Preparation of 6-aminocyclohepta-2,4-dien-1-one Derivatives via Tricarbonyl(tropone)iron
概要
Representative experimental procedures for the addition of amine nucleophiles to tricarbonyl(tropone)iron and subsequent demetallation of the resulting complexes are presented in detail.
Abstract
aza-Michael adducts of tricarbonyl(tropone)iron are synthesized by two different methods. Primary aliphatic amines and cyclic secondary amines participate in a direct aza-Michael reaction with tricarbonyl(tropone)iron under solvent-free conditions. Less nucleophilic aniline derivatives and more hindered secondary amines add efficiently to the cationic tropone complex formed by protonation of tricarbonyl(tropone)iron. While the protocol utilizing the cationic complex is less efficient overall for accessing the aza-Michael adducts than the direct, solvent-free addition to the neutral complex, it allows the use of a broader range of amine nucleophiles. Following protection of the amine of the aza-Michael adduct as a tert-butyl carbamate, the diene is decomplexed from the iron tricarbonyl fragment upon treatment with cerium(IV) ammonium nitrate to provide derivatives of 6-aminocyclohepta-2,4-dien-1-one. These products can serve as precursors to diverse compounds containing a seven-membered carbocyclic ring. Because the demetallation requires protection of the amine as a carbamate, the aza-Michael adducts of secondary amines cannot be decomplexed using the protocol described here.
Introduction
Structurally complex amines containing a seven-membered carbocyclic ring are common to a number of biologically active molecules. Notable examples include the tropane alkaloids1 and several members of the Lycopodium2, Daphniphyllum3, and monoterpenoid indole alkaloid4 families. However, such compounds are often more difficult to synthesize compared to compounds of similar complexity containing only five- or six-membered rings. Thus, we sought to develop a new avenue towards such compounds by attaching diverse amine nucleophiles to tropone5. The resulting adduct contains several functional handles for subsequent synthetic elaboration to diverse complex seven-membered ring-containing scaffolds that would be otherwise difficult to access.
While previous work with tropone6,7 suggests that it would not be suitable for such a transformation, the related organometallic complex tricarbonyl(tropone)iron8 (1, Figure 1) has proven to be a versatile synthetic building block that has been utilized in the synthesis of a number of natural products and complex molecules9,10,11,12,13. Furthermore, the uncomplexed double bond of tricarbonyl(tropone)iron has been shown to behave similar to an α,β-unsaturated ketone in reactions with, for example, dienes14,15, tetrazines16, nitrile oxides17, diazoalkanes8,10, and organocopper reagents11. Thus, we envisioned that an aza-Michael reaction of tricarbonyl(tropone)iron would provide an efficient entry to synthetically valuable aminated tropone derivatives.
Eisenstadt had previously reported that, following protonation of tricarbonyl(tropone)iron, the resulting cationic complex 2 (Figure 1) could undergo nucleophilic attack by aniline or tert-butylamine to produce aminated derivatives of the tropone iron complex.18 However, the synthetic potential of this method remains unrealized. Indeed, no additions of other amines had been reported, and the demetallation of those products was not explored in Eisenstadt’s report. We have adapted this protocol to demonstrate the addition of a wide variety of amine nucleophiles.
We also describe a method for direct aza-Michael additions to tricarbonyl(tropone)iron (Figure 2), which does not require synthesis of the cationic complex and generally proceeds in higher yields compared to the previously reported method. We also report herein a protocol for the demetallation of the resulting adducts. Overall, this protocol provides formal aza-Michael adducts of tropone in four steps from tropone (and three steps from the known iron complex).
Protocol
1. Synthesis of tricarbonyl(tropone)iron (1)19
- In an argon-atmosphere glovebox, weigh out 4.1 g of diiron nonacarbonyl into an oven-dried 20 mL vial. Cap the vial and remove it from the glovebox.
CAUTION: Prolonged storage of diiron nonacarbonyl leads to some deterioration to give triiron dodecacarbonyl and finely divided metallic iron20. This deterioration is evidenced by the presence of a black solid within the shiny orange diiron nonacarbonyl. The iron impurity is pyrophoric and can ignite upon exposure to air. Storing the diiron nonacarbonyl under argon at 2-8 °C in a bottle sealed with electrical tape appears to minimize this deterioration. The pyrophoric iron impurities can be destroyed via addition of dilute hydrochloric acid. - Add an oven-dried PTFE stir bar, 0.5 mL of tropone, and 10 mL of dry benzene to an oven-dried 50 mL round bottom flask.
NOTE: A round bottom flask with a 24/40 ground glass joint is preferred so that the solid diiron nonacarbonyl may be added rapidly with minimal spilling (see Step 1.5). - Degas the contents of the round bottom flask via three freeze-pump-thaw cycles as follows.
- Submerge the flask in a dry ice-acetone bath until the contents solidify completely. Then, with the flask still submerged in the cold bath, evacuate the flask under vacuum for 2-3 min.
- Allow the contents to thaw under static vacuum.
- Repeat steps 1.3.1 and 1.3.2 twice.
- After the final thaw, backfill the flask with argon and cover the flask with a rubber septum. Keep the flask under a positive pressure of argon.
- Cover the flask with aluminum foil and commence vigorous magnetic stirring.
- Briefly remove the rubber septum and add the previously weighed diiron nonacarbonyl in a single portion and replace the septum.
- Immerse the flask in an oil bath at 55-60 °C and stir for 30 min.
- After 30 min, remove the flask from the oil bath and allow to cool to room temperature.
- Isolate the tropone complex via alumina column chromatography as follows.
- Pack a chromatography column (~30 mm diameter) with 12 cm of alumina (Activity II/III) and hexanes.
- Pipette the crude reaction mixture directly onto the alumina. Rinse the flask with a small amount (1-3 mL) of hexanes and add to the top of the column.
- Drain the column until the solvent is level with the top of the alumina and add ~2 cm of sand.
- Elute with hexanes until the blue-green band (triiron dodecacarbonyl) comes off the column.
- Elute with 1:1 hexanes:methylene chloride until the red-orange tropone iron complex elutes completely.
- Remove the solvent from the red-orange solution via rotary evaporation to obtain the tropone complex as a dark red oil that solidifies on standing.
NOTE: The tropone complex isolated in this fashion is occasionally contaminated with paramagnetic, iron-based impurities, as evidenced by severely broadened peaks in the 1H NMR spectrum. These impurities can be removed by redissolving the complex in methylene chloride and passing through a short plug of alumina, eluting with 1:1 hexanes:methylene chloride.
2. Synthesis of tricarbonyl(5-ketocycloheptadienyl)iron tetrafluoroborate (2)21
- Add a PTFE magnetic stir bar, 432 mg of tricarbonyl(tropone)iron, and 10 mL of methylene chloride to a 50 mL round bottom flask.
- Cool the flask in an ice bath and commence vigorous magnetic stirring.
- Add 3.2 mL of concentrated sulfuric acid dropwise.
- Vigorously stir the mixture at 0 °C for 30 min.
- To a separate 100 mL round bottom flask, add a PTFE stir bar, 2.0 g of anhydrous sodium carbonate, and 10 mL of methanol.
- Cool the flask containing the sodium carbonate mixture in an ice bath and vigorously stir it magnetically.
- Upon completion of the 30-min period (step 2.4), cease magnetic stirring. Two layers should form.
- Using a Pasteur pipette, transfer the viscous, brown lower layer to the rapidly stirring sodium carbonate suspension.
- Stir for ~5 min, and then carefully and slowly add 50 mL of deionized water.
CAUTION: Vigorous bubbling is involved in this step. - Pour the mixture into a 250 mL separatory funnel and extract with methylene chloride (2x 50 mL).
- Sequentially wash the combined organic layers with water (50 mL) and brine (50 mL).
- Dry the organic layers over anhydrous magnesium sulfate.
- Remove the magnesium sulfate via gravity or vacuum filtration and concentrate the filtrate via rotary evaporation to obtain a red-brown oil.
NOTE: The protocol may be paused at this point. - Add 3 mL of acetic anhydride to a 25 mL Erlenmeyer flask and cool it in an ice bath.
- Add 1 mL of 48% aqueous tetrafluoroboric acid to the cold acetic anhydride dropwise.
CAUTION: The addition is highly exothermic. However, the exotherm is readily contained by controlling the temperature and rate of addition. - In a 100 mL round bottom flask submerged in an ice bath, add the mixture obtained from step 2.15 to the oil obtained in step 2.13.
- Agitate the mixture with a stainless steel spatula for 5 min.
NOTE: The mixture generally takes on a gummy consistency upon agitation and the color becomes lighter. - Add 50 mL of diethyl ether to the mixture. Collect the resulting pale yellow solid via vacuum filtration using a Buchner funnel to obtain the cationic complex as its tetrafluoroborate salt.
3. Synthesis of aza-Michael adduct 4: Tricarbonyl[(2-5-h)-6-((2-phenylethyl)amino)cyclohepta-2,4-dien-1-one]iron
- Add a PTFE magnetic stir bar, 150 mg of tricarbonyl(tropone)iron (1), and 0.154 mL of phenethylamine to a 1-dram vial. Cap the vial under an air atmosphere and commence magnetic stirring.
NOTE: Phenethylamine will be oxidized by air upon prolonged storage resulting in a yellow-brown color. Phenethylamine should be distilled prior to use if it is not colorless. - Monitor the reaction periodically by removing a small (~1 drop) aliquot from the reaction mixture, dissolving in CDCl3, and acquiring a 1H NMR spectrum.
NOTE: While this particular reaction is usually complete within 1 h, the reaction may be left to stir overnight. - Upon disappearance of the signals for tricarbonyl(tropone)iron in the 1H NMR spectrum (see Representative Results and Figure 3 and Figure 4), purify the crude reaction mixture via chromatography on basic alumina (Activity II/III) as follows.
- Pack a 30 mm diameter chromatography column with alumina (10-15 cm) and hexanes and apply the crude reaction mixture to the top of the column.
- Elute the column with 1:1 hexanes:diethyl either to remove the excess phenethylamine from the column. Monitor the elution via thin layer chromatography (TLC).
NOTE: The column was monitored using alumina TLC plates and a 1:1 diethyl ether:methylene chloride mixture as the mobile phase. If alumina TLC plates are not available, silica gel plates may be used (use 5% methanol in methylene chloride as the mobile phase). - After the excess amine has finished eluting, change the eluting solvent to 1:1 diethyl ether:methylene chloride to elute the product.
NOTE: The title compound elutes as a yellow band. - Combine the product-containing fractions (as judged by thin layer chromatography) and remove the solvent on a rotary evaporator to obtain the purified product as a dark yellow oil.
4. Synthesis of tricarbonyl[(2-5-h)-6-(2-methylanilino)cyclohepta-2,4-dien-1-one]iron (3)
- Add a PTFE stir bar, 0.021 mL of o-toluidine, and 1.0 mL of diethyl ether to a 1-dram vial. Commence vigorous magnetic stirring.
- Carefully add 33 mg of the cationic complex to the mixture. Allow the suspension to stir for 12 h.
- Pour the reaction mixture into 5 mL of deionized water in a separatory funnel and extract the aqueous layter with 5 mL of ethyl acetate three times.
- Wash the combined organic layers with 10 mL of brine before drying over anhydrous sodium sulfate.
- Remove the sodium sulfate by gravity filtration and concentrate the filtrate via rotary evaporation to obtain the crude product.
- Purify the crude product via column chromatography on basic alumina using a gradient of 30-50% diethyl ether in hexanes to obtain the pure product as a yellow solid.
5. Protection of amine 4 as a tert-butyl carbamate
- Dissolve 76 mg of amine 4 in 2 mL of absolute ethanol in a 25 mL round bottom flask under air atmosphere.
- Add 104 mg of di-tert-butyl dicarbonate followed by 40 mg of solid sodium bicarbonate to the reaction mixture.
- Cap the flask with a rubber septum and sonicate the mixture for 1 h.
NOTE: This reaction may be allowed to run overnight. - Filter the crude reaction mixture through a bed of diatomaceous earth using a Buchner funnel. Wash the diatomaceous earth with ethanol until no more brown-colored solution comes out the bottom of the funnel.
- Transfer the filtrate to a round bottom flask and concentrate on a rotary evaporator. Dissolve the resulting oil in ~2.5 mL of methylene chloride.
- Add ~1.3 g of silica gel to the solution and remove the methylene chloride on the rotary evaporator until a fine, free-flowing solid is obtained.
- Pack the silica gel into a 10 g silica cartridge for automated flash chromatography.
NOTE: An automated purification system was used in this protocol. However, conventional flash chromatography with silica gel may also be employed. - Run the column using a gradient starting from 90:10 hexanes:ethylacetate and ending at 20:80 hexanes:ethyl acetate over a period of 20 min. Collect the fractions containing the product (as indicated by the major peak detected at 254 nm absorbance) in a round bottom flask. Evaporate the hexanes and ethyl acetate on a rotary evaporator to obtain the purified product as a yellow oil.
6. Synthesis of tert-butyl (6-oxocyclohepta-2,4-dien-1-yl)(2-phenylethyl) carbamate (6)
- In a 10 mL round bottom flask, dissolve 27 mg of iron complex 5 in 1 mL of methanol under air atmosphere and immerse the flask in an ice bath.
- Commence magnetic stirring and add 33 mg of cerium(IV) ammonium nitrate.
- After 30 min, add a second 33 mg portion of cerium(IV) ammonium nitrate, followed by a third 33 mg portion after an additional 30 min of stirring.
- After adding the third portion of cerium(IV) ammonium nitrate, dilute the reaction mixture with ethyl acetate (5 mL).
- Pour the mixture into a 30 mL separatory funnel containing 5 mL of saturated aqueous sodium bicarbonate. Separate the layers.
- Re-extract the aqueous layer with ethyl acetate (2x 5 mL). Dry the combined organic layers over anhydrous sodium sulfate.
- Remove the sodium sulfate via gravity or vacuum filtration and concentrate the filtrate on a rotary evaporator.
- Dissolve the crude product in ~2.5 mL of methylene chloride, add ~1.3 g of silica gel, and remove the solvent on a rotary evaporator.
- Pack the silica gel with the adsorbed crude product into a 10 g silica gel column for automated flash chromatography.
NOTE: An automated purification system was used in this protocol. However, conventional flash chromatography with silica gel may also be employed. - Run the column using a gradient starting from 90:10 hexanes:ethylacetate and ending at 20:80 hexanes:ethyl acetate over a period of 20 min. Collect the fractions containing the product (as indicated by the major peak detected at 254 nm absorbance) in a round bottom flask. Evaporate the hexanes and ethyl acetate on a rotary evaporator to obtain the purified product as a pale brown oil.
Representative Results
All novel compounds in this study were characterized by 1H and 13C NMR spectroscopy and high resolution mass spectrometry. Previously reported compounds were characterized by 1H NMR spectroscopy. NMR data for representative compounds are described in this section.
The 1H NMR spectrum of tricarbonyl(tropone)iron is shown in Figure 3. The protons of the η4-diene ligand give rise to the signals at 6.39 ppm (2 H), 3.19 ppm, and 2.75 ppm. The protons from the uncomplexed double bond appear at 6.58 and 5.05 ppm.
The progress of the aza-Michael addition is monitored via 1H NMR by observing the disappearance of the signals from the uncomplexed double bond and a characteristic change in the chemical shift of the two furthest downfield η4-diene protons from around 6.4 ppm to two well separated signals that typically appear between 5.3 and 6.0 ppm (see Figure 3 and Figure 4). Furthermore, the aza-Michael adduct features signals corresponding to the two diastereotopic methylene protons (adjacent to the ketone within the seven-membered ring), which typically appear between 1.5 and 2.5 ppm.
Direct aza-Michael additions to tricarbonyl(tropone)iron generally proceeded in 60-95% yield, depending on the amine substrate (see Discussion). Secondary cyclic amines tend to give somewhat higher yields than primary aliphatic amines, possibly due to a greater resistance to decomposition during purification.
1H NMR data for the cationic complex (in CD3CN) is shown in Figure 5 and features seven distinct multiplets. It should be noted that the complex decomposes over time in CD3CN. However, the dried solid tetrafluoroborate complex can be stored indefinitely under ambient conditions. Figure 6 shows 1H and 13C NMR data for the o-toluidine adduct 3, prepared via the cationic complex 2 (Figure 1), which contains the same features described above for the phenethylamine adduct 4.
Figure 7 shows 1H and 13C NMR spectra of tert-butyl carbamate 5. The 1H NMR spectrum is characterized by its broad peaks, caused by slow rotation of the carbamate C-N bond relative to the NMR time scale. In addition, the presence of the tert-butyl carbamate is evident from the large singlet at 1.5 ppm from the tert-butyl protons, as well as the signal at 154.3 ppm in the 13C NMR spectrum corresponding to the carbonyl carbon of the carbamate group.
Upon decomplexation of the diene from the iron, the most notable aspect of the 1H NMR spectrum (Figure 8) is the presence of four signals between 5.75 and 6.75 ppm, corresponding to the protons from the uncomplexed diene.
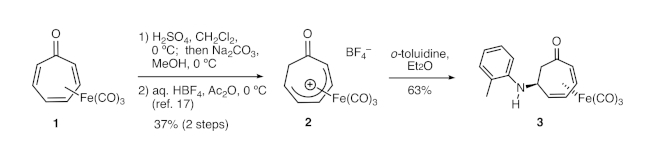
Figure 1. Synthesis of 3 from tricarbonyl(tropone)iron via cationic complex 2. Tricarbonyl(tropone)iron is converted to cationic complex 2 in two steps, which was followed by nucleophilic addition of ortho-toluidine to the complex. Please click here to view a larger version of this figure.
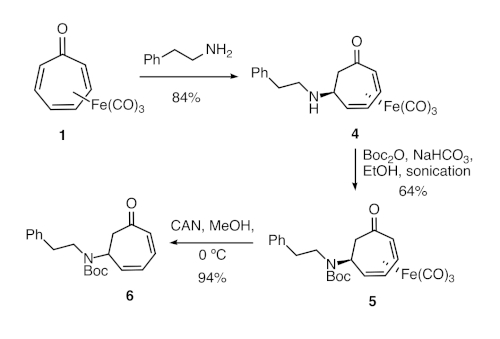
Figure 2. Synthesis of formal tropone aza-Michael adduct 6. Direct aza-Michael reaction of tricarbonyl(tropone)iron and phenethylamine was followed by amine protection and oxidative demetallation. Please click here to view a larger version of this figure.
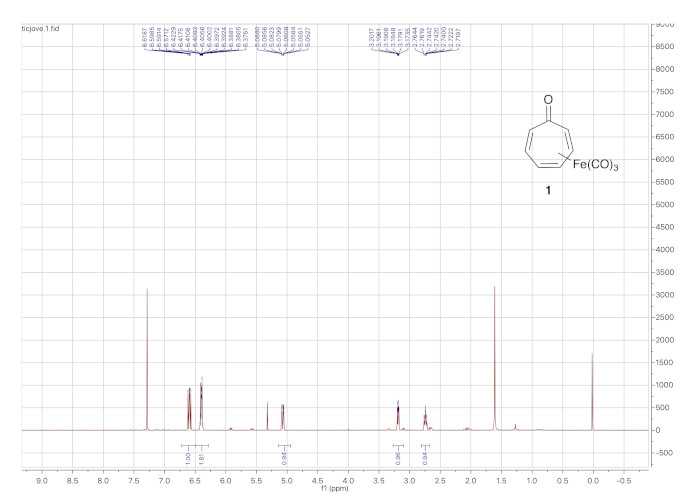
Figure 3. 1H NMR spectrum (solvent: CDCl3) of tricarbonyl(tropone)iron 1. The peaks at 6.59 ppm and 5.05 ppm correspond to the uncomplexed alkene hydrogens while those 6.39 ppm (2H), 3.19 ppm, and 2.75 ppm arise from the iron-complexed diene. Please click here to view a larger version of this figure.
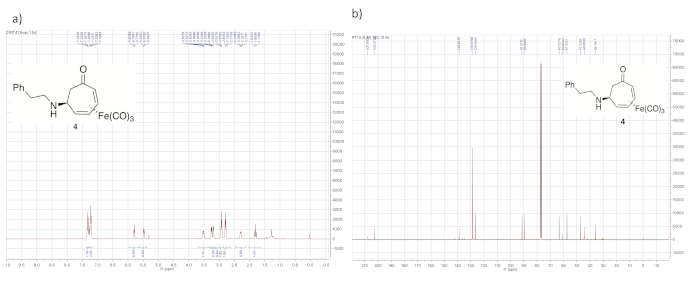
Figure 4. Spectral data for iron complex 4. (a) 1H NMR spectrum; (b) 13C NMR spectrum (solvent: CDCl3). Notable peaks in the 1H NMR spectrum include those from the iron-complexed diene (5.75, 5.48, 3.30, and 3.20 ppm) and the diastereotopic α-methylene protons (2.30 and 1.70 ppm). Please click here to view a larger version of this figure.
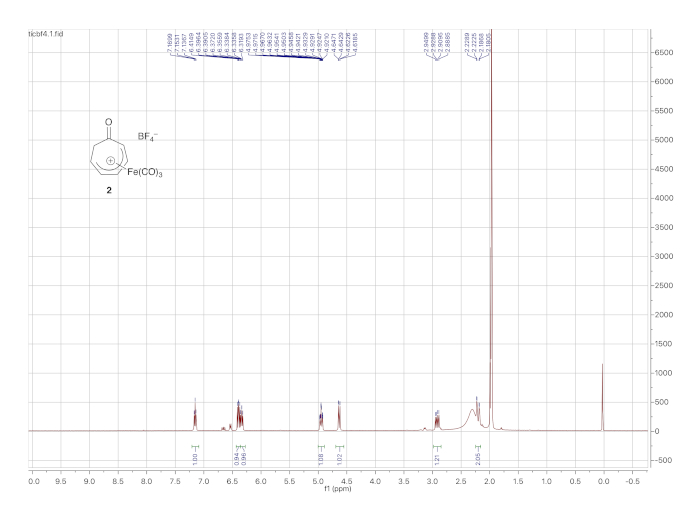
Figure 5. 1H NMR spectrum (solvent: CD3CN) of cationic iron complex 2. The most notable difference from the 1H NMR spectrum of 1 (the precursor to 2) is the signals arising from the diastereotopic α-methylene protons (2.85 and 2.23 ppm). Please click here to view a larger version of this figure.
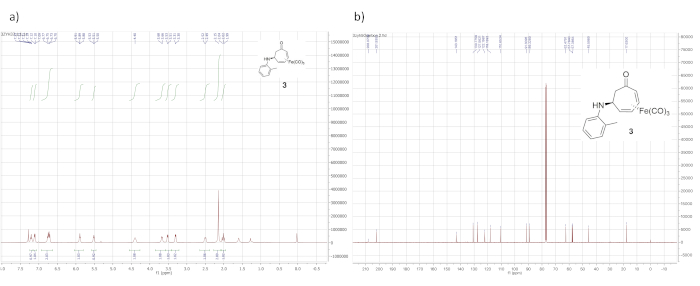
Figure 6. Spectral data for iron complex 3. (a) 1H NMR spectrum; (b) 13C NMR spectrum (solvent: CDCl3). Similar to the 1H NMR spectrum of 4, the 1H NMR spectrum of 3 is characterized by signals arising from the iron-complexed diene (5.89, 5.51, 3.53, and 3.30 ppm) and the diastereotopic α-methylene protons (2.50 and 2.02 ppm). Please click here to view a larger version of this figure.
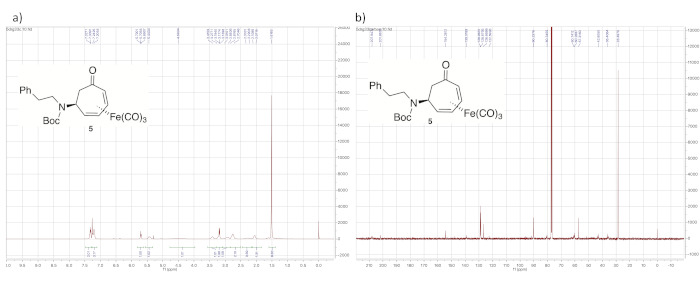
Figure 7. Spectral data for tert-butyl carbamate 5. (a) 1H NMR spectrum; (b) 13C NMR spectrum (solvent: CDCl3). The signal corresponding to the protons of the tert-butyl group of the carbamate appear at 1.52 ppm. Many signals also show characteristic broadening. Please click here to view a larger version of this figure.
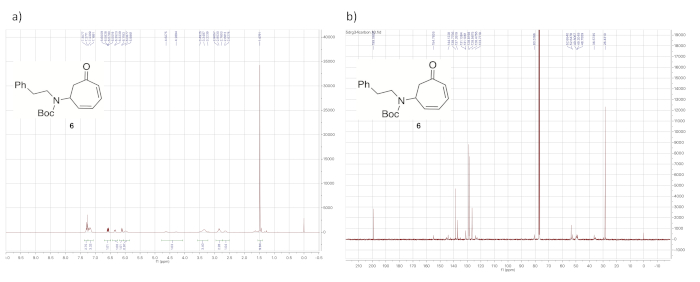
Figure 8. Spectral data for demetallated diene 6. (a) 1H NMR spectrum; (b) 13C NMR spectrum (solvent: CDCl3). The most notable aspect of the 1H NMR spectrum compared to those of the iron complexes in Figure 4a, Figure 6a, and Figure 7a is that all of the signals corresponding to the diene protons now appear above 5.75 ppm (6.57, 6.34, 6.10, and 5.99 ppm). Please click here to view a larger version of this figure.
Discussion
Whether the solvent-free protocol involving direct addition to tricarbonyl(tropone)iron (Figure 2) or the indirect method utilizing the corresponding cationic complex as the electrophile (Figure 1) is to be employed depends on the amine substrate used. In general, the direct addition method is preferable since it requires fewer steps to generate the aza-Michael adducts from tropone and the overall yields are generally higher. However, this more direct method is generally limited to reasonably unhindered primary aliphatic amines and cyclic secondary amines (e.g., piperidine). Less nucleophilic substrates such as arylamines or more sterically hindered amines such as acyclic secondary amines or tert-butylamine do not directly add to tricarbonyl(tropone)iron. On the other hand, these substrates efficiently add to the corresponding cationic complex (2, Figure 1). Thus, the two protocols complement one another in that the direct addition reaction is generally more efficient and higher yielding, while the addition to the cationic complex enjoys a broader substrate scope.
For the direct addition to tricarbonyl(tropone)iron, reaction times tend to be substrate-dependent. Some additions are complete within minutes as judged by 1H NMR analysis (e.g., unhindered primary amines) while some must be left overnight (e.g., morpholine). Upon completion, excess amine is removed via chromatography over Activity II/III alumina. However, for sufficiently volatile amine substrates, the excess amine may be removed via rotary evaporation and the crude material can then be subjected to protection as the corresponding carbamate (if applicable).
Adducts of primary aliphatic amines should be purified without delay and should be protected as carbamates as soon as is practicable, as we have generally experienced that such adducts will degrade over time. The degradation is generally accompanied by a color change from bright yellow to orange-brown. NMR analysis of such partially degraded samples showed the presence of tricarbonyl(tropone)iron, indicating that elimination of the amine had occurred.
We screened a variety of known protocols for removing the iron tricarbonyl group from the diene of the aza-Michael adducts22,23,24,25,26,27. The only successful protocol in our hands involved oxidative demetallation via treatment of the carbamate-protected adducts with cerium(IV) ammonium nitrate28. A representative result is described for demetallation of a tert-butyl carbamate-protected adduct. However, benzyl carbamates can also be demetallated using this protocol (no other carbamates were examined). Since tertiary amines cannot be protected as carbamates, we have thus far been unable to successfully demetallate those substrates despite extensive experimentation, including attempts to temporarily protect the nitrogen from oxidation by quantitatively protonating it with trifluoroacetic acid.
This protocol represents an extension of a method reported by Eisenstadt18 for addition of amines to cationic complex 2. However, addition of only two amines to the complex was reported, and demetallation of the complex was not described. The work described herein more fully explores the scope of addition to the cationic complex. Furthermore, the protocol for the direct addition of certain amines to tricarbonyl(tropone)iron constitutes a more efficient method for synthesizing such amine adducts. In addition, successful demetallation of the complexes opens the way for diverse subsequent reactions to access more complex molecular architectures containing a seven-membered carbocyclic ring. Notably, the addition of diverse amine nucleophiles with different functionalized side chains can potentially enable an even more diverse set of downstream reactions. Exploration of such newly-opened synthetic routes to complex alkaloid-like architectures is currently under investigation in our laboratory.
開示
The authors have nothing to disclose.
Acknowledgements
Acknowledgement is made to the Donors of the American Chemical Society Petroleum Research Fund for support of this research. We acknowledge the Lafayette College Chemistry Department and the Lafayette College EXCEL Scholars program for financial support.
Materials
| 10 g SNAP Ultra silica gel columns | Biotage | for automated column chromatography | |
| Acetic anhydride | Fisher Scientific | A10-500 | |
| Acetone | Fisher Scientific | A-16S-20 | for cooling baths |
| Acetonitrile-D3 | Sigma Aldrich | 366544 | |
| Benzene, anhydrous, 99.8% | Sigma Aldrich | 401765 | |
| Biotage Isolera Prime | Biotage | ISO-PSF | for automated chromatography |
| Celite; 545 Filter Aid | Fisher Scientific | C212-500 | diatomaceous earth |
| Cerium(IV) ammonium nitrate, ACS, 99+% | Alfa Aesar | 33254 | |
| Chloroform-D | Acros | 209561000 | |
| Di-tert-butyl dicarbonate, 99% | Acros | 194670250 | |
| Ethyl acetate | Fisher Scientific | E145-4 | |
| Ethyl alcohol, absolute – 200 proof | Greenfield Global | 111000200PL05 | |
| Ethyl ether anhydrous | Fisher Scientific | E138-1 | |
| Hexanes | Fisher Scientific | H302-4 | |
| iron nonacarbonyl 99% | Strem | 26-2640 | air sensitive, synonymous with diiron nonacarbonyl |
| Magnesium sulfate | Fisher Scientific | M65-500 | |
| Methanol | EMD Millipore | MX0475-1 | |
| Methylene chloride | Fisher Scientific | D37-4 | |
| MP alumina, Act. II-III acc. To Brockmann | MP Biomedicals | 4691 | for column chromatography |
| o-toluidine 98% | Sigma Aldrich | 466190 | |
| Phenethylamine 99% | Sigma Aldrich | 128945 | distill prior to use if not colorless |
| Sodium bicarbonate | Fisher Scientific | S233-500 | |
| Sodium carbonate anhydrous | Fisher Scientific | S263-500 | |
| Sodium chloride | Fisher Scientific | S271-500 | dissolved in deionized water to perpare a saturated aqueous solution |
| Sodium sulfate anhydrous | Fisher Scientific | S415-500 | |
| Sonicator | Branson | model 2510 | |
| Sulfuric acid | Fisher Scientific | A300C-212 | |
| Tetrafluoroboric acid solution, 48 wt.% | Sigma Aldrich | 207934 | aqueous solution |
| TLC Aluminium oxide 60 F254, neutral | EMD Millipore | 1.05581.0001 | for thin layer chromatography |
| Tropone 97% | Alfa Aesar | L004730-06 | Light sensitive |
参考文献
- Pollini, G. P., Benetti, S., De Risi, C., Zanirato, V. Synthetic Approaches to Enantiomerically Pure 8-Azabicyclo[3.2.1]octane Derivatives. Chemical Reviews. 106, 2434-2454 (2006).
- Ma, X., Gang, D. R. The Lycopodium alkaloids. Natural Product Reports. 21 (6), 752 (2004).
- Kobayashi, J., Kubota, T. The Daphniphyllum alkaloids. Natural Product Reports. 26 (7), 936-962 (2009).
- Leonard, J. Recent progress in the chemistry of monoterpenoid indole alkaloids derived from secologanin. Natural Product Reports. 16, 319-338 (1999).
- Huang, Z., Phelan, Z. K., Tritt, R. L., Valent, S. D., Griffith, D. R. Formal aza-Michael additions to tropone: Addition of diverse aryl- and alkylamines to tricarbonyl(tropone)iron and [(C7H7O)Fe(CO)3]BF4. Tetrahedron Letters. 59 (37), 3432-3434 (2018).
- Pauson, P. L. Tropones and Tropolones. Chemical Reviews. 55 (1), 9-136 (1955).
- Pietra, F. Seven-Membered Conjugated Carbo-and Heterocyclic Compounds and Their Homoconjugated Analogs and Metal Complexes. Synthesis, Biosynthesis, Structure, and Reactivity. Chemical Reviews. 73 (4), 293-364 (1973).
- Johnson, B. F. G., Lewis, J., Wege, D. Transition metal carbonyl complexes derived from cycloocta-2,4,6-trienone and cyclohepta-2,4,6-trienone. Journal of the Chemical Society, Dalton Transactions. 1976, 1874-1880 (1976).
- Franck-Neumann, M., Brion, F., Martina, D. Friedel-Crafts acylation of tropone-irontricarbonyl. Synthesis of β-thujaplicin and β-dolabrin. Tetrahedron Letters. 19 (50), 5033-5036 (1978).
- Saha, M., Bagby, B., Nicholas, K. M. Cobalt-mediated propargylation/annelation: Total synthesis of (±)-cyclocolorenone. Tetrahedron Letters. 27 (8), 915-918 (1986).
- Yeh, M. -. C. P., Hwu, C. -. C., Ueng, C. -. H., Lue, H. -. L. Michael Addition Reactions of the Highly Functionalized Zinc-Copper Reagents RCu(CN)ZnI to (Tropone)iron Tricarbonyl Promoted by Boron Trifluoride Etherate. Organometallics. 13 (5), 1788-1794 (1994).
- Pearson, A. J., Srinivasan, K. Approaches to the synthesis of heptitol derivatives via iron-mediated stereocontrolled functionalization of cycloheptatrienone. The Journal of Organic Chemistry. 57 (14), 3965-3973 (1992).
- Soulié, J., Betzer, J. -. F., Muller, B., Lallemand, J. -. Y. General access to polyhydroxylated nortropane derivatives through hetero diels -alder cycloaddition. Tetrahedron Letters. 36 (52), 9485-9488 (1995).
- Rigby, J. H., Ogbu, C. O. Tricarbonyl(tropone)iron as a useful functionalized enone equivalent. Tetrahedron Letters. 31 (24), 3385-3388 (1990).
- Franck-Neumann, M., Martina, D. Cycloadditions de la tropone avec le cyclopentadiene synthese d’un intermediaire potentiel par utilisation de complexe metallique. Tetrahedron Letters. 18 (26), 2293-2296 (1977).
- Ban, T., Nagai, K., Miyamoto, Y., Harano, K., Yasuda, M., Kanematsu, K. Periselective cycloaddition of tricarbonyliron complexes of seven-membered unsaturated compounds with 1,2,4,5-tetrazine. Masking and activating effects of tricarbonyliron complexes. The Journal of Organic Chemistry. 47 (1), 110-116 (1982).
- Bonadeo, M., Gandolfi, R., De Micheli, C. Reactions of nitrile oxides and of 2,5-dimethyl-3,4-diphenylcyclopentadienone with tricarbonyltroponeiron and oxidation of the adducts with cerium(IV). Gazzetta Chimica Italiana. 107, 577-578 (1977).
- Eisenstadt, A. The reactivity of cycloheptadienyl-1-one iron tricarbonyl cation towards nucleophilic attack. Journal of Organometallic Chemistry. 113 (2), 147-156 (1976).
- Rosenblum, M., Watkins, J. C. Cyclopentannulation reactions with organoiron reagents. Facile construction of functionalized hydroazulenes. Journal of the American Chemical Society. 112 (17), 6316-6322 (1990).
- Pearson, A. J. . Iron Compounds in Organic Synthesis. , (1994).
- Eisenstadt, A. Fluxional behaviour of protonated substituted troponeiron tricarbonyls. Journal of Organometallic Chemistry. 97 (3), 443-451 (1975).
- Shvo, Y., Hazum, E. A Simple Method for the Disengagement of Organic Ligands from Iron Complexes. Journal of the Chemical Society, Chemical Communications. , 336-337 (1974).
- Thompson, D. J. Reaction of tricarbonylcyclohexadieneiron complexes with cupric chloride. Journal of Organometallic Chemistry. 108 (3), 381-383 (1976).
- Franck-Neumann, M., Heitz, M. P., Martina, D. Une methode simple de liberation des ligands organiques de leurs complexes de fer carbonyle. Tetrahedron Letters. 24 (15), 1615-1616 (1983).
- Birch, A. J., Kelly, L. F., Liepa, A. J. Lateral control of skeletal rearrangement by complexation of thebaine with Fe(CO)3. Tetrahedron Letters. 26 (4), 501-504 (1985).
- Ripoche, I., Gelas, J., Grée, D., Grée, R., Troin, Y. A new stereoselective synthesis of chiral optically pure 4-piperidones. Tetrahedron Letters. 36 (37), 6675-6678 (1995).
- Williams, I., Kariuki, B. M., Reeves, K., Cox, L. R. Stereoselective Synthesis of 2-Dienyl-Substituted Pyrrolidines Using an η4-Dienetricarbonyliron Complex as the Stereodirecting Element: Elaboration to the Pyrrolizidine Skeleton. Organic Letters. 8, 4389-4392 (2006).
- Coquerel, Y., Depres, J. -. P., Greene, A. E., Cividino, P., Court, J. Synthesis of Substituted Cycloheptadienes by Catalytic Hydrogenation of Cycloheptatrieneiron Complexes. Synthetic Communications. 31, 1291-1300 (2001).

