A Modified Roller Tube Method for the Long-Term Culture of Rodent Brain Slices
Abstract
Source: Fixman, B. B., et al. Modified Roller Tube Method for Precisely Localized and Repetitive Intermittent Imaging During Long-term Culture of Brain Slices in an Enclosed System. J. Vis. Exp. (2017).
This video demonstrates a modified roller tube method for the long-term culturing of brain slices from a rodent model. First, the brain slices are attached to coverslips. Then, flat-sided roller tubes with a hole on the flat side are taken, and the coverslips are positioned on the hole using adhesive discs. Finally, a nutrient medium and a carbon dioxide and air mixture are added to the tube, and the tubes are incubated with a rolling motion.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
NOTE: The protocol below describes the preparation and culture method for the long-term incubation and intermittent imaging of hippocampal slices. A single hippocampal slice is attached to a specially prepared photoetched coverslip using a plasma clot, and then the coverslips are sealed onto the flat side of a drilled-out roller tube, which is maintained in a roller incubator.
1. Preparation of Roller Tube Rack
- Use the template shown in Figure 1A printed to the size shown on the scale bar. With a nail, punch small holes (large enough for a fine point marker) in the template centered on the holes.
- Set the template on the bottom of a 15 cm tissue culture dish (nominal diameter of 14 cm) and mark the position of the holes. Repeat this on a second dish.
- With drill bits designed for use on plastic, drill six 1.5 cm diameter holes on each dish in a hexagonal array (4.8 cm center-to-center) with hole centers 2.5 cm from the edge of the dish. Drill three holes (3 mm in diameter) 12 mm from the edge that are placed equidistantly between two of the larger holes as shown in Figure 1A.
- With the bottoms of each dish facing each other, place a 2.5-inch-long machine screw (3/16-inch diameter) with a flat washer through one of the small holes followed by a second flat washer, a piece of polyethylene tubing (spacer, 4.7 cm), another flat washer, the second tissue culture dish, another flat washer, a locking washer, and a nut.
- Repeat step 1.4 on the other two machine screws and tighten only loosely until all machine screws are in place. Then tighten the nuts securely.
- Work the grommets (5/16 inch thick, 5/8-inch hole diameter) into the holes of the bottom dish to obtain the final roller tube rack (Figure 1B; shown with two tubes in place). Place a sticker on each rack with a unique number.
2. Preparation of Roller Tubes and Coverslips
- Making the jig for drilling the hole in roller tubes
- Drill a 1.5 cm hole 8 cm deep in the center side of a 2 x 4 x 5.5-inch wooden block at an angle such that the flat side of the roller tube will be nearly parallel with the block when inserted (Figure 2A).
- Enlarge the hole using a round wood file to both widen and taper the hole to allow tube insertion (roller tubes are slightly larger in diameter near the cap end) (Figure 2B).
- Drill a 1.5 cm diameter vertical hole, 5.5 cm from the side of the block, and centered over the side hole (Figure 2C).
- When the side hole is tapered enough, insert a roller tube that is marked at the desired spot to center the hole for the slice and position the tube so the marked spot is centered in the 1.5 cm vertical hole.
- Remove the tube and measure the distance from the spot to the end of the tube. Mark this distance from the center of the hole in the jig and insert a nail to provide a stop to correctly position the tube for drilling (arrow in Figure 2C).
- Use a hacksaw to cut the nail off flush with the surface of the wood block to prevent injury.
- Add spring clips on the bottom of the jig if there is a drill press with slots that allow it to be anchored (Figure 2D black arrow). Otherwise, use C-clamps to hold the jig securely onto the drill press.
- Using the jig described above to hold and position a flat sided 11 cm plastic culture tube with the flat side up (Figure 2A), drill a 6 mm diameter hole with the center 1.0 cm from the bottom and centered between the sides of the tube.
NOTE: A drill bit designed for plastic should be used. - With a swiveling deburring tool, smooth the edges of the hole (Figure 2E) and make 4 grooves on the inside edge of the hole (Figure 2E, inset) to facilitate draining of the hole during rotation.
- With a 12 mm hole punch, cut 12 mm diameter disks from non-toxic double sided adhesive silicon rubber sheets. Using a standard one-hole paper punch (6 mm diameter), make a hole in the center of each disk.
- Rinse the drilled tubes with 70% ethanol, air dry them in a biological safety cabinet, and sterilize the tubes and the punched adhesive discs for 40 min under the ultraviolet (UV) lamp (30 W at 70 cm average distance) in the biological safety cabinet.
- Reposition the tubes and discs after 20 min so that all exposed surfaces are sterilized. Under sterile conditions, peel off the white backing from an adhesive disc and affix the silicone rubber to the outside of a tube, aligning the holes (Figure 2F).
Caution: To avoid UV exposure, wear eye protection and close the cabinet before turning on the UV lamp. - Clean 12 mm diameter photoetched (100 center numbered 1 mm squares) German glass coverslips. Hold the coverslips gently with forceps and dip in absolute ethanol, followed by water, followed by absolute ethanol again, and finally dip the coverslips in a flame to burn off the ethanol. Allow coverslips to cool.
- Holding the coverslips with forceps, dip into 2% 3-aminopropyltriethoxysilane in acetone for 10 s. Rinse the coverslips with ultrapure water and allow to air dry.
- Set the coverslips on sterile filter paper inside of a biological safety cabinet and turn on the UV light. Expose each side of the coverslips for 20 min.
Caution: To avoid UV exposure, wear eye protection and close the cabinet before turning on the UV lamp.
3. Hippocampal Slice Preparation
- Before starting the dissection, prepare halves of double-edged razor blades for the tissue chopper. Fold the blades lengthwise carefully with fingers and snap in half.
- Rinse the blade halves with acetone using a cotton swab to clean them, followed by rinsing in absolute ethanol and air drying. Before mounting a half blade on the tissue chopper, sterilize it by rinsing with 70% ethanol.
- Follow protocols approved by the institutional Animal Care and Use Committee; after isoflurane anesthesia, euthanize a 4-7 day old mouse or rat pup by decapitation and remove the head with scissors or guillotine.
NOTE: The brain slice culture protocol is independent of mouse or rat strain or genotype. Many transgenic mouse lines with different genetic backgrounds have been used. - Rinse the head with 70% ethanol, and place it in a 60 mm Petri dish. Until the final mounting of the brain slice onto the roller tube, all of the following steps are performed in a laminar flow hood to maintain sterility.
- Using a #21 surgical blade, make a sagittal cut through the skin and skull. With a #5 Dumont forceps, peel back the skin and skull to expose the brain.
- With closed forceps, gently tease out the whole brain, releasing it by pinching with the forceps through the brain stem behind the cerebellum. Place the brain in a sterile 60 mm dish containing 4 °C Gey's Balanced Salt Solution/0.5% glucose (GBSS/glucose).
- Using a dissection microscope to visualize the brain (Figure 3A), place the brain dorsal side up and cut off the front third of the brain and the cerebellum with a surgical blade (Figure 3B).
- With forceps, hold the trimmed brain posterior side up and ventral side against the side of the Petri dish for stability. Gently tease away meninges around the sagittal midline and remove the midbrain tissue using a fine tipped Dumont #5 forceps (Figure 3C, dashed circle).
- Make two cuts along the side of the brain to spread it open (Figure 3C, dashed lines). Once the brain is placed dorsal side down and spread open, the hippocampal fissure should be visible (Figure 3D, arrow).
- Transfer the spread open brain to a piece of polychlorotrifluoroethylene plastic film and position it for slicing on the stage of a tissue chopper. Wet the blade with GBSS/glucose and chop the hippocampus into ~300 µm thick slices.
- With a transfer pipette, flush the sliced brain off the plastic film into a fresh 60 mm dish containing GBSS/glucose (Figure 3E). Gently pinch off and tease away, with fine tipped forceps, the remaining meninges and other non-hippocampal tissue (Figure 3F) from the slices (Figure 3G, H).
4. Plating Slices
- Once slices have been obtained, place 2 µL of chicken plasma on the center of the photoetched side of a prepared coverslip. Spread the plasma slightly to achieve a 3-4 mm diameter spot.
NOTE: The photoetched side is the top side of the coverslip when viewed through a dissection microscope such that the numbers are oriented correctly. - Transfer 1 brain slice with a sterile narrow-tip spatula (Figure 4A) to the plasma spot (Figure 4B). Use closed forceps to keep the slice on the spatula tip while lifting the slice from the GBSS/glucose.
- Touch the spatula to the plasma spot on the coverslip, and with closed forceps, push the slice onto the coverslip.
- Mix 2.5 µL of plasma with 2.5 µL of thrombin in a separate tube. Quickly place 2.5 µL of this mixture over and around the slice and pipet up and down gently to mix it (Figure 4B).
NOTE: The plasma will clot within 10-15 s, so this must be done quickly. If slice adhesion is a problem, mix 5 µL plasma with 5 µL thrombin and use 4-5 µL on the slice, removing some after mixing so that the slice lies flat on the coverslip. - Remove the clear plastic covering from the exposed side of the silicone rubber adhesive previously affixed to a roller tube and place the coverslip with the brain slice onto the adhesive aligning the slice within the hole (Figure 4C).
- To ensure adhesion, apply soft, even pressure to the coverslip with the thumb by pressing the coverslip down evenly and holding it for about 1 min while transferring it to the biological safety cabinet.
- In a biological safety cabinet, add 0.8 mL of complete Neurobasal A culture medium (Table of Materials) to each tube (Figure 4D).
- Flow a 5% CO2/95% air mixture through a sterile cotton-plugged Pasteur pipette held securely by a clamp. Flush the roller tube with the gas mixture and rapidly cap the tube as it is withdrawn from around the pipette.
- Label the tubes with the slice number and rack number. Insert tubes into a roller rack, ensuring they are geometrically balanced. If there is an odd number of tubes, add tubes to balance.
- Place the racks in a 35 °C roller incubator with rollers turning the roller rack at about 10-13 Revolutions Per Hour (RPH) (Figure 4E). To keep the medium in the bottom of the tubes, tilt the incubator back approximately 5° by raising its front on a board.
Representative Results
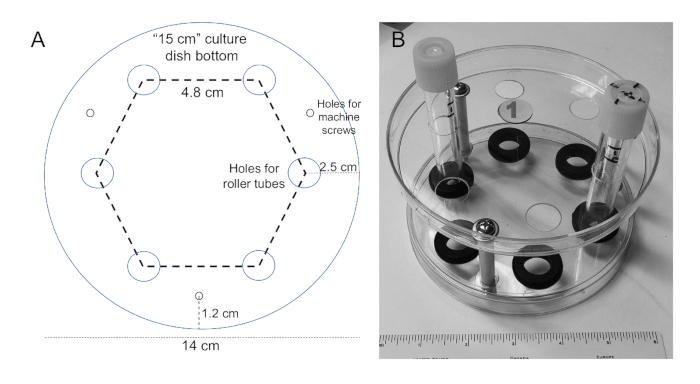
Figure 1: Preparation of roller tube rack. (A) Template for marking hole positions for drilling out the 15 cm tissue culture dish bottoms. If the figure is printed to the size of the scale bar shown, it can be cut out and used for marking the positions on a 15 cm culture dish for drilling the holes shown. (B) Completed roller tube rack with two tubes inserted. Each rack is numbered on a sticker easily visible on the top of the rack.
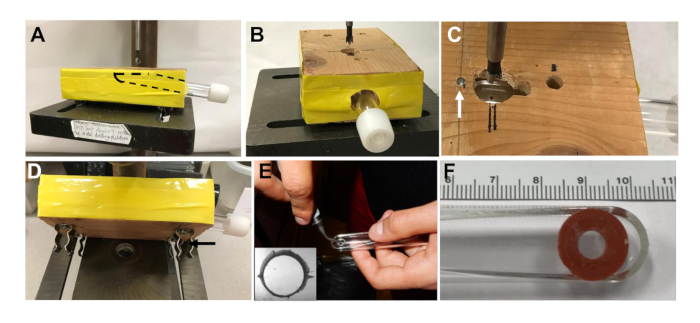
Figure 2: Preparation of the roller culture tubes. (A) Front view of the roller tube inserted in a jig on a drill press for drilling out the 6 mm hole in the tube. Dashed line shows the position of the flat sided roller tube in the jig. (B) End view of the jig with the tube inserted and drill bit aligned over hole. (C) Top view of the hole in the jig for drilling out roller tubes with a 6 mm drill bit. White arrow shows the position of the cut-off nail inserted as a stop for positioning the tubes, and black double lines are for bit alignment. (D) Spring clips (black arrow) installed on the jig bottom to securely hold it in position when drilling tubes. (E) After drilling out the hole, the edges are smoothed with a deburring tool and grooves are cut on the inner side of the hole (inset shows the hole viewed through a dissection microscope) to enhance medium draining from the hole during tube rotation. (F) Culture tube with the hole aligned to a hole in the silicone rubber adhesive, to which the coverslip will be attached.
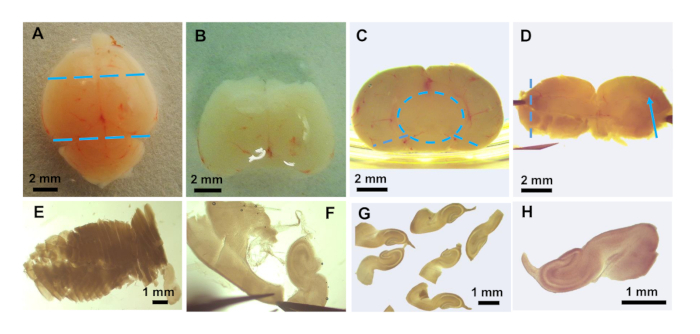
Figure 3: Preparation of hippocampal brain slices. Photos taken with a dissection microscope showing: (A) intact mouse brain. Position of cuts to remove forebrain and cerebellum are shown as blue dashed lines. (B) after removal of forebrain and cerebellum. (C) piece of brain from B is flipped 90° with posterior region (toward the cerebellum) facing up. Positioning the piece next to the side of the dish helps with the removal of the midbrain (blue dashed circle) which can be teased away from the remaining hippocampus, thalamus, and hypothalamus. Two cuts with the forceps (blue dashed lines) allow the remaining piece containing the hippocampus from both hemispheres to be spread flat. (D) The flattened brain piece showing the blood vessel running along the hippocampal fissure (blue arrow). This tissue is placed on plastic film and transferred to the tissue chopper for slicing in the direction of the dashed line. (E) Sliced tissue showing slightly more than half the hippocampus after being returned to GBSS/glucose. (F) Final dissection of the hippocampus and cleaning of the slices to remove non-hippocampal material. (G) Several floating slices after final clean-up. (H) Enlarged photo of a single slice for transfer to coverslip.
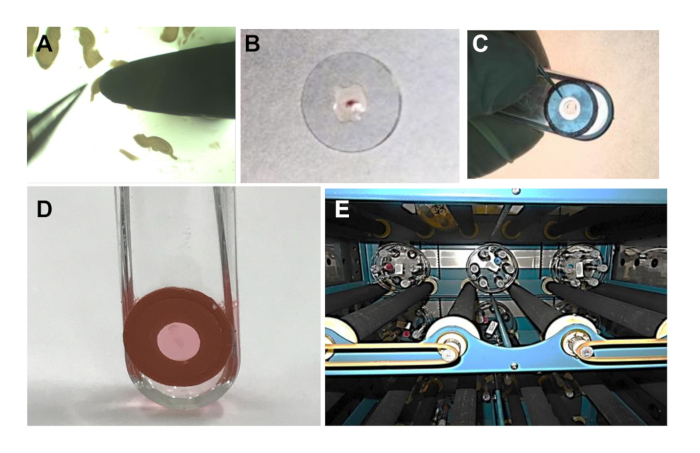
Figure 4: Plating and incubating slices. (A) A mouse hippocampal slice is removed from the culture dish on the tip of a spatula using the tip of forceps to help lift it free from the solution. (B) The slice is placed flat in the center of a photoetched and treated 12 mm coverslip on 2 µL of chicken plasma and another 2.5 µL of a 1:1 plasma/thrombin mixture is added to generate a clot. (C) After the clot is set (about 1-2 min), the covering is removed from the silicone rubber adhesive circle on a roller tube and the coverslip is positioned with the clot centered into the hole; then the coverslip is pressed in place with a thumb and held in position for about 1 min. (D) Add 0.8 mL of complete culture medium. (E) Roller tube holders inside of a large roller incubator with the front raised to tilt 5° to keep the medium at the bottom of the tubes.
開示
The authors have nothing to disclose.
Materials
| Bottoms from 15 cm culture dishes | VWR Scientific | 25384-326 | |
| Phillips Head Machine Screws (#10-32) | Ace Hardware | 2.5" long and 3/16" in diameter | |
| Flat Washers #10 | Ace Hardware | ||
| Machine Screw Nuts (#10-32) | Ace Hardware | ||
| Rubber Grommets | Ace Hardware | 5/16", thick; 5/8", hole diameter; 1.125", OD | |
| Polyethylene tubing (5/16"; OD, 3/16"; ID) | Ace Hardware | Cut to 1.8" length | |
| Lock Washer #10 | Ace Hardware | ||
| Drill Press, 5 speed | Ace Hardware | ||
| Nunclon Delta Flat-Sided Tubes | VWR | 62407-076 | |
| Drill bits, 3 mm, 6 mm and 15 mm | Ace Hardware | Diablo freud brand | Drill bits for cutting plastic |
| Drill bits for wood, 1.5 cm and 1 mm | Ace Hardware | ||
| Wood file, 1/4" round | Ace Hardware | ||
| Spring clips, 16 mm snap holder | Ace Hardware | ||
| Swivel Head Deburring Tool, 5" | Ace Hardware | 26307 | |
| Adhesive Silicone Sheet (Secure Seal) | Grace Bio-Labs | 666581 | 0.5 mm Thickness |
| 6 mm hole punch | Office Max | ||
| 12 mm hole punch | thepunchbunch.com | ||
| 70% Ethanol | |||
| Phototeched Coverslips, 12 mm diameter | Bellco Glass, Inc. | ||
| Bunsen Burner | |||
| Absolute Ethanol | |||
| Nanopure Water | |||
| 3-aminopropyltriethoxylane | Sigma-Aldrich | A3648 | |
| Acetone | Sigma-Aldrich | 179124 | |
| #5 Dumont Forceps | Fine Science Tools | 11251-30 | |
| McIlwain Tissue Chopper | Ted Pella, Inc. | 10180 | |
| Double Edge Razor Blades | Ted Pella, Inc. | 121-6 | |
| Whatman Filter Paper | VWR | 28450-182 | Cut into 5.8 cm diameter circles |
| Poly-chloro-trifluoro-ethylene (Aclar) | Ted Pella, Inc. | 10501-10 | Cut into 5.8 cm diameter circles |
| #21 Surgical Blade | VWR Scientific | 25860-144 | |
| #5 Dumont Forceps | Fine Science Tools | 11251-30 | |
| Spatula, stainless with tapered end | VWR | 82027-518 | |
| Gey's Balanced Salt Solution | Sigma-Aldrich | G9779 | |
| Glucose | ThermoFisher Scientific | 15023-021 | 25% (w/v) Solution, 0.2 mm filter sterilized |
| Chicken Plasma | Cocalico Biologicals | 30-0300-5L | Rehydrate in sterile water, centrifuge at 2500 x g 30 min at 4 °C, quick freeze aliquots in liquid nitrogen and store at -80 °C. |
| Thrombin, Topical (Bovine) | Pfizer | Thrombin-JMI | Quick freeze aliquots in liquid nitrogen at 1,000 international units/mL in diluent provided and store at -80°C. Use at 250 units/ mL. |
| Cell Roller System | Bellco Biotech | SciERA | |
| Roller Incubator | Forma | Model 3956 |

