Protocols for Vaginal Inoculation and Sample Collection in the Experimental Mouse Model of Candida vaginitis
概要
Key techniques to be used in the evaluation of Candida vaginitis in an experimental animal model are described. The methods will allow rapid collection of vaginal specimens and lymphocytes from draining lumbar lymph nodes. These techniques could give rise to mouse models of other diseases in the female lower genital tract.
Abstract
Vulvovaginal candidiasis (VVC), caused by Candida species, is a fungal infection of the lower female genital tract that affects approximately 75% of otherwise healthy women during their reproductive years18,32-34. Predisposing factors include antibiotic usage, uncontrolled diabetes and disturbance in reproductive hormone levels due to pregnancy, oral contraceptives or hormone replacement therapies33,34. Recurrent VVC (RVVC), defined as three or more episodes per year, affects a separate 5 to 8% of women with no predisposing factors33.
An experimental mouse model of VVC has been established and used to study the pathogenesis and mucosal host response to Candida3,4,11,16,17,19,21,25,37. This model has also been employed to test potential antifungal therapies in vivo13,24. The model requires that the animals be maintained in a state of pseudoestrus for optimal Candida colonization/infection6,14,23. Under such conditions, inoculated animals will have detectable vaginal fungal burden for weeks to months. Past studies show an extremely high parallel between the animal model and human infection relative to immunological and physiological properties3,16,21. Differences, however, include a lack of Candida as normal vaginal flora and a neutral vaginal pH in the mice.
Here, we demonstrate a series of key methods in the mouse vaginitis model that include vaginal inoculation, rapid collection of vaginal specimens, assessment of vaginal fungal burden, and tissue preparations for cellular extraction/isolation. This is followed by representative results for constituents of vaginal lavage fluid, fungal burden, and draining lymph node leukocyte yields. With the use of anesthetics, lavage samples can be collected at multiple time points on the same mice for longitudinal evaluation of infection/colonization. Furthermore, this model requires no immunosuppressive agents to initiate infection, allowing immunological studies under defined host conditions. Finally, the model and each technique introduced here could potentially give rise to use of the methodologies to examine other infectious diseases of the lower female genital tract (bacterial, parasitic, viral) and respective local or systemic host defenses.
Protocol
1. Vaginal inoculation with Candida albicans
- Three days prior to inoculation, while restraining the animal to expose the abdomen, inject 100 μl of sesame oil containing 0.1-0.5 mg of β-estradiol subcutaneously in the lower abdomen. Advance the needle about 5 to 10 mm lateral to the skin to minimize leakage from the injection site.
The subcutaneous administration of estrogen in the lower abdomen is optimal in this model due to the close proximity to the genital tract. Effective doses may vary by mouse strains, ages or estrogen derivatives. In previous studies using CBA-J (H-2κ), C3H/HeN (H-2κ), C57BL/6 (H-2b), Balb/c (H-2d), DBA/2 (H-2d), SJL (H-2s) mice at 6-8 weeks of age, 0.1 mg/100 μl was found effective evidenced by thickening of the vaginal wall, reduced vaginal mucus and increased epithelial cell sloughing. Mice above treated with estrogen at this concentration exhibit consistent vaginal colonization with Candida. For inoculation in mice of other strains and ages, a pilot study is recommended to ensure the effectiveness of estrogen under the modified conditions and increase the estrogen dose if necessary.
The estrogen solution should be prepared fresh each time on the day of injection. To ensure complete solubility of estrogen in sesame oil, thoroughly mix the solution using a vortex mixer and heat intermittently at 37°C. Repeat the injection weekly throughout the study period. - To prepare the inoculum, add a loopful of C. albicans blastoconidia from a recent subculture preparation on Sabouraud-dextrose agar (SDA) into 10 ml of Phytone-peptone broth supplemented with 0.1% glucose. Incubate the broth culture to stationary phase for 18 h at 25°C in a shaking water bath.
- Following incubation, collect the broth culture into a 15 ml conical tube and centrifuge at 800 x g for 5 min. Wash the pellet twice with sterile PBS.
- Enumerate viable blastoconidia on a hemocytometer by trypan blue dye exclusion. Adjust the cell concentration to 2.5 x 106/ml (or to a desired inoculum concentration) in sterile PBS.
- To stabilize the mouse, hold the base of the tail with two fingers and lift the hip upward so that the vaginal opening faces toward you (Figure 1A). It is ideal if the mouse is placed on a flat grated surface (e.g. cage top) so that the mouse can provide resistance against the tail restraint.
- Pipette 20 μl (or a desired volume not to exceed 20 μl) of the inoculum suspension by inserting the pipette tip about 5 mm deep into the vaginal lumen (Figure 1B). Complete this step as quickly and gently as possible to minimize distress in the mouse.
2. Vaginal lavages
- Following euthanasia (or anesthesia), hold the mouse downward by the base of the tail with two fingers so that the vaginal opening becomes exposed.
- Lavage the vaginal lumen by introducing 100 μl of sterile PBS with repeated aspiration and agitation with a pipette tip. The pipette tip may become clogged with cells. If this occurs, dispense the obstructing cells and continue lavaging with remaining PBS in the vagina. Collect the lavage fluid into a microcentrifuge tube.
- Alternatively, vaginal lavages can be performed on anesthetized mice with isoflurane inhalant anesthesia. For this, expose the mice to vaporized isoflurane until they are fully sedated (~30 sec.). Hold the mice downward by the base of the tail and gently lavage the vaginal lumen using 50 μl of sterile PBS. Make sure to avoid harsh agitation with a pipette tip to minimize trauma to the vagina during this procedure. Sedated mice should recover from anesthesia within 30 sec of exposure to ambient air.
Isoflurane can be vaporized using an isoflurane vaporizer and O2 (preferred) or a standard drop system closed anesthetic chamber without the vaporizer system (requires close monitoring of the animal while sedated to avoid respiratory distress).
Vaginal lavages on anesthetized mice should be the method of choice for longitudinal lavage samples on the same mice. Consecutive lavaging does not influence assessment of fungal burden over time41. - For wet-mount preparations, transfer 10 μl of the lavage fluid onto a glass slide and observe at 400-1000x magnification by light microscopy. Additionally, cellular fractions of the lavage fluid can be stained to examine cell and nuclear morphologies. For smear preparations, transfer 10 μl of the lavage fluid onto a glass slide and gently spread using the outer wall of a pipette tip. Preserve the smear samples with CytoPrep fixative and stain by the standard Papanicolaou technique (Pap smear). Observe at 400 × magnification by light microscopy.
3. Quantification of vaginal fungal burden
- In a 96 well round-bottom plate, transfer the lavage fluid into one well of the top row and 180 μl of sterile PBS into the following 5 wells of that column (down the plate).
- Make 1:10 serial dilutions of vaginal lavage fluid by transferring the 20 μl of the fluid to the next well in the column. Mix thoroughly by repeated aspiration before each transfer. Serial dilutions of up to 12 lavage samples (one full horizontal row) can be performed simultaneously using a 12-channel pipette.
- Starting with the lowest dilution, transfer 10 μl of the sample onto Sabouraud-dextrose agar (SDA). Plating of up to 36 samples can be performed on 1 plate using SDA prepared in square petri dishes with grid and an adjustable spacing multichannel pipette.
- Enumerate colony forming units (CFUs) after incubation at 34°C for 48 h.
4. Vaginal tissue extraction
- Following the vaginal lavage procedure, lay the euthanized mouse on its back and saturate the groin area with 70% ethanol. Using a pair of forceps, lift the urinary orifice upward so that the vaginal opening becomes exposed.
- Insert a pair of curved forceps into the vaginal lumen and locate the cervix. While maintaining a firm grip with the forceps, extract the cervix through the vaginal cavity (Figure 2A-C).
- Excise the vagina at the base of the vaginal opening and then remove the cervix from the vagina with surgical scissors. Keep in mind that the vaginal tissue is involuted (inner epithelium-side of the vagina is exposed outward). The tissue can be either laterally inverted to maintain the original orientation of the vagina or opened into a sheet by making a lateral incision.
Excised vaginal tissues can be used for 1) lymphocyte extraction following collagenase digestion (~1 ×104/mouse)40, 2) epithelial cell isolation following dispase digestion (~5 ×104/mouse)28, 3) frozen or paraffin-embedded preparations for histological analyses25.
5. Lumbar lymph node excision
- Following the vaginal lavage procedure, lay the euthanized mouse on its back and saturate the abdomen with 70% ethanol. Make a lateral incision starting from the lower abdomen to the chest and expose the internal organs. Using a pair of forceps in both hands, move the intestines upward so that the central blood vessels become visible.
- Locate the inferior vena cava and abdominal aorta. Normally, a pair of lumbar lymph nodes can be identified adjacent to the abdominal aorta, located about halfway between the origin of the renal and common iliac arteries39. These lymph nodes can be visually distinguished from fat tissue by the elastic texture and are lighter and more opaque in color compared to fat tissue (Figure 3). These lymph nodes are noticeably larger in infected animals compared to uninoculated animals.
- Excise the lymph nodes by placing microforceps under the node and then pull up gently to separate from surrounding tissue.
6. Isolation of lymphoid cells in single-cell suspensions
- Transfer lymph nodes onto a sterile wire mesh screen (approximately 3 x 3 cm2 in size) placed inside a sterile glass petri dish containing ˜10 ml of Hanks’ balanced salt solution (HBSS) (Figure 4).
- With the petri dish inclined slightly, press the lymph nodes against the screen with a syringe plunger head. Make sure to break all lymph nodes so that the cellular contents of the nodes pass through the screen while the non-cellular components of the nodes (i.e., membranes, stroma, fat) remain on the screen.
- Using the same plunger and syringe, aspirate the HBSS containing cells. Wash the screen with ˜5 ml HBSS and collect the remaining fluid into a 15 ml conical tube.
- Centrifuge at 800 x g for 10 min. Aspirate any fatty deposits at the top of the fluid with a pipette prior to discarding the fluid. Wash the cell pellet three times with HBSS. Resuspend the pellet in 1 ml of HBSS and enumerate viable cells by trypan blue dye exclusion.
7. Representative Results:
The cellular fractions of vaginal lavage fluid from >4-day inoculated mice typically consist of Candida, epithelial cells and cellular infiltrates (Figure 5). By wet-mount microscopy, Candida can be visually identified by the presence of hyphae as well as yeast (Figure 5A). Smear preparations of vaginal lavage fluid can be stained by Papanicolaou technique to examine epithelial cells and infiltrating leukocytes, of which the principal cells are neutrophils identified by the tri-nuclear lobes (Figure 5B). Very few neutrophils, if any, are detected in uninoculated mice (Figure 5C)41.
An example of vaginal fungal burden is shown in Figure 6. Vaginal lavage fluid collected at specific time points are cultured for CFU enumeration (Figure 6A). Vaginal colonization/infection with Candida persists for weeks in estrogen-treated inoculated mice (Figure 6B), while Candida fails to establish vaginal colonization in non-estrogen-treated inoculated mice (Figure 6C). Estrogen-treated uninoculated mice remain negative for Candida throughout the time (data not shown). In addition, vaginal lavages can be performed either one time on separate mice at each time point or longitudinally in the same mice under anesthesia.
The lumbar lymph nodes are the primary draining lymph nodes of the genital tract and the most relevant site to evaluate for systemic immune responses to a vaginal challenge. Note that these lymph nodes may become enlarged in inoculated mice while they normally appear quite small in uninoculated mice. Leukocyte cellular recoveries typically range from 8 × 105/uninoculated mouse to 5 ×106/inoculated mouse. In addition to lumbar lymph nodes, inguinal, popliteal and mesenteric lymph nodes can also be used.
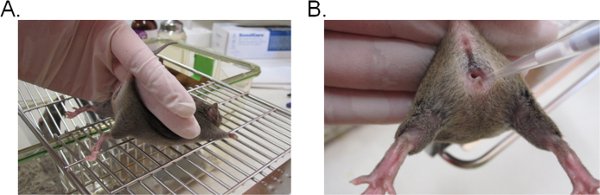
Figure 1. Vaginal inoculation with Candida. A) A mouse restrained for inoculation. The mouse is placed on a wire cage insert and held by the base of the tail, slightly upward to lift the legs and expose the vaginal opening. The hip of the mouse can be stabilized with the same hand as it attempts to resist the tail restraint. B) Introduction of the inoculum into the vaginal lumen. A pipette tip is gently inserted about 5 mm deep into the vaginal lumen. The suspension inoculum is then deposited.
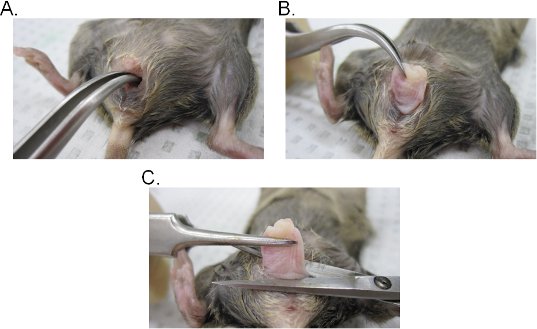
Figure 2. Vaginal tissue extraction. A-B) Extraction of the cervix. The cervix is located with curved forceps and exposed outward through the vaginal cavity. Once out of the vaginal cavity, the cervix is further pulled outward to fully expose the vagina. C) Extraction of the vagina. The vagina is excised from the vulva with scissors. Once detached, remove the cervix from the vagina.
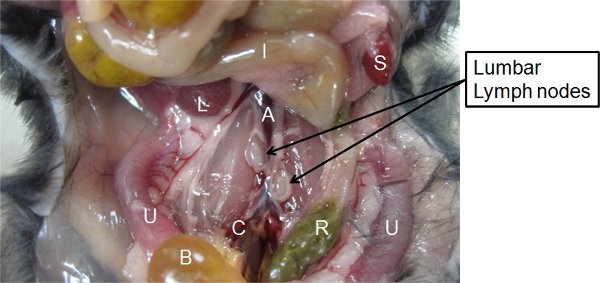
Figure 3. Identification of the lumbar lymph nodes. The location of the lumbar lymph nodes among the surrounding organs/blood vessels in the vicinity of the pelvis is indicated. A, abdominal aorta. B, urinary bladder. C, common iliac artery. I, intestines. L, liver. R, rectum. S, spleen. U, Uteri.

Figure 4. The lumbar lymph nodes placed on a wire mesh screen. The lymph nodes are pooled onto the screen placed in a petri dish with HBSS. The lymph nodes are pressed against the screen with a syringe plunger to obtain lymphoid cells in single-cell suspensions.
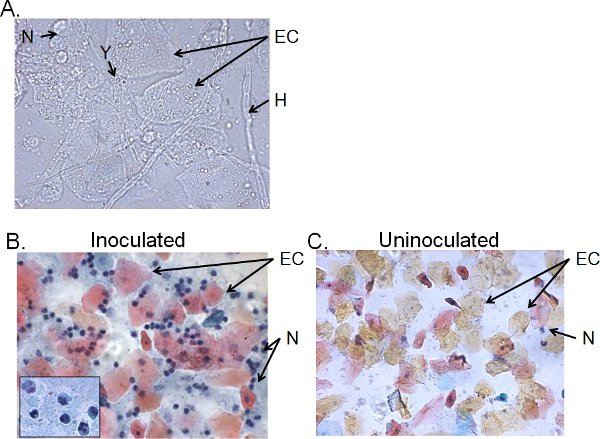
Figure 5. Cellular fractions of vaginal lavage fluid from inoculated mice. A) Wet-mount and B) Pap smear preparations of vaginal lavage samples collected 4 days post-inoculation and C) from uninoculated mice. Images are shown at 1000× (A) or 400× (B, C) magnification. The insert in B shows the nuclear morphology of vaginal neutrophils at 1000× . Candida yeast (Y) and hyphae (H), epithelial cells (EC) and neutrohils (N) are indicated.
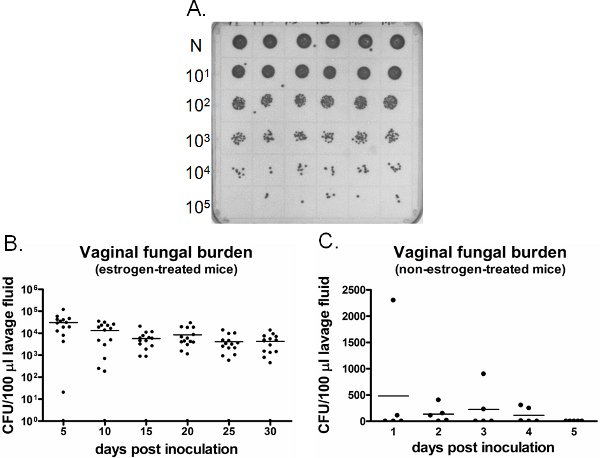
Figure 6. Detection of vaginal fungal burden. A) Representative C. albicans colonies grown on a SDA plate. Neat (N) lavage samples from six different inoculated mice (top row) were serially diluted and cultured for CFU enumeration. B) Quantification of vaginal fungal burden in estrogen-treated and C) non-estrogen-treated mice. CFU/100 μl of lavage fluid from inoculated mice was assessed on indicated time points.
Discussion
An experimental mouse model of Candida vaginitis has been established and historically used for the past few decades to study mucosal host response to Candida as well as for testing antifungal therapies3,4,11,13,16,17,19,21,24,25,37. The protocols presented here incorporate efficient and less labor-intensive methods, and appear to be one of the most optimized model systems of Candida vaginitis described to date. These techniques enable rapid quantification of fungal burden and collection of vaginal specimens. Furthermore, previous studies testing several haplotypic strains of mice (n=13) and various time points post-inoculation showed the similar levels of variability in fungal burden and host responses to vaginal colonization with Candida2,6,41. Hence, this model can be adapted to existing protocols without restriction on mouse strains or the duration of infection. However, one should recognize that the variability in vaginal fungal burden can be high between animals given the same inoculum (Figure 6B). There is evidence supporting that the variability occurs due to differing degrees of early adherence to vaginal epithelium41. Therefore, 7-10 mice/group is suggested for statistical purposes.
Variability in vaginal fungal burden has also been evidenced between different strains of C. albicans, suggesting that not all strains of C. albicans have the capacity to similarly colonize the mouse vagina. For instance, C. albicans 3153A (lab strain) used in this protocol has been reported to show higher vaginal colonization than SC5314 (clinical isolate), where a higher inoculum (> one log) would be required to obtain equivalent levels of vaginal fungal burden seen with 3153A27. In fact, a highly variable vaginal colonization with several clinical isolates has been documented36. Hence, care should be taken when determining an optimal inoculum for each C. albicans strain to ensure consistent colonization in mice. Typically, mean CFUs ranging from 5 x 104 to 5 x 105/100 μl lavage fluid should be detected for consistent colonization. For assessment of vaginal fungal burden, quantification of CFUs by lavages is appropriate for this model as Candida blastoconidia and hyphae normally do not penetrate beyond the superficial layer of vaginal epithelium. Our previous histological evaluation of post-lavage vaginal tissues rarely showed residual Candida25,41. However, we recognize the possibility that hyphae could be miscounted as they grow as a single colony on the agar plate and might not reflect accurate fungal burden. As an alternative method to CFU enumeration, a newly developed in vivo imaging technique has been reported to successfully assess vaginal fungal burden when using genetically engineered luminescent C. albicans9,29. Furthermore, the protocol can be modified to induce C. glabrata colonization in diabetic animals15,20. To date, mouse models for other non-C. albicans-induced vaginitis have not been reported.
Estrogen administration is critical for this model, initiating robust vaginal colonization with Candida6,14,23. In addition to subcutaneous injection of estradiol in sesame oil suspension, injection of water-soluble estradiol in PBS and intradermal implantation of a controlled-release estradiol pellet are alternative methods for estrogen administration and have been employed in other mouse models of female genital tract infections10,35. Requirement of high estrogen in this model may be explained by two physiological factors. One is that elevated estrogen promotes stratification of vaginal epithelium5,8,30. Thickened epithelium from increased epithelial cell proliferation may allow Candida to gain better adherence to the vaginal wall and subsequent colonization. Secondly, vaginal epithelial cells are known to have high glycogen content. An increased tissue estrogen level results in deposition of glycogen into the vaginal walls30. Elevated glycogen in the vaginal microenvironment may in turn allow Candida to flourish by providing additional nutrients. Previous immune analyses showed that exogenous estrogen did not affect cell adhesion molecule expression40. A drawback of treating the animals with exogenous estrogen is that elevated estrogen has also been known to promote overgrowth of vaginal commensal flora30. Since the composition of flora may vary significantly between animals, this may add a variable where commensal organisms could influence Candida growth or modulate host responses. In consideration of this issue, the model requires estrogen-treated control (uninoculated) animals to be included in all experiments and analyzed in parallel.
In this mouse model, the efficacy of anti-fungal agents can be accurately assessed by the protocols described herein, which may provide crucial in vivo testing leading to the development of potential therapies for clinical use. In fact, the robust nature of the model provides good indications of clinical efficacy. In addition to the use of estrogen, neutral vaginal pH in mice promotes growth of hyphae, a pathogenic form of Candida seen in all inoculated mice. Similar to clinical observations, a robust vaginal neutrophil migration occurs in a subset of mice without affecting fungal burden31,41. The presence of neutrophils is prominent in women during symptomatic vaginitis and appears to parallel mice following inoculation12,41. Since other clinical symptoms (i.e., itching, swelling) cannot be precisely measurable in mice, the assessment of vaginal neutrophils serves as a simple yet reliable indication of inflammation (symptoms) in this model.
The microscopy of the vaginal lavage fluid shown in Figure 5 is routinely performed in our laboratory to assess Candida colonization and vaginal inflammation. Moreover, hyphal scoring can also be used as a measure of infection. Thereafter, the cellular and soluble fractions of the lavage fluid can be preserved and cryo-archived for future analyses. Of note, an advantageous feature of the protocol is that vaginal lavages can be performed longitudinally in the same mice under anesthesia. Our previous studies confirmed that longitudinal evaluation does not influence assessment of vaginal fungal burden. This approach is particularly advantageous in cases where longitudinal analyses of infection are desired.
Finally, we have developed a less-invasive excision method for the vagina. This effective and quick technique requires no incision in the abdomen or any internal organs, leaving the rest of the genital tract intact. Another useful feature of this model is the lack of requirement for immunosuppressive agents to initiate Candida colonization. This is particularly important because maintaining natural immune status of the host is a critical aspect of immunological studies and host responses to a microbial challenge. Hence, tissues and cells isolated by these methods could be applied in various in vitro immune assays.
In conclusion, we provide several important features and representative results of the experimental model of vaginal candidiasis. In addition to C. albicans-induced vaginitis, infections by other pathogens of the female lower genital tract, including Neisseria gonorrhoeae, Trichomonas vaginalis and Herpes simplex virus have been studied using mouse models where the organisms are intravaginally introduced in hormone-treated mice1,7,10,22,26,35,38. Therefore, the techniques that are useful for studies involving Candida vaginitis can be applied to and potentially advance methodologies to study pathogenesis and host immune responses for other infectious diseases of the female lower genital tract.
開示
The authors have nothing to disclose.
Acknowledgements
This work was supported by R01 AI32556 (NIAID, National Institute of Health). This work was also supported in part by Louisiana Vaccine Center and South Louisiana Institute for Infectious Disease Research sponsored by the Louisiana Board of Regents.
Materials
| Name of the reagent | Company | Catalogue number | コメント |
| Female CBA/J mice | Charles River Laboratories | 01C38 | 5-6 weeks of age |
| Candida albicans (3153A) | National Collection of Pathogenic Fungi, UK | NCPF3153 | |
| Sesame oil | Sigma-Aldrich | S3547 | Does not need to be pre-sterilized before use |
| Β-estradiol 17-valerate | Sigma-Aldrich | E1631 | 0.1-0.5mg in sesame oil |
| Phytone peptone | Becton Dickinson | 211906 | Supplement with 0.1% glucose |
| Trypan blue solution | Sigma-Aldrich | T8154 | |
| Sabouraud dextrose agar | Becton Dickinson | 211584 | |
| Collagenase type IV | Sigma-Aldrich | C5138 | 0.25% |
| Dispase | Invitrogen | 17105-041 | 1.7 U/ml |
| Wire mesh screens | TWP | 060X060S0065W36T | No. 60 mesh, stainless |
| Hanks’ balanced salt solution | Invitrogen | 24020-117 | |
| CytoPrep fixative | Fisher Scientific | 12-570-10 | Preserves smear slides |
| Papanicolaou stain EA-65 | EMD Chemicals | 7054X-85 | |
| Papanicolaou stain OG-6 | EMD Chemicals | 7052X-85 | |
| Harris’ Alum hematoxylin | EMD Chemicals | 638A-85 | |
| Isoflurane | Baxter Healthcare | NDC 10019-773-60 | Used with isoflurane vaporizer or in a drop system closed anesthetic chamber |
参考文献
- Abraham, M. C. Inducible immunity to Trichomonas vaginalis in a mouse model of vaginal infection. Infect. Immun. 64, 3571-3571 (1996).
- Black, C. A. Major histocompatibility haplotype does not impact the course of experimentally induced murine vaginal candidiasis. Lab. Anim. Sci. 49 (6), 668-668 (1999).
- Black, C. A. Acute neutropenia decreases inflammation associated with murine vaginal candidiasis but has no effect on the course of infection. Inf. Immun. 66, 1273-1273 (1998).
- Black, C. A. Increased severity of Candida vaginitis in BALB/c nu/nu mice versus the parent strain is not abrogated by adoptive transfer of T cell enriched lymphocytes. J. Reprod. Immunol. 45, 1-1 (1999).
- Buchannan, D. L. Role of stromal and epithelial estrogen receptors in vaginal epithelial proliferation, stratification, and cornification. Endocrinology. 139 (10), 4345-4345 (1998).
- Clemons, K. V. Genetic susceptibility of mice to Candida albicans vaginitis correlates with host estrogen sensitivity. Infect. Immun. 72, 4878-4878 (2004).
- Conrady, C. D., Halford, W. P., Carr, D. J. Loss of the type I interferon pathway increases vulnerability of mice to genital Herpes simplex virus 2 infection. J. Virol. 85 (4), 1625-1625 (2011).
- Cunha, G. R., Cooke, P. S., Kurita, T. Role of estromal-epithelial interaction in hormonal responses. Arch Histol Cytol. 67 (5), 417-417 (2004).
- Enjalbert, B. A multifunctional, synthetic Caussia princeps luciferase reporter for live imaging of Candida albicans infections. 77 (11), 4847-4847 (2009).
- Feinen, B. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol. 3 (3), 312-312 (2010).
- Fidel, P. L. Distinct protective host defenses against oral and vaginal candidiasis. Med. Mycol. 40, 359-359 (2002).
- Fidel, P. L. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect. Immun. 72, 2939-2939 (2004).
- Fidel, P. L., Cutright, J. L., Sobel, J. D. Efficacy of D0870 treatment of experimental Candida vaginitis. Antimicrob. Agents. Chemother. 41, 1455-1455 (1997).
- Fidel, P. L., Cutright, J. L., Steele, C. Effects of Reproductive hormones on experimental vaginal candidiasis. Infect. Immun. 68, 651-651 (2000).
- Fidel, P. L. A murine model of Candida glabrata vaginitis. J. Inf. Dis. 173, 425-425 (1996).
- Fidel, P. L. Analysis of vaginal cell populations during experimental vaginal candidiasis. Inf. Immun. 67, 3135-3135 (1999).
- Fidel, P. L., Lynch, M. E., Sobel, J. D. Candida-specific cell-mediated immunity is demonstrable in mice with experimental vaginal candidiasis. Infect. Immun. 61, 1990-1990 (1993).
- Fidel, P. L., Sobel, J. D. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin. Microbiol. Rev. 9. 9, 335-335 (1996).
- Fidel, P. L., Sobel, J. D., Zak, O., Sande, M. . Murine Models of Candida Vaginal Infections, In Experimental models in antimicrobial chemotherapy. , 741-748 (1999).
- Fidel, P. L., Vazquez, J. A., Sobel, J. D. Candida glabrata: Review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 12, 80-80 (1999).
- Fulurija, A., Ashman, R. B., Papadimitriou, J. M. Neutrophil depletion increases susceptibility to systemic and vaginal candidiasis in mice, and reveals differences between brain and kidney in mechanisms of host resistance. 微生物学. 142, 3487-3487 (1996).
- Gill, N. NK cells require type I IFN receptor for antiviral responses during genital HSV-2 infection. Cell Immunol. 269 (1), 29-29 (2011).
- Hamad, M., Abu-Elteen, K. H., Ghaleb, M. Estrogen-dependent induction of persistent vaginal candidosis in naive mice. Cell. Immunol. 47 (7), 304-304 (2004).
- Hamad, M. Utility of the oestrogen-dependent vaginal candidosis murine model in evaluating the efficacy of various therapies against vaginal Candida albicans infection. Mycoses. 49 (2), 104-104 (2006).
- LeBlanc, D. M., Barousse, M. M., Fidel, P. L. A role for dendritic cells in immunoregulation during experimental vaginal candidiasis. Infect. Immun. 74, 3213-3213 (2006).
- McGrory, T., Garber, G. E. Mouse intravaginal infection with Trichomonas vaginalis and role of Lactobacillus acidophilus in sustaining infection. Infect. Immun. 60, 2375-2379 (1992).
- Naglik, J. R., Fidel, P. L., Odds, F. C. Animal models of mucosal Candida infection. FEMS. Microbiol. Lett. 283 (2), 129-129 (2008).
- Nomanbhoy, F. Vaginal and oral epithelial cell anti-Candida activity. Inf. Immun. 70, 7081-7081 (2002).
- Pietrella, D. A beta-glucan-conjugate vaccine and anti-beta-glucan antibodies are effective against murine vaginal candidiasis as assessed by a novel in vivo imaging technique. Vaccine. 28 (7), 1717-1717 (2010).
- Redondo-Lopez, V., Cook, R. N., Sobel, J. D. Emerging role of Lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis. 12 (5), 856-856 (1990).
- Saavedra, M. Local production of chemokines during experimental vaginal candidiasis. Inf. Immun. 67, 5820-5820 (1999).
- Sobel, J. D. Pathogenesis and epidemiology of vulvovaginal candidiasis. Ann. N. Y. Acad. Sci. 544, 547-547 (1988).
- Sobel, J. D. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin. Infect. Dis. 14, S148-S153 (1992).
- Sobel, J. D. Vulvovaginal candidiasis: Epidemiologic, diagnostic, and therapeutic considerations. Am. J. Obstet. Gynecol. 178 (2), 203-203 (1998).
- Song, W. Local and humoral immune responses against primary and repeat Neisseria gonorrhoeae genital tract infections of 17β-estradiol-treated mice. Vaccine. 26, 5741-5741 (2008).
- Taylor, B. N. In vivo virulence of Candida albicans isolates causing mucosal infections in people infected with the human immunodeficiency virus. J. Infect. Dis. 182, 955-955 (2000).
- Taylor, B. N., Saavedra, M., Fidel, P. L. Local Th1/Th2 cytokine production during experimental vaginal candidiasis. Med. Mycol. 38, 419-419 (2000).
- Tirabassi, R. S. A mucosal vaccination approach for herpes simplex virus type 2. Vaccine. 29 (5), 1090-1090 (2011).
- Broeck, W. V. a. n. d. e. n., Derore, A., Simoens, P. Anatomy and nomenclature of murine lymph nodes: descriptive and nomenclatory standardization in BALB/cAnNCrl mice. J. Immunol. Methods. 312 (1-2), 12-12 (2006).
- Wormley, F. L., Chaiban, J., Fidel, P. L. Cell adhesion molecule and lymphocyte activation marker expression during experimental vaginal candidiasis. J. Immunol. Methods. 69, 5072-5072 (2001).
- Yano, J. Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect. Immun. 78 (12), 5126-5126 (2010).

