 in the spinal motor neurons
- Open the image file in ImageJ/Fiji21 (Version: 2.1.0/1.53c), an open-source Java image processing program developed by NIH Image, which can be downloaded from https://imagej.net/Fiji/Downloads.
- Use the Z scrollbar to move through the focal planes. Create a maximum intensity projection of multiple slices by clicking Image | Stacks | Z project | Max Intensity and setting Start slice and Stop slice that cover the hemisegments of the spinal motor column.
- Split the multichannel image into two single-channel images by clicking Image | Color | Split Channels.
- Enhance the EGFP-TDP-43z signal to ensure that cytoplasmic EGFP-TDP-43z is visible clearly by clicking Image | Adjust | Brightness/Contrast and adjusting Minimum
- Open the ROI manager by clicking Analyze | Tools | ROI manager. Find the cells that are identifiable in both images at 48 hpf and 72 hpf, based on the relative positions of the cell bodies. Set the ROIs by outlining the contours of cell bodies of single spinal motor neurons visualized by the EGFP-TDP-43z signal using Freehand selections, and then clicking Add[t] in the ROI manager.
- Set a major axis of the soma by drawing a straight line using Straight and clicking Analyze | Plot Profile for EGFP-TDP-43z (C1) and opTDP-43h (C2) images at 48 and 72 hpf.
- Normalize the Plot Profile by dividing the values by the greatest value for each EGFP-TDP-43z and opTDP-43h signal. Plot the normalized values with x-y coordinates, where x and y represent the major axis of the soma and relative fluorescent intensities, respectively, using XY graph in a statistical software.
7. Ratiometric comparison between opTDP-43h and EGFP-TDP-43z signals using ImageJ/Fiji
- Open the image file in ImageJ/Fiji and set ROIs for single mnr2b-positive cells using a maximum intensity projection image of EGFP-TDP-43z as in 6.1-6.5. Add a ROI outside the ventral spinal cord (e.g., notochord) to represent the background signal (background ROI).
- For each EGFP-TDP-43z and opTDP-43 image, create a projection of multiple slices by clicking Image | Stacks | Z project | Sum Slices and set Start slice and Stop slice that cover the hemispinal motor column.
- Open the ROI manager created with the maximum intensity projection image. Display the ROIs on the Sum Slices image by selecting the image window for EGFP-TDP-43z and then clicking Show All in the ROI manager. Click Measure in the ROI manager to obtain mean values for each ROI.
- Acquire mean values for opTDP-43h following the same procedure provided in 7.3.
- For each ROI, after subtracting the mean for the background ROI, divide the subtracted mean for opTDP-43h by the subtracted mean for EGFP-TDP-43z to obtain the ratiometric value. The ratiometric values can be compared between different time points and presented using Column graph in Prism software.
Representative Results
Live imaging of optogenetic and non-optogenetic TDP-43 proteins in the mnr2b+ spinal motor neurons of zebrafish larvae
To induce TDP-43 phase transition in the spinal motor neurons in zebrafish, a human TDP-43h that is tagged with mRFP1 and CRY2olig22 at the N- and C-termini, respectively, was constructed and designated as opTDP-43h14 (Figure 1A). The opTDP-43h gene fragment was introduced into a BAC containing the mnr2b locus (Figure 1B). The resulting BAC, designated as mnr2b-hs:opTDP-43h, was introduced into the zebrafish genome by Tol2 transposon-mediated BAC transgenesis19. To monitor the localization of non-optogenetic TDP-43 in the spinal motor neurons, a zebrafish TDP-43 encoded by tardbp gene was tagged with EGFP at the N-terminus (Figure 1A) and the EGFP-TDP-43z gene fragment was introduced into the mnr2b BAC, similar to opTDP-43h (Figure 1B). The resulting BAC, designated as mnr2b-hs:EGFP-TDP-43z, was introduced into the zebrafish genome by Tol2 transposon-mediated BAC transgenesis. The fish injected with each BAC construct was crossed with a wild-type fish and the resulting F1 fish were screened on day 3 for red (Tg[mnr2b-hs:opTDP-43h]) or green (Tg[mnr2b-hs:EGFP-TDP-43z]) fluorescence in the ventral spinal cord. The isolated red or green fluorescence-positive F1 fish were designated as Tg[mnr2b-hs:opTDP-43h] or Tg[mnr2b-hs:EGFP-TDP-43z], respectively.
Tg[mnr2b-hs:opTDP-43h] Tg[mnr2b-hs:EGFP-TDP-43z] double-transgenic fish at 48 hpf were anesthetized and embedded in a low melting agarose on their side; after agarose solidification, they were covered with E3 buffer to allow observations of the lateral side of the spinal cord from above. Confocal laser scanning microscopy was performed with a 20x water immersion objective lens and a combination of excitation/emission wavelengths: 473/510 nm for EGFP and 559/583 nm for mRFP1. The scanned fish was immediately and carefully taken from the agarose, transferred into E3 buffer in a six-well plate, and illuminated by a blue LED light by placing the plate on a LED panel at 28 °C (Figure 2A,B). After a 24-h illumination, the fish was anesthetized and embedded again in agarose before being scanned with a confocal microscopy using the same imaging conditions, except that the ROI was adjusted to include the corresponding spinal cord region imaged at 48 hpf (Figure 2B). The whole hemispinal cord of 4-5 contiguous spinal segments was imaged during the microscopy session (typically 20-30 min), generating z-series images containing 20-40 slices depending on the horizontalness of the longitudinal axis of the spinal cord of the mounted fish relative to the confocal scanning plane.
Visualization of cytoplasmic relocation of optogenetic TDP-43 in spinal motor neurons
To visualize the cytoplasmic relocation of opTDP-43h in single spinal motor neurons, the z-series images (typically 20-40 slices) acquired at 48 and 72 hpf were opened with Fiji and separated into each EGFP-TDP-43z and opTDP-43h channel. Max intensity projection images of EGFP-TDP-43z were created for images at 48 and 72 hpf and a single isolated spinal neuron identifiable in both images at 48 and 72 hpf was selected (Figure 2B, arrows). Spinal motor neurons with cell bodies located on the dorsal side of the motor column were considered suitable for measurements of the cell body shape because of their sparse distribution patterns.
After adjusting the intensity of the EGFP signal in EGFP-TDP-43z images, ROIs covering the somas of the motor neurons were set by tracing the edge of the EGFP signal at 48 and 72 hpf (Figure 2C). The fluorescent intensity along the major axis of the soma was measured for the EGFP-TDP-43z and opTDP-43h signals at 48 and 72 hpf (Figure 2C, right). At 48 hpf, the patterns of opTDP-43h and EGFP-TDP-43z signals largely overlapped each other.
In contrast, at 72 hpf, the peak of the opTDP-43h signal was found in the region where the EGFP-TDP-43z signal was low, namely in the cytoplasm, indicating the cytoplasmic relocation of opTDP-43h. The light-dependent cytoplasmic opTDP-43h relocation is initiated largely independently of non-optogenetic TDP-4314. In this assay, Max intensity projection images were used to estimate the position of the nucleus/cytoplasm boundary at the expense of quantitative measurements.
Ratiometric comparison of the fluorescence intensity of optogenetic and non-optogenetic TDP-43 in the spinal motor neurons
To evaluate the effects of the blue light stimulation on the fluctuating opTDP-43h protein levels, opTDP-43h signals in the cell body were measured before and after the light stimulation with reference to the EGFP-TDP-43z signal. The z-series images, including the spinal hemisegment, were opened with Fiji. To set ROIs covering single spinal motor neurons, Max intensity projection images of the EGFP-TDP-43z signal were created for 48 and 72 hpf, and single isolated spinal neurons that were identifiable in both 48 and 72 hpf images were selected (Figure 3A). Using freehand selections, ROIs were drawn and registered in a ROI manager for each of the 48 and 72 hpf images (Figure 3A). Max intensity projection of the EGFP-TDP-43z signal was considered suitable to set the ROIs because of its high contrast.
To quantify the opTDP-43h and EGFP-TDP-43z signals, Sum slices projection images for each channel of the same z-series images were produced for 48 and 72 hpf (Figure 3A). Using the registered ROI sets, the fluorescent intensities of opTDP-43h and EGFP-TDP-43z were measured for each time point. Relative amounts of opTDP-43h to EGFP-TDP-43z (RFP/GFP values) were calculated for each cell and time point by dividing the RFP value by the GFP value (Figure 3B). Of the four mnr2b-positive cells that were investigated, cell #1 notably displayed a saturated EGFP-TDP-43z signal (Figure 3A). Cells with saturated fluorescent signals should be excluded from the data sets when conducting quantitative analyses. Cells #2-#4 displayed decreasing trends of relative opTDP-43h levels to EGFP-TDP-43z after blue light illumination (Figure 3B), although a larger-scale analysis previously demonstrated that the decrease in relative opTDP-43h levels to EGFP-TDP-43z was not statistically significant (65 cells from three independent animals)14.
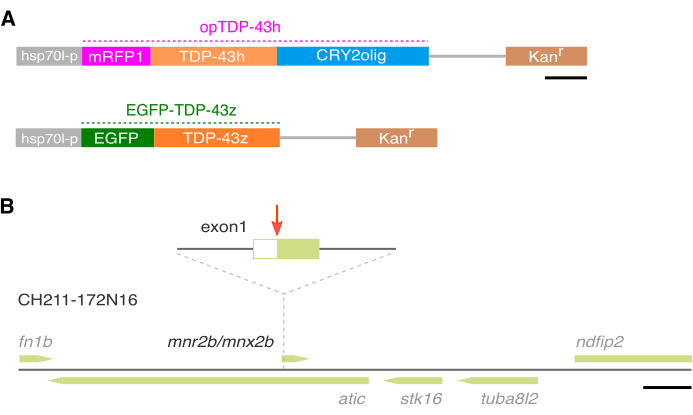
Figure 1: Construction of BAC DNA using the mnr2b locus. (A) Structures of the expression cassettes for opTDP-43h and EGFP-TDP-43z. (B) The zebrafish genomic region carried by the CH211-172N16 BAC DNA. The PCR-amplified expression cassettes for opTDP-43h and EGFP-TDP-43z are inserted downstream of the 5′-untranslated region (UTR) of the mnr2b (red arrow) in the first exon of mnr2b by homologous recombination. The bars indicate 500 (A) and 10k (B) bp. Please click here to view a larger version of this figure.
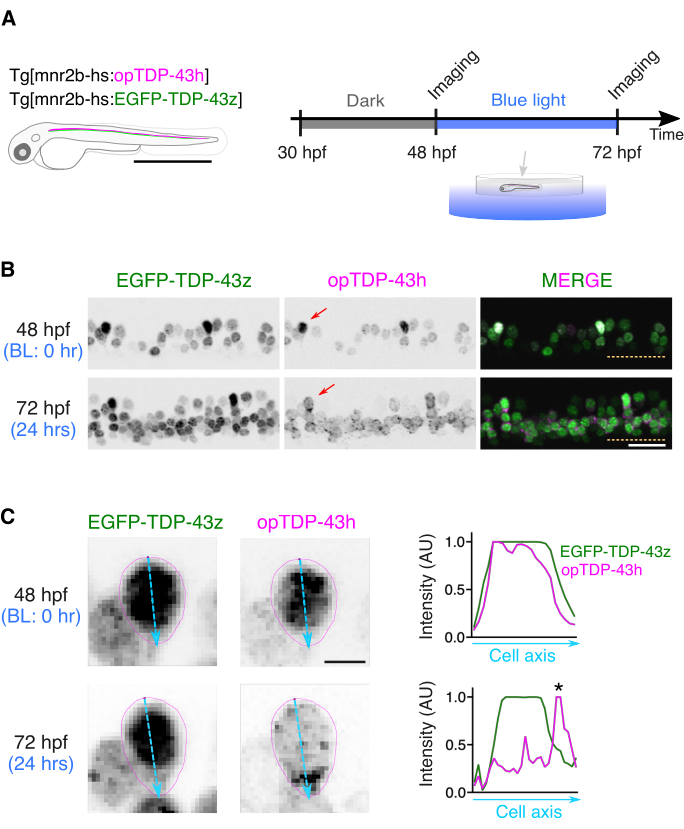
Figure 2: Live imaging of opTDP-43h before and after light stimulation. (A) A scheme of the light stimulation of opTDP-43h expressed in the spinal motor neurons of unrestrained Tg[mnr2b-hs:opTDP-43h] Tg[mnr2b-hs:EGFP-TDP-43z] double-transgenic fish through a field illumination of blue LED light from 48 to 72 hpf. (B) Max intensity projection images of the z-series confocal images of the ventral spinal cord before (48 hpf) and after (72 hpf) the light stimulation. The dashed lines demarcate the ventral limit of the spinal cord. Figures are adapted from Asakawa et al.14 (C) Cytoplasmic mislocalization of opTDP-43 after the 24-h blue light illumination. The contours of the soma of an mnr2b-positive cell (red arrows in B) observed at 48 and 72 hpf are shown in magenta. The major axes of the somas were shown with light blue. The normalized fluorescent intensity along the major axes was plotted. The asterisk indicates a cluster of strong opTDP-43 signals that do not display a strong EGFP-TDP-43z overlapping signal, indicating cytoplasmic opTDP-43h mislocalization. The bars indicate 1 mm (A), 20 µm (B), and 4 µm (C). Please click here to view a larger version of this figure.
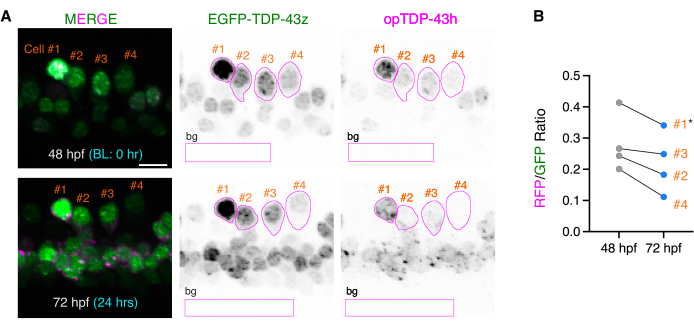
Figure 3: Ratiometric comparisons of opTDP-43h and EGFP-TDP-43z before and after light stimulation. (A) ROIs covering the somas of four single mnr2b-positive cells at 48 and 72 hpf were drawn based on the EGFP-TDP-43z signal and are shown in magenta. The rectangular ROIs (bg) were used to subtract the background signal (background ROI). Figures are adapted from Asakawa et al.14 (B) The relative intensities of opTDP-43h to EGFP-TDP-43z were plotted for each cell at each time point as the RFP/GFP ratio. Cell #1, indicated by the asterisk, was not suitable for the ratiometric comparison because its EGFP-TDP-43z signal was saturated and its RFP/GFP value was overestimated. The bar indicates 10 µm. Please click here to view a larger version of this figure.
Discussion
The mnr2b-BAC-mediated expression of opTDP-43h and EGFP-TDP-43z in zebrafish provides a unique opportunity for live imaging of TDP-43 phase transition in the spinal motor neurons. The optical transparency of body tissues of zebrafish larvae allows for the simple and noninvasive optogenetic stimulation of opTDP-43h. Comparisons between single spinal motor neurons over time demonstrated that the light-dependent oligomerization of opTDP-43h causes its cytoplasmic clustering, which is reminiscent of ALS pathology.
One of the critical parameters that define the phase behavior of an intrinsically disordered protein is intracellular concentration. A high level of opTDP-43h expression could potentially lead to light-independent opTDP-43h oligomerization, phase transition, and aggregation. Thus, the induction of a stable, nontoxic level of opTDP-43h expression in the spinal motor neurons is key to the successful evaluation of the physiological and pathological consequences of opTDP-43 phase transition on the spinal motor neurons. The mnr2b-BAC-mediated expression of opTDP-43 h appears to be suitable for this purpose because Tg[mnr2b-hs:opTDP-43h] fish display predominantly nuclear opTDP-43, they are capable of free swimming with an inflated swim bladder and they maintain full viability even after being raised under the continuous dark conditions from 1-5 dpf. In zebrafish, a conventional approach used to exogenously express a gene of interest is mRNA injection at the one-cell stage. However, a high dose of the TDP-43 protein translated from the injected mRNA all at once causes early developmental defects14 that preclude analyses of later differentiating spinal motor neurons. However, lowering the amount of injected mRNA to a tolerable level fails to supply sufficient opTDP-43h for optogenetic modulation in the spinal motor neurons by the time of its differentiation, indicating that mRNA injection is not a suitable method by which to deliver opTDP-43h to the spinal motor neurons of zebrafish. Another possible approach used to express opTDP-43h in the spinal motor neurons is injecting, at the one-cell stage, a plasmid or BAC DNA harboring the opTDP-43h construct under the control of a promoter that is active in the spinal motor neuron. Although the injection of mnr2b-hs:opTDP-43h BAC DNA can direct the expression of opTDP-43h in the spinal motor neurons, the number of opTDP-43h-positive motor neurons is low, and in such numerically restricted opTDP-43h-positive cells, the expression level is usually high, often associated with light-independent opTDP-43h aggregation. These observations collectively suggest that transgenic expression is an irreplaceable strategy by which to stably express opTDP-43h in the spinal motor neurons at a nontoxic level. Notably, although the mnr2b-BAC labels almost all spinal motor neurons in zebrafish20,23, the expression levels of opTDP-43h could vary among individual mnr2b-positive cells. This variation may be partly due to the intrinsic expression pattern of mnr2b associated with its regulatory role in motor neuron differentiation. Another potential cause for the variegated expression may result from a chromosomal position effect on the mnr2b-BAC inserts. Whatever the cause, the varied opTDP-43 expression levels among mnr2b-positive cells could cause variation in cytotoxicity associated with light-dependent opTDP-43h phase transition.
In zebrafish larvae, the somas of spinal motor neurons are densely aligned in the ventral spinal cord, and the somal cytoplasm has a much lower volume than that of the axonal cytoplasm. This anatomical feature limits quantitative analyses of cytoplasmic opTDP-43h in the spinal motor neurons: opTDP-43h is only quantitatively measurable in the nucleus and somal cytoplasm, but not in the entire cell of motor neurons. Quantitative imaging of a protein in the whole cell of spinal motor neurons, including the soma and the peripheral nerve terminal, remains a challenging task. Despite the restrictions on quantitative assays, the opTDP-43h/EGFP-TDP-43z ratio in the soma was shown to be elevated by the ALS-causing mutation in the IDR (A315T)14. Therefore, the present method is applicable to evaluating the effects of the TDP-43 mutation on protein stability associated with phase transition in the soma of spinal motor neurons.
In principle, the mnr2b-BAC approach can be extended to modulate the LLPS and evaluate the cytotoxicity of other ALS-related RNP proteins with IDRs beyond TDP-43. Several protocol steps may need to be adjusted to obtain an optimal expression level of such optogenetic probes in the spinal motor neurons. First, in the Tg[mnr2b-hs:opTDP-43h] transgenic construct, the hsp70l promoter was used as a basal promoter to boost opTDP-43h expression driven by mnr2b enhancers. The hsp70l promoter may be removed from the BAC construct if the expression level of a protein of interest is so high that it causes light-independent ectopic phase transition. Second, opTDP-43h is equipped with CRY2olig tag, which is a photolyase homology (PHR) domain carrying a point mutation enhancing the clustering capacity on blue light illumination22. The wild-type protein CRY2PHR may be used as a light-dependent oligomerization tag if there is a need to attenuate light-dependent clustering activity. Finally, to more faithfully recapitulate ALS pathology in zebrafish, it is desirable to establish an illumination protocol where fish physiology is minimally affected by the field illumination of blue light during the juvenile and adult stages. The present method uses a continuous blue light illumination from 48 to 72 hpf (for 24 h), and the illumination duration can be extended until 120 hpf (for 72 h) without losing fish viability14, although light-responsive physiological functions, such as vision, may be affected. By developing a protocol for intermittent light illumination, much longer-term light stimulation may become possible, which may in turn facilitate the development of opTDP-43h into more mature pathological aggregates. To achieve this, other optogenetic probes for modulating protein-protein interactions using different light wavelengths that are less physiologically disturbing24 may also be worth investigating further. Combinations of such improved optogenetic TDP-43 probes, appropriate promoters targeting disease-vulnerable cell types, and illumination protocols with minimal effects on physiological functions would open avenues for faithfully modeling the pathologies of TDP-43 proteinopathies not only in the spinal cord but also in the brain.
開示
The authors have nothing to disclose.
Acknowledgements
This work was supported by SERIKA FUND (KA), KAKENHI Grant numbers JP19K06933 (KA) and JP20H05345 (KA).
Materials
| Confocal microscope | Olympus | FV1200 | |
| Epifluorescence microscope | ZEISS | Axioimager Z1 | |
| Fluorescence stereomicroscope | Leica | MZ16FA | |
| Glass base dish | IWAKI | 3910-035 | |
| Incubator | MEE | CN-25C | |
| LED panel | Nanoleaf Limited | Nanoleaf AURORA smarter kit | |
| Mupid-2plus | TAKARA | AD110 | |
| NucleoBond BAC100 | MACHEREY-NAGEL | 740579 | |
| NuSieve GTG Agarose | LONZA | 50181 | |
| Objective lens | Olympus | XLUMPlanFL N 20×/1.00 | |
| Objective lens | ZEISS | Plan-Neofluar 5x/0.15 | |
| Optical power meter | HIOKI | 3664 | |
| Optical sensor | HIOKI | 9742-10 | |
| Phenol red solution 0.5% | Merck | P0290-100ML | |
| PrimeSTAR GXL DNA Polymerase | TAKARA | R050A | |
| QIAquick Gel Extraction Kit | Qiagen | 28704 | |
| Six-well dish | FALCON | 353046 | |
| Spectrometer probe BLUE-Wave | StellerNet Inc. | VIS-50 | |
| Syringe needle | TERUMO | NN-2725R | |
| TaKaRa Ex Taq | TAKARA | RR001A | |
| Tricane | Sigma-Aldrich | A5040 | |
| Zebrafish BAC clone CH211-172N16 | BACPAC Genomics | CH211-172N16 |
参考文献
- Brangwynne, C. P. Phase transitions and size scaling of membrane-less organelles. Journal of Cell Biology. 203 (6), 875-881 (2013).
- Hyman, A. A., Weber, C. A., Julicher, F. Liquid-liquid phase separation in biology. Annual Review of Cell and Developmental Biology. 30, 39-58 (2014).
- Lennox, A. L., et al. Pathogenic DDX3X mutations impair rna metabolism and neurogenesis during fetal cortical development. Neuron. 106 (3), 404-420 (2020).
- Nedelsky, N. B., Taylor, J. P. Bridging biophysics and neurology: aberrant phase transitions in neurodegenerative disease. Nature Reviews Neurology. 15 (5), 272-286 (2019).
- Ramaswami, M., Taylor, J. P., Parker, R. Altered ribostasis: RNA-protein granules in degenerative disorders. Cell. 154 (4), 727-736 (2013).
- Nguyen, H. P., Van Broeckhoven, C., vander Zee, J. ALS genes in the genomic era and their implications for FTD. Trends in Genetics. 34 (6), 404-423 (2018).
- Pakravan, D., Orlando, G., Bercier, V., Van Den Bosch, L. Role and therapeutic potential of liquid-liquid phase separation in amyotrophic lateral sclerosis. Journal of Molecular Cell Biology. 13 (1), 15-28 (2021).
- Santamaria, N., Alhothali, M., Alfonso, M. H., Breydo, L., Uversky, V. N. Intrinsic disorder in proteins involved in amyotrophic lateral sclerosis. Cellular and Molecular Life Sciences. 74 (7), 1297-1318 (2017).
- Lagier-Tourenne, C., Cleveland, D. W. Rethinking ALS: the FUS about TDP-43. Cell. 136 (6), 1001-1004 (2009).
- Asakawa, K., Handa, H., Kawakami, K. Multi-phaseted problems of TDP-43 in selective neuronal vulnerability in ALS. Cellular and Molecular Life Sciences. 78 (10), 4453-4465 (2021).
- Shin, Y., et al. Spatiotemporal control of intracellular phase transitions using light-activated optodroplets. Cell. 168 (1-2), 159-171 (2017).
- Zhang, P., et al. Chronic optogenetic induction of stress granules is cytotoxic and reveals the evolution of ALS-FTD pathology. Elife. 8, 39578 (2019).
- Mann, J. R., et al. RNA Binding Antagonizes Neurotoxic Phase Transitions of TDP-43. Neuron. 102 (2), 321-338 (2019).
- Asakawa, K., Handa, H., Kawakami, K. Optogenetic modulation of TDP-43 oligomerization accelerates ALS-related pathologies in the spinal motor neurons. Nature Communications. 11 (1), 1004 (2020).
- Otte, C. G., et al. Optogenetic TDP-43 nucleation induces persistent insoluble species and progressive motor dysfunction in vivo. Neurobiology of Disease. 146, 105078 (2020).
- Wendik, B., Maier, E., Meyer, D. Zebrafish mnx genes in endocrine and exocrine pancreas formation. 発生生物学. 268 (2), 372-383 (2004).
- Seredick, S. D., Van Ryswyk, L., Hutchinson, S. A., Eisen, J. S. Zebrafish Mnx proteins specify one motoneuron subtype and suppress acquisition of interneuron characteristics. Neural Development. 7, 35 (2012).
- Warming, S., Costantino, N., Court, D. L., Jenkins, N. A., Copeland, N. G. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Research. 33 (4), 36 (2005).
- Suster, M. L., Abe, G., Schouw, A., Kawakami, K. Transposon-mediated BAC transgenesis in zebrafish. Nature Protocols. 6 (12), 1998-2021 (2011).
- Asakawa, K., Abe, G., Kawakami, K. Cellular dissection of the spinal cord motor column by BAC transgenesis and gene trapping in zebrafish. Frontiers in Neural Circuits. 7, 100 (2013).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2012).
- Taslimi, A., et al. An optimized optogenetic clustering tool for probing protein interaction and function. Nature Communications. 5, 4925 (2014).
- Asakawa, K., Kawakami, K. Protocadherin-mediated cell repulsion controls the central topography and efferent projections of the abducens nucleus. Cell Reports. 24 (6), 1562-1572 (2018).
- Redchuk, T. A., et al. Optogenetic regulation of endogenous proteins. Nature Communications. 11 (1), 605 (2020).

