Measuring Compound Muscle Action Potentials in Mouse Forelimb Muscles In Vivo
Abstract
Source: Pollari, E. et al., In Vivo Electrophysiological Measurement of Compound Muscle Action Potential from the Forelimbs in Mouse Models of Motor Neuron Degeneration. J. Vis. Exp. (2018).
This video demonstrates an in vivo technique for measuring compound muscle action potential (CMAP) in mice. It outlines the steps for setting up electrodes, stimulating forelimb neurons that innervate muscle fibers, and recording muscle responses. The technique assesses neuronal demyelination and functional neuron loss.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Animal Preparation and Anesthesia
- Induce anesthesia in the mouse with isoflurane/oxygen inhalation. Use 4% of isoflurane for the induction of the anesthesia and 2-3% for the maintenance at 2.5 L/min flow of oxygen. Adjust the isoflurane percentage for the maintenance of the anesthesia according to the condition of the mouse, i.e., small and weak mice require less anesthetics. Confirm adequate anesthesia e.g., by applying mild pressure to the hindlimb walking pad to check the absence of a pain withdrawal reflex.
- Control the mouse body temperature using a thermostatic heating plate at 37 °C to prevent the decrease of body temperature during anesthesia.
- Fit the mouse with the nosecone for maintenance of the anesthesia. Ensure that the animal has sufficient delivery of oxygen by checking that the nosecone does not block airways and that the animal is breathing steadily.
- During the recording, monitor whether the mouse is sufficiently anesthetized by observing the breathing rate (approximately 1 Hz in anesthesia) and the absence of a withdrawal reflex on mild pressure. Increase the isoflurane concentration manually if the anesthesia is not deep enough.
- After the measurements, leave the mouse to recover on the heating plate or in the warmth of an infrared lamp until it has regained sufficient consciousness to maintain sternal recumbency, for approximately 2-5 min. Do not leave the mouse unattended and in the company of other mice until it has fully recovered from anesthesia.
2. Measurement of CMAP in Forelimbs
- Use the 27 G needle electrodes for forelimb CMAP measurements. See Figure 1 for recommended places of electrode positioning.
- Place electrodes on the forelimbs as follows.
- Position the mouse on the heating pad in the supine position and use adhesive tape to extend both forelimbs on the sides of the body (Figure 1B).
- Place the stimulating electrodes (1 = anode and 2 = cathode) subcutaneously on both sides of the forelimb to align with the brachial plexus nerve. Lift the skin to insert the needle perpendicularly through the skin and push approximately 5 mm of the needle under the skin without puncturing the underlying muscles.
- Place the recording electrode (3) subcutaneously on top of the biceps brachii muscle by lifting the skin. Place the reference electrode (4) on the walking pads in 3 mm depth at a 30-degree angle. Place the ground electrode (5) subcutaneously on the side of the mouse.
NOTE: Electrodes are in close proximity of each other in this setup. Prevent electrodes from touching each other as this distorts the recording.
3. Data Acquisition
- Start the stimulation by pushing the recurrent stimulus button in the controller unit and turn the intensity controller knob to increase the stimulus. Stimulate all axons using 1 pulse/s with 0.1 ms stimulus duration. Select the correct frequency and duration from dropdown menus in the software.
- To reach supramaximal stimuli (5-20 mA; in demyelinating conditions up to 60 mA), apply increasing stimuli by turning the intensity controller knob until the amplitude of the CMAP response ceases to increase. From there, further increase the stimulus by 20% to ensure that the CMAP amplitude has reached its maximal response. End the stimulation by pushing the recurrent stimulus button again.
- Use the marker tool to indicate the following points in the recording: initiation of the stimulus, initiation of the response, maximum positive peak, and maximum negative peak (Figure 2).
- Determine the latency (in ms) as a delay from the initiation of the stimulus to the initiation of the response (Figure 2). Define the initiation of the response as the earliest point where the amplitude begins to increase. Use the latency to evaluate demyelination in the axons.
- Measure the amplitude (mV) from the maximum negative to the maximum positive peak (Figure 2). Use the magnitude of the amplitude to correlate the number of functional axons.
Representative Results
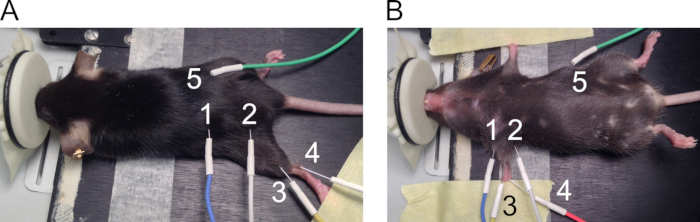
Figure 1. Positioning of the electrodes for CMAP measurements. The position of the electrodes is presented for hind- (A) and forelimbs (B). The electrodes are numbered as follows: 1: anode and 2: cathode stimulating electrodes, 3: active recording electrode, 4: reference electrode, and 5: grounding electrode.
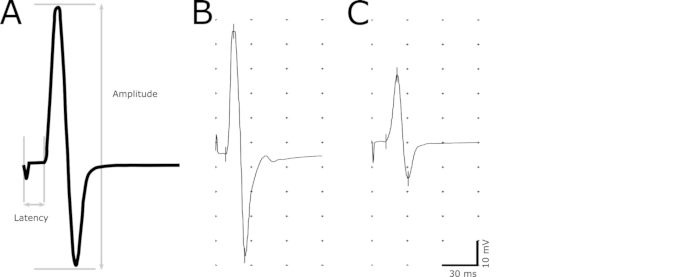
Figure 2. Representative image of CMAP response. A descriptive CMAP response indicating the points used for calculating the amplitude and latency (A). Latency is determined by the delay from the stimulation to the onset of the CMAP response. Peak-to-peak amplitude is measured from the maximum negative to the maximum positive peak of the biphasic wave. Representative recordings of a healthy non-transgenic animal (B) and a diseased animal with prolonged latency and reduced amplitude (C).
開示
The authors have nothing to disclose.
Materials
| Resuable subdermal needle electrode, Pl/Ir | Technomed | TE/S61-434 | The Needle is 13 mm (0.51") in length, 0.4 mm (27G) in diameter |
| Natus electrodiagnostic system | Natus Neurology | UltraPro S100 | EMG device |
| Synergy | Natus Neurology | version 20.1.0.100 | EMG software for UltraPro S100 |
| Physitem Controller | Rothacher-Medical GmbH | TCAT-2LV | Heating pad |
| combi-vet Base Anesthesia System Digital Flowmeter with TEC 3 Vaporize | Rothacher & Partner | CV 30-301-D | Isoflurane Vaporizer and flowmeter |
| Iso-Vet 1000 mg/g | Piramal Healthcare UK Limited | AP/DRUGS/220/96 | Isoflurane |
| SOD1-G93A mice | The Jackson Laboratory | #002726 | ALS tg and non-tg control littermates, only females |
| PrP-hFUS-WT3 mice | The Jackson Laboratory | #017916 | ALS tg and non-tg control littermates, all groups balanced for males and females |
| C57BL/6Jax mice | The Jackson Laboratory | #000664 | Non-tg mice for axotomy, male and female |
| C61-PMP22 mice | Mouse line was generously donated by Prof. M. Sereda (The Max Planck Institute of Experimental Medicine, Göttingen, Germany). | CMT tg and non-tg control littermates, all groups balanced for males and female |

