Assessment of Pulmonary Capillary Blood Volume, Membrane Diffusing Capacity, and Intrapulmonary Arteriovenous Anastomoses During Exercise
概要
To assess the pulmonary diffusion and vasculature responses to exercise, we describe the multiple-inspired oxygen diffusion capacity technique to determine capillary blood volume and membrane diffusing capacity, as well as agitated saline contrast echocardiography to assess the recruitment of intrapulmonary arteriovenous anastomoses.
Abstract
Exercise is a stress to the pulmonary vasculature. With incremental exercise, the pulmonary diffusing capacity (DLCO) must increase to meet the increased oxygen demand; otherwise, a diffusion limitation may occur. The increase in DLCO with exercise is due to increased capillary blood volume (Vc) and membrane diffusing capacity (Dm). Vc and Dm increase secondary to the recruitment and distension of pulmonary capillaries, increasing the surface area for gas exchange and decreasing pulmonary vascular resistance, thereby attenuating the increase in pulmonary arterial pressure. At the same time, the recruitment of intrapulmonary arteriovenous anastomoses (IPAVA) during exercise may contribute to gas exchange impairment and/or prevent large increases in pulmonary artery pressure.
We describe two techniques to evaluate pulmonary diffusion and circulation at rest and during exercise. The first technique uses multiple-fraction of inspired oxygen (FIO2) DLCO breath holds to determine Vc and Dm at rest and during exercise. Additionally, echocardiography with intravenous agitated saline contrast is used to assess IPAVAs recruitment.
Representative data showed that the DLCO, Vc, and Dm increased with exercise intensity. Echocardiographic data showed no IPAVA recruitment at rest, while contrast bubbles were seen in the left ventricle with exercise, suggesting exercise-induced IPAVA recruitment.
The evaluation of pulmonary capillary blood volume, membrane diffusing capacity, and IPAVA recruitment using echocardiographic methods is useful to characterize the ability of the lung vasculature to adapt to the stress of exercise in health as well as in diseased groups, such as those with pulmonary arterial hypertension and chronic obstructive pulmonary disease.
Introduction
During exercise, cardiac output can increase up to six-fold above resting values1. Given that the lungs are the only organ to receive 100% of the cardiac output, exercise presents a considerable stress to the pulmonary system. With incremental exercise, pulmonary diffusing capacity (DLCO) must increase to meet the increased oxygen demand2. From rest to peak exercise, DLCO can increase to up to 150% of resting values without reaching an upper limit with respect to cardiac output3,4,5. The increase in diffusing capacity occurs as a result of increases in membrane diffusing capacity (Dm) and capillary blood volume (Vc), secondary to the recruitment and distension of pulmonary capillaries6.
Roughton and Forster (1957) developed a technique to partition Dm and Vc7 by modulating the fraction of inspired oxygen (FIO2) during a standard diffusion capacity for carbon monoxide test (DLCO). Oxygen and carbon monoxide (CO) competitively bind to heme sites on hemoglobin, such that increasing FIO2 will decrease the DLCO8,9. By modulating the FIO2 during a standard DLCO maneuver, this relationship can be exploited to measure Vc and Dm7. We have recently adapted this technique to be used during exercise5. Similar to previous work, we have found that DLCO continuously increases up to peak exercise secondarily to increases in both Vc and Dm5. Interestingly, we have found that in endurance-trained athletes who have a greater oxygen consumption and thus a greater need for diffusing capacity, there is an increase in the DLCO at peak exercise, secondary to an increased Dm, and not Vc, suggesting a potential adaptation in the pulmonary membrane of the athlete5.
The increases in Vc and Dm during exercise are accomplished by an increase in pulmonary artery pressure, which results in the recruitment and distension of pulmonary capillaries previously hypo-perfused at rest4,10. This results in an increase in the cross-sectional area of the pulmonary capillary network, thereby decreasing pulmonary vascular resistance and attenuating the increase in pulmonary artery pressure.
Studies using agitated saline contrast echocardiography have shown evidence of intrapulmonary arteriovenous anastomoses (IPAVA) recruitment during exercise11,12,13,14. The significance of IPAVA recruitment is not yet clear, and while some studies suggest that they may contribute to gas exchange impairment12,14 and may serve to unload the right ventricle11,12, the topic remains controversial15,16. Further, while the exact mechanism of IPAVA recruitment is not known, we have found that increasing cardiac output, as well as exogenous dopamine, causes IPAVA recruitment at rest17. An acutely-increasing pulmonary artery pressure18 or dopamine blockade does not appear to significantly affect IPAVA recruitment during exercise11. There is speculation that these larger-diameter IPAVA vessels may help to protect the pulmonary capillaries from the large increases in pulmonary artery pressure by reducing pulmonary vascular resistance12,17,19,20,21.
When combined with the evaluation of Vc and Dm, agitated saline contrast echocardiography is a valuable tool to examine the adaptation of the pulmonary circulation to the stress of exercise22,23.
Protocol
This protocol follows the guidelines of the human research ethics board at the University of Alberta and conforms to the standards set by the latest revision of the Declaration of Helsinki.
1. Graded Exercise Test (VO2peak)
- Obtain written, informed consent from the subject. Have the subject read and answer the questions listed on the Physical Activity Readiness Questionnaire+ (PAR-Q+) to determine their readiness for exercise24.
- Adjust the seat height of the cycle ergometer in accordance to subject preference. Place four electrocardiogram (ECG) electrodes on the back of the patient according to standard 3-lead ECG placement, with modified limb leads to measure the heart rate (HR)25.
- Insert the mouthpiece into the subject's mouth to measure the exhaled gas and ventilation throughout the test using a metabolic measurement system25.
NOTE: The metabolic system will measure real-time oxygen consumption (VO2), carbon dioxide production (VCO2), ventilation (VE), heart rate (HR), and end tidal CO2 (PETCO2). - Following 2 min of collection of baseline data, instruct the subject to start cycling with an initial workload of 50 watt, to maintain a consistent cadence of ≥60 RPM. Increase the workload in 25 W steps every 2 min, until the subject reaches volitional exhaustion or requests to stop the test25.
2. Multiple Fraction of Inspired Oxygen (FIO2 ) Diffusing Capacity (DLCO ) Method7
- Calculate the workloads corresponding to 30%, 50%, 70%, and 90% of the VO2peak using the peak VO2 obtained in the graded exercise test. At least 48 h after the graded exercise test, have the subject return to the laboratory for DLCO maneuvers.
- Do not exceed 12 DLco tests per day, as carboxyhemoglobin (COHb) build-up can occur with repeated testing5. Therefore, perform testing on multiple days based on the number of exercise workloads to be conducted and the quality of the DLCO data.
- Prepare pre-breathing gases by attaching a tank of 100% O2 gas and a tank of medical-grade air (21% O2 and 79% N2) to an air blender system. Fill two 60 L non-diffusing Douglas bags, one containing 40% O2, and one containing 60% O2, using the air blender system.
- Set up two large-bore, three-way stopcock valves that will allow for the modulation of inhaled gas mixtures. These will be referred to as the "pre-breath valves."
- Connect the Douglas bags to the valve system using flexible, non-compressible tubing. Connect the valve system to a two-way, T-shaped non-rebreathing valve connected to the test gas intake assembly of the mass flow sensor of the metabolic measurement system.
- For resting measurements, have the subject seated upright, with both feet flat on the floor. For exercise trials, ensure that the subject is in a steady state by monitoring HR using the ECG (HR ± 3 bpm for steady state).
NOTE: Steady state may not be reached at 90% of the VO2peak; thus, begin the measurement once the subject has reached the HR equivalent to 90% of the VO2peak on the graded exercise test. - Collect a single drop of capillary blood via a finger prick and analyze it for hemoglobin concentration. Then, adjust all subsequent DLCO for [Hb] using the following equation26:
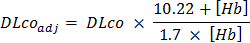
- Select an FIO2 (21%, 40%, or 60%) at random by switching the pre-breathe valves to the desired orientation. Choose the corresponding FIO2-DLCO gas by turning the DLCO gas valve selector (see Figure 1C).
- Instruct the subject to affix the nose clips and to breathe normally into the mouthpiece for five breaths from the Douglas bag corresponding to the respective FIO2.
- Instruct the subject to expire to residual volume. When the lung volume plateaus at residual volume, have the subject inhale the DLCO gas mixture to total lung capacity and hold their breath for 6 s before exhaling to residual volume.
- Monitor the methane tracing during the exhalation to ensure that the slope is horizontal, as this indicates that the CO test gas is well equilibrated in the lung.
NOTE: Alveolar volume (VA) and breath hold time are calculated automatically and reported by the metabolic measurement system. - Ensure that the VA for each DLco maneuver is within 5% of previous trials. Similarly, breath hold time should be 6.0 ± 0.3 s. If not, repeat the maneuver.
- Wait 4 min to allow residual carbon monoxide to wash out, and then repeat steps 2.8 – 2.11 for each remaining FIO2 at rest.
- At least 48 h later, repeat steps 2.9 – 2.15 during steady state at each exercise intensity (30%, 50%, 70%, and 90% of the VO2peak) for each FIO2. Reduce the workload between the breath holds at 90% of the VO2peak workload to recover the subject.
- Wait 2 min between DLco tests during exercise to clear alveolar CO during exercise. Do not exceed 12 DLco tests per day to avoid carboxyhemoglobin (COHb) build-up5.
3. Calculating Pulmonary Capillary Blood Volume and Membrane Diffusing Capacity
- Calculate the alveolar partial pressure of O2 (PAO2) using the following equation
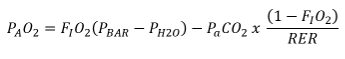
NOTE: FIO2 is the fraction of inspired O2, PBAR is the atmospheric pressure, PH2O is the water vapor pressure, PaCO2 is the pressure of arterial CO2, and RER is the respiratory exchange ratio. - Estimate the RER and PaCO2 using the measured 30-s average PETCO2 and RER for the respective exercise intensity from the data obtained in the previous graded exercise test.
- Calculate θCO using the following equation7.
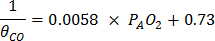
- Graph the relationship between 1/DLcoadj and 1/θCO for each FIO2 and calculate the regression equation.
NOTE: The minimum acceptable r2 value is 0.95, and DLCO maneuvers should be repeated when r2 values are outside of this range21.
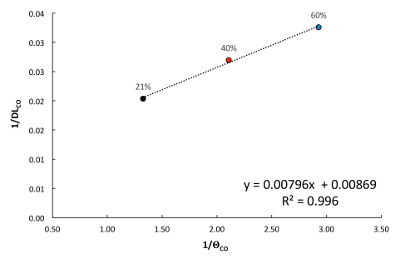
Figure 2: Representative Graph of 1/DLCO versus 1/θCO at Peak Exercise. The relationship between 1/DLCO and 1/θCO is plotted for three breath holds at the various FIO2 (21%, 40%, and 60%). The calculation of Vc and Dm are derived from the regression equation for the relationship above. The inverse of the slope (1/0.00796) of the line gives the value for Vc (125.5 mL), and the inverse of the y-intercept (1/0.00869) gives the value for Dm (115.0 mL·min-1·mmHg-1). Please click here to view a larger version of this figure.
- Calculate Vc by taking the inverse of the slope of the regression equation between 1/DLCO and 1/θCO. Calculate Dm by taking the inverse of the y-intercept of the equation.
4. Intrapulmonary Arteriovenous Anastomosis Recruitment
- On a separate day from the DLCO data collection, insert a 20-gauge intravenous (IV) catheter into an antecubital vein and attach it to a three-way stopcock via a 6-in IV extension tube for the injection of agitated saline for contrast echocardiography11,17.
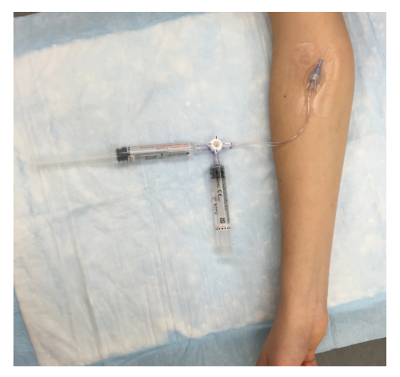
Figure 3: Agitated Saline Contrast Setup. An IV catheter is placed in the antecubital space and is connected to a three-way stopcock via a 6-in extension. Two 10 mL syringes are attached to the stopcock to create the contrast solution, which contains 10 mL of saline and 0.5 mL of room air. Please click here to view a larger version of this figure.
- Connect two 10 mL syringes to the three-way stopcock. Combine 10 mL of 0.9% sterile saline with 0.5 mL of air, and forcefully agitate it through the three-way stopcock, back and forth between the two syringes, to form fine, suspended bubbles until the sonographer is ready for contrast.
- Have an experienced sonographer or cardiologist obtain a standard apical four-chamber view of the heart. At rest, have the echocardiographer evaluate the intra-atrial septum and ventricular septum for an intra-cardiac shunt with standard echocardiographic and color Doppler imaging.
- If no intra-cardiac shunt is detected, instruct the subject to perform a Valsalva maneuver during the contrast injection to evaluate for a patent foramen ovale (PFO)11,17. Repeat the measurement during non-Valsalva.
- Inject the contrast while the sonographer maintains the four-chamber view. Record 15 cardiac cycles following the detection of contrast in the right ventricle.
- Repeat the contrast-enhanced imaging during steady-state exercise at 30%, 50%, and 70% of the VO2peak. As steady state cannot be reached at 90% of the VO2peak, begin the imaging once the target HR, identified by the HR at 90% of the VO2peak during the graded exercise test, is reached.
NOTE: The time between exercise intensities depends on the clearance of contrast from both ventricles, ≥ 2 min. - Have an echocardiographer who is blinded to experimental conditions interpret the agitated saline contrast echocardiograms according to a previously-described scoring system17,27.
NOTE: Scoring is based on the maximum number of contrast bubbles visible within the left ventricle (LV) in a single echocardiographic frame, as follows: no contrast bubbles in the LV = 0, ≤3 bubbles = 1, 4 – 12 bubbles = 2, > 12 bubbles = 3.
NOTE: The appearance of contrast in the left ventricle after five cardiac cycles suggests an IPAVA. An intracardiac shunt is graded by the appearance of contrast in less than five cardiac cycles27.
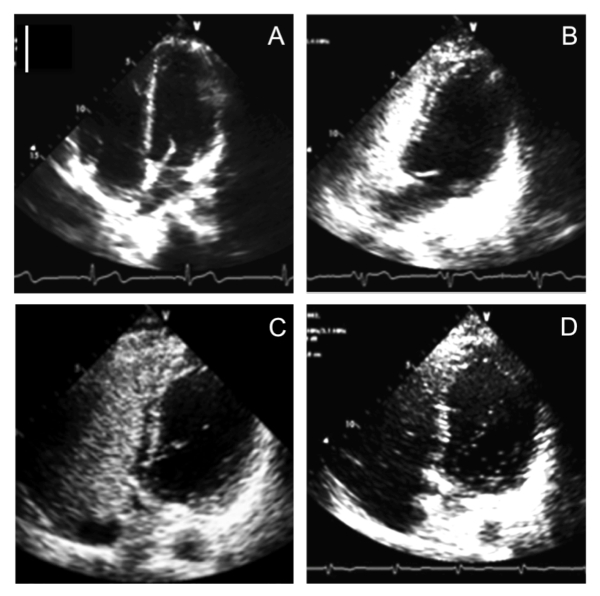
Figure 4: Representative Images for IPAVA Scoring. The scale is 5 cm (solid white line). (A) Pre contrast injection. (B) IPAVA score = 0. (C) IPAVA score = 1. (D) IPAVA score = 3. Please click here to view a larger version of this figure.
Representative Results
The effect of increasing exercise intensity on oxygen consumption, diffusing capacity, pulmonary capillary blood volume, membrane diffusing capacity, and IPAVA score is shown in Table 1. VO2, DLCO, Vc, and Dm increase in response to increasing power output.
Figure 2 shows a representative calculation of Vc and Dm using the multiple FIO2-DLCO technique during exercise. DLCO decreases with increasing FIO2, and this relationship is exploited to partition Vc and Dm. Calculating the inverse of the slope of 1/DLCO versus 1/θCO results in the Vc, and the inverse of the y-intercept yields the value for the Dm. As expected, both the Vc and Dm increase during exercise compared to resting values.
The results show that these techniques can be used to assess the pulmonary vasculature response during exercise. The multiple-FIO2 DLCO and agitated saline contrast echocardiography method provides investigators with more insight into the contributions of pulmonary capillary and membrane recruitment to the overall diffusion capacity and could supplement traditional pulmonary function testing in the clinical setting. Failure to increase Vc or Dm during exercise would lead to a diffusion limitation and hypoxemia. For example, a low DLCO secondary to a low Vc would indicate changes to the pulmonary capillaries; similarly, a decreased Dm would indicate changes to the pulmonary membrane.
Figure 4 shows representative tracings of four-chamber contrast echocardiographs. With increasing exercise intensity, the IPAVA score increases from 0 (i.e., no evidence of IPAVAs) at rest to 3 at the highest exercise intensity (Table 1). Previous work has shown that exercise increases the IPAVA score11,12,14, but there is no consensus as to how these IPAVAs are recruited. There is evidence that IPAVAs can be recruited pharmacologically at rest with dopamine17,28, as well as by increasing cardiac output with dobutamine17,28 and epinephrine28. Inotropes such as dopamine and epinephrine are of particular interest, as they increase endogenously during exercise29. Furthermore, there is some evidence that IPAVA recruitment may be important to exercise hemodynamics, in that the absence of IPAVAs appears to result in greater pulmonary artery pressure, decreased cardiac output, and decreased peak power output12. Thus, this technique may be used in studies examining individuals with pulmonary artery hypertension.
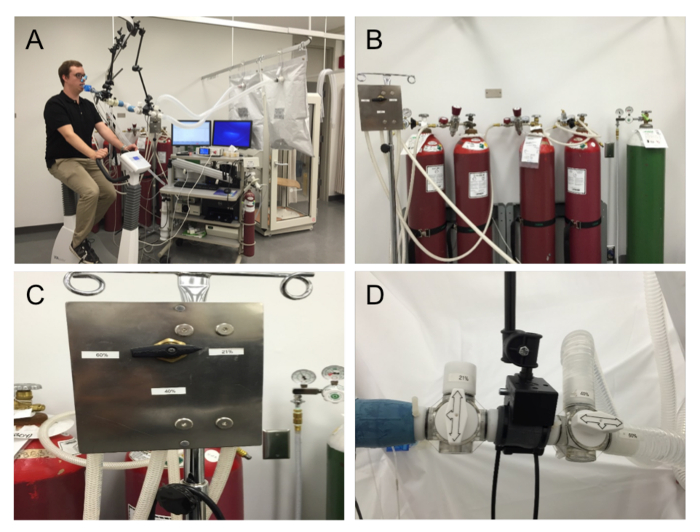
Figure 1: Multiple FIO2 DLCO Setup. (A) Setup overview. (B) Compressed-gas cylinders containing 21%, 40%, and 60% O2 with 0.3% CO, 0.3% methane, and balance nitrogen, as well as a supplemental oxygen compressed-gas cylinder. (C) Three-way valve selector for the three FIO2 DLCO tanks. (D) Valve switch for three-way valves in the series for the selection of FIO2 for pre-breathing. Please click here to view a larger version of this figure.

Table 1: Representative Data for One Subject at Rest and During Exercise at 30, 50, 70, and 90% of the VO2peak. VO2, volume of oxygen consumption relative to body mass; DLCO, diffusing capacity for carbon monoxide; Vc, pulmonary capillary blood volume; Dm, membrane diffusing capacity; IPAVA score, scoring of contrast appearance in the left ventricle after five cardiac cycles. Data modified from Tedjasaputra et al. 2016.
Discussion
This method enables the evaluation of the pulmonary diffusing capacity and intrapulmonary arteriovenous anastomosis recruitment during exercise.
Critical steps within the protocol
Although the DLCO breath hold is relatively simple at rest, breath holding during exercise presents a unique challenge to the subject, as it is counter-intuitive, and subjects have a high drive to breathe during exercise. Thus, a good-quality determination of Vc and Dm relies on the rapport and clear communication between the tester and the subject. The tester's technical ability can be quantified with the variability of the alveolar volume (± 5% of previous trials) and a breath-hold time (BHT) of 6.0 ± 0.3 s.
Modifications and troubleshooting
At the conclusion of a Vc/Dm measurement, the tester should quickly graph the three DLCO maneuvers to determine the best-fit line of the data points; the DLCO measured with 21% FIO2 should always be greater than that with 40%, which should be greater than that with 60%. If not, it is recommended to check if the valve switch corresponds to the correct testing gas. Similarly, check that the pre-breathing bags are filled with the correct FIO2 gas corresponding to the testing gas (Figure 1B-1D). Caution should be taken when testing a participant who is a smoker, as elevated COHb levels may underestimate DLco.
For the IPAVA recruitment assessment, the position of the subject is critical to ensure high-quality image acquisition. It is possible to replace the upright cycle ergometer with a recumbent cycle ergometer to minimize the movement of the subject. However, recumbent cycle exercise will elicit a different metabolic response for a given work rate, and thus the graded exercise test should be repeated on the recumbent cycle ergometer. Scanning of the upper chest may be uncomfortable to some women; in this case, a female sonographer is recommended. Finally, the recommended exercise protocol is designed for a young, healthy individual; accordingly, the exercise protocol can be modified for a different target population.
Limitations of the technique
The principal limitations of the multiple FIO2 DLCO technique are the skill of the tester and the ability of the subject to follow commands and to remain calm during the breath hold, as Valsalva or Müllerian maneuvers will affect the measurements. Secondly, the number of breath holds in one session should be limited to 12, due to an increase in CO backpressure, which may affect the Vc and Dm measurement5,30 and pose a health risk to the subject. Depending on the research design, it may be necessary to complete the testing across multiple sessions to allow for the clearance of CO and to limit participant fatigue. With good participant coaching and good technical ability, we have determined a satisfactory coefficient of variation between trials for DLco, Vc, and Dm to be 7%, 8%, and 15%, respectively.
The multiple FIO2 DLCO technique assumes that the alveolar O2 is the same as the capillary O2, and thus, caution should be exercised when interpreting the data in individuals with known gas exchange impairment.
Agitated saline contrast echocardiographic imaging is limited by the technical ability of the sonographer and the ability of the subject to minimize thoracic movement while exercising. It is also critical that the interpreter of the images be familiar with the scale for scoring IPAVA recruitment according to established procedures (Figure 4)27. The significance of a positive saline contrast echocardiography during exercise remains a topic of debate15,16, and there is some discussion that a positive agitated saline contrast in the left ventricle may be secondary to capillary distention, and not IPAVA recruitment. Ongoing work is attempting to resolve this issue.
Significance of the technique with respect to existing/alternative methods
By utilizing these physiological techniques, it is possible to assess the pulmonary vasculature during exercise in a variety of conditions, including in health, in disease, and in drug interventions. Although the quality relies with the ability of the tester, these skills are easily and quickly acquired with proper mentorship and training. The multiple FIO2 DLCO method is considered the "gold standard" in the measurement of Dm and Vc31. While these measures are not calculated clinically, the values could be used to determine the mechanisms for hypoxemia and exercise intolerance, to predict patient outcomes, and to further characterize diagnosis31,32. Likewise, the agitated saline echocardiography technique is the most widely-used method in determining the recruitment of IPAVAs.
Future applications or directions after mastering this technique
These techniques are applicable for use in a range of experimental conditions and interventions. We demonstrate these techniques during exercise, but they can easily be modified to measure pulmonary vascular responses during a drug infusion, such as dobutamine or dopamine, inotropes known to increase cardiac output17. Furthermore, it is possible to use these techniques in clinical populations, such as in those with heart failure34 or chronic obstructive pulmonary disease (COPD), in which the DLCO is lower compared to age-matched control subjects35.
開示
The authors have nothing to disclose.
Acknowledgements
Funding was provided by the Natural Sciences and Engineering Research Council of Canada and The Heart and Stroke Foundation of Canada.
Materials
| Metabolic Measurement System | SensorMedics Inc. | Encore 299 Vmax | |
| Cycle Ergometer | Ergoline | Ergoselect II 1200 | |
| 60L Douglas Bags | Hans Rudolph | 6100 Series | |
| Two-way T Valve | Hans Rudolph | 2700 Series | |
| Hemoglobin Measurement System | HemoCue | Hb 201+ | |
| 22-gauge Intravenous Catheter | BD | Insyte-W | |
| Ultrasound | Vivid Q | ECHOpac | |
| Compressed gas 21% O2, 0.3% CO, 0.3% CH4, balance nitrogen | Praxair | ||
| Compressed gas 40% O2, 0.3% CO, 0.3% CH4, balance nitrogen | Praxair | ||
| Compressed gas 60% O2, 0.3% CO, 0.3% CH4, balance nitrogen | Praxair | ||
| Nose-clip | Vacu-Med | snuffer #1008 |
参考文献
- Naeije, R., Chesler, N. Pulmonary Circulation at Exercise. Comp Physiol. 2 (1), (2012).
- Stickland, M. K., Lindinger, M. I., Olfert, I. M., Heigenhauser, G. J. F., Hopkins, S. R. Pulmonary gas exchange and acid-base balance during exercise. Comp Physiol. 3 (2), 693-739 (2013).
- Hsia, C. C., Herazo, L. F., Ramanathan, M., Johnson, R. L. Cardiac output during exercise measured by acetylene rebreathing, thermodilution, and Fick techniques. J Appl Physiol. 78 (4), 1612-1616 (1995).
- Hsia, C. C. W. Recruitment of lung diffusing capacity: update of concept and application. Chest. 122 (5), 1774-1783 (2002).
- Tedjasaputra, V., Bouwsema, M. M., Stickland, M. K. Effect of aerobic fitness on capillary blood volume and diffusing membrane capacity response to exercise. J Physiol. 594 (15), 4359-4370 (2016).
- Johnson, R. L., Spicer, W. S., Bishop, J. M., Forster, R. E. Pulmonary capillary blood volume, flow and diffusing capacity during exercise. J Appl Physiol. 15 (5), 893-902 (1960).
- Roughton, F. J., Forster, R. E. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol. 11 (2), 290 (1957).
- Forster, R. E., Roughton, F. J., Cander, L., Briscoe, W. A., Kreuzer, F. Apparent pulmonary diffusing capacity for CO at varying alveolar O2 tensions. J Appl Physiol. 11 (2), 277-289 (1957).
- Roughton, F. J., Forster, R. E., Cander, L. Rate at which carbon monoxide replaces oxygen from combination with human hemoglobin in solution and in the red cell. J Appl Physiol. 11 (2), 269-276 (1957).
- Johnson, R. L., Hsia, C. C. Functional recruitment of pulmonary capillaries. J Appl Physiol. 76 (4), 1405-1407 (1994).
- Tedjasaputra, V., Bryan, T. L., et al. Dopamine receptor blockade improves pulmonary gas exchange but decreases exercise performance in healthy humans. J Physiol. 593 (14), 3147-3157 (2015).
- Stickland, M. K., Welsh, R. C., et al. Intra-pulmonary shunt and pulmonary gas exchange during exercise in humans. J Physiol. 561 (1), 321-329 (2004).
- Stickland, M. K., Lovering, A. T. Exercise-induced intrapulmonary arteriovenous shunting and pulmonary gas exchange. Exerc Sport Sci Rev. 34 (3), 99-106 (2006).
- Eldridge, M. W., Dempsey, J. A., Haverkamp, H. C., Lovering, A. T., Hokanson, J. S. Exercise-induced intrapulmonary arteriovenous shunting in healthy humans. J Appl Physiol. 97 (3), 797-805 (2004).
- Hopkins, S. R., Olfert, I. M., Wagner, P. D. Point:Counterpoint: Exercise-induced intrapulmonary shunting is imaginary. J Appl Physiol. 107 (3), 993-994 (2009).
- Lovering, A. T., Eldridge, M. W., Stickland, M. K. Counterpoint: Exercise-induced intrapulmonary shunting is real. J Appl Physiol. 107 (3), 994-997 (2009).
- Bryan, T. L., van Diepen, S., Bhutani, M., Shanks, M., Welsh, R. C., Stickland, M. K. The effects of dobutamine and dopamine on intrapulmonary shunt and gas exchange in healthy humans. J Appl Physiol. 113 (4), 541-548 (2012).
- Stickland, M. K., Welsh, R. C., et al. Effect of acute increases in pulmonary vascular pressures on exercise pulmonary gas exchange. J Appl Physiol. 100 (6), 1910-1917 (2006).
- Berk, J. L., Hagen, J. F., Tong, R. K., Maly, G. The use of dopamine to correct the reduced cardiac output resulting from positive end-expiratory pressure. A two-edged sword. Crit Care Med. 5 (6), 269 (1977).
- Lalande, S., Yerly, P., Faoro, V., Naeije, R. Pulmonary vascular distensibility predicts aerobic capacity in healthy individuals. J Physiol. 590 (17), 4279-4288 (2012).
- Tedjasaputra, V., Collins, S. &. #. 2. 0. 1. ;., Bryan, T. L., van Diepen, S., Bouwsema, M. M., Stickland, M. K. Is there a relationship between pulmonary capillary blood volume and intrapulmonary arteriovenous anastomosis recruitment during exercise?. FASEB J. 30 (1), (2016).
- Reeves, J. T., Linehan, J. H., Stenmark, K. R. Distensibility of the normal human lung circulation during exercise. Am J Physiol. Lung cellular and molecular physiology. 288 (3), 419-425 (2005).
- Thadani, U., Parker, J. O. Hemodynamics at rest and during supine and sitting bicycle exercise in normal subjects. Am J Card. 41 (1), 52-59 (1978).
- Warburton, D. E. R., Jamnik, V. K., Bredin, S. S. D., Gledhill, N. The Physical Activity Readiness Questionnaire for Everyone (PAR-Q) and Electronic Physical Activity Readiness Medical Examination (ePARmed-X+). The Health & Fitness Journal of Canada. 4 (2), (2011).
- Wasserman, K. . Principles of Exercise Testing and Interpretation. , (2012).
- Wasserman, K. Determinants and detection of anaerobic threshold and consequences of exercise above it. Circulation. 76 (6), (1987).
- Marrades, R. M., Diaz, O., et al. Adjustment of DLCO for hemoglobin concentration. Am J Resp Crit Care Med. 155 (1), 236-241 (2011).
- Lovering, A. T., Romer, L. M., Haverkamp, H. C., Pegelow, D. F., Hokanson, J. S., Eldridge, M. W. Intrapulmonary shunting and pulmonary gas exchange during normoxic and hypoxic exercise in healthy humans. J Appl Physiol. 104 (5), 1418-1425 (2008).
- Weyman, A. E. . Principles and Practice of Echocardiography. , (1994).
- Laurie, S. S., Elliott, J. E., Goodman, R. D., Lovering, A. T. Catecholamine-induced opening of intrapulmonary arteriovenous anastomoses in healthy humans at rest. J Appl Physiol. 113 (8), 1213-1222 (2012).
- Hopkins, S. R., Bogaard, H. J., Niizeki, K., Yamaya, Y., Ziegler, M. G., Wagner, P. D. β-Adrenergic or parasympathetic inhibition, heart rate and cardiac output during normoxic and acute hypoxic exercise in humans. J Physiol. 550 (2), 605-616 (2009).
- Zavorsky, G. S. The rise in carboxyhemoglobin from repeated pulmonary diffusing capacity tests. Respir Physiol Neurobiol. 186 (1), 103-108 (2013).
- Coffman, K. E., Taylor, B. J., Carlson, A. R., Wentz, R. J., Johnson, B. D. Optimizing the calculation of DM,CO and VC via the single breath single oxygen tension DLCO/NO method. Respir Physiol Neurobiol. 221, 19-29 (2015).
- Guazzi, M., Pontone, G., Brambilla, R., Agostoni, P., Rèina, G. Alveolar-capillary membrane gas conductance: a novel prognostic indicator in chronic heart failure. Eur Heart J. 23 (6), 467-476 (2002).
- Ofir, D., Laveneziana, P., Webb, K. A., Lam, Y. -. M., O’Donnell, D. E. Mechanisms of Dyspnea during Cycle Exercise in Symptomatic Patients with GOLD Stage I Chronic Obstructive Pulmonary Disease. Am J Resp Crit Care Med. 177 (6), 622-629 (2008).

