Microinjection of CRISPR/Cas9 Protein into Channel Catfish, Ictalurus punctatus, Embryos for Gene Editing
Summary
A simple and efficient microinjection protocol for gene editing in channel catfish embryos using the CRISPR/Cas9 system is presented. In this protocol, guide RNAs and Cas9 protein were microinjected into the yolk of one-cell embryos. This protocol has been validated by knocking out two channel catfish immune-related genes.
Abstract
The complete genome of the channel catfish, Ictalurus punctatus, has been sequenced, leading to greater opportunities for studying channel catfish gene function. Gene knockout has been used to study these gene functions in vivo. The clustered regularly interspaced short palindromic repeats/CRISPR associated protein 9 (CRISPR/Cas9) system is a powerful tool used to edit genomic DNA sequences to alter gene function. While the traditional approach has been to introduce CRISPR/Cas9 mRNA into the single cell embryos through microinjection, this can be a slow and inefficient process in catfish. Here, a detailed protocol for microinjection of channel catfish embryos with CRISPR/Cas9 protein is described. Briefly, eggs and sperm were collected and then artificial fertilization performed. Fertilized eggs were transferred to a Petri dish containing Holtfreter’s solution. Injection volume was calibrated and then guide RNAs/Cas9 targeting the toll/interleukin 1 receptor domain-containing adapter molecule (TICAM 1) gene and rhamnose binding lectin (RBL) gene were microinjected into the yolk of one-cell embryos. The gene knockout was successful as indels were confirmed by DNA sequencing. The predicted protein sequence alterations due to these mutations included frameshift and truncated protein due to premature stop codons.
Introduction
Microinjection is a common laboratory technique used to deliver a small amount of a substance such as DNA, RNA, protein, and other macromolecules into cells or embryos through a glass capillary1. Microinjection is performed using special equipment setup including a microinjector, micromanipulator, and a microscope2. The technique has been used by researchers to genetically modify many organisms through the generation of transgenics, gene knockouts, and gene therapy, with the aim of understanding the dynamics of intracellular components3,4,5.
The channel catfish (Ictalurus punctatus) is the most popular catfish species in aquaculture and recreational fishing activities in the United States. There is an increasing need to study functional genomics in channel catfish, and sequencing of the complete genome of channel catfish enhances the utility of recent advances in genome editing tools6,7. Understanding gene function would not only enrich the research that is being conducted on catfish, but could also lead to more effective genetic improvement programs to enhance the catfish industry. Once critical genes for a given trait of interest are identified, they can be used to genetically improve catfish production through genome editing to generate beneficial alleles, selection for these alleles, suppressing the detrimental alleles, transferring the beneficial alleles through transgenesis, or some combination of these options. Combining the best genes for different commercially beneficial traits from different species in one fish would greatly improve productivity and profitability of fish production operations8.
Gene knockout is a direct method to study gene functions in vivo. Mutations at the DNA level will be inherited by future generations which will facilitate the study of their effects in different generations. Different genome editing tools have been developed including zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9) system9,10,11,12.
The CRISPR/Cas9 system is a powerful, efficient tool that has been used to edit genomic DNA sequences including gene knockout in fish through RNA-guided site-specific DNA cleavage5,13. The system consists of a guide RNA (gRNA), which determines the targeted sequence in the genome and a DNA endonuclease enzyme, Cas9. The CRISPR/Cas9 system can be designed to target any sequence in the genome with several advantages over ZFNs and TALENs: (1) lower cost (2) easier engineering (3) more specific binding of guide RNA to the target sequence, and reduced off-target mutations (4) multiple sequences can be targeted with different gRNA at the same time (5) high mutagenesis rate in genes that could not be mutated by TALENs, and (6) improved germline transmission rate of mutations for up to 6-fold when compared to ZFNs and TALENs14,15,16,17,18.
The main alternative method for microinjection is electroporation, in which electric impulses are applied to embryos or cells to increase membrane permeability and the uptake of biological molecules19,20. Several transgenic fish were generated using electroporation such as medaka (Oryzias latipes), zebrafish (Danio rerio), chinook salmon (Oncorhynchus tshawytscha), channel catfish, sea bream (Sparus sarba), and common carp (Cyprinus carpio)21,22,23,24,25,26.
Electroporation has been used to deliver plasmid DNA, RNA, and Cas9 protein for gene knockout. In mammalian cells, Cas9/gRNA plasmid DNA, Cas9 mRNA/gRNA, and Cas9 protein/gRNA complexes were delivered using electroporation and the mutagenesis rates were highest using Cas9 protein/gRNA complexes for most electroporation conditions tested27. In the ascidian chordate (Ciona intestinalis), TALENs expressing constructs were electroporated into fertilized eggs to induce knockout of multiple genes28. ZFNs expressing plasmid constructs were electroporated to knock out luteinizing hormone in channel catfish29. However, once introduced into the cell, plasmid constructs will need to be transcribed to RNA and translated to functional proteins before they can target the desired DNA sequence, which would likely delay the time of mutagenesis compared to microinjection of RNA/protein. As an embryo is developing, delayed mutagenesis increases the mosaicism of injected founders. In addition, transgenic expression is unlikely to achieve the expression level possible with microinjection, lowering the mutagenesis efficiency.
As a means for introducing genome editing tools into cells, microinjection has several advantages over electroporation. Genome editing molecules can be reliably introduced into cells or embryos through microinjection30. Less injection material is needed. It is easy to determine the amounts of material injected. Higher mutation rates with low mosaicism in the founder fish can be achieved which would improve germline transmission of mutations to F1. Fewer founder fish need to be analyzed to produce the first generation, due to the higher mutation rate. In electroporation, more founder fish need to be analyzed which will increase costs. However, the survival of channel catfish microinjected embryos is lower than electroporated ones, though this can be overcome by injecting more embryos31,32. In channel catfish, survival of yolk-microinjected embryos ranged from 16 to 55%, depending on the genes being targeted and the dosage of gRNA/Cas9 protein32.
Regular microinjection of catfish embryos is technically demanding and time consuming, however, a rapid and efficient CRISPR/Cas9 protein microinjection protocol is presented for channel catfish embryos. This protocol requires less time and expertise since the CRISPR/Cas9 protein solution is injected into the yolk of one cell embryos. Hundreds of fertilized eggs can be injected in 1 h (approximately the time needed for the first cell division to occur). To validate the protocol, two disease susceptibility-related genes were knocked out in channel catfish, the toll/interleukin 1 receptor domain-containing adapter molecule (TICAM1) gene and the rhamnose binding lectin (RBL) gene. TICAM 1 is involved in the signaling pathway initiated by toll-like receptor (TLR) 3. In channel catfish, TICAM 1 was dramatically upregulated following bacterial challenge with Edwardsiella ictaluri, while it was downregulated in blue catfish, a species resistant to E. ictaluri33. RBL plays an important role in early infection with Flavobacterium columnare, the causative agent of columnaris disease, where acute and robust upregulation was recorded in a columnaris-susceptible channel catfish strain compared to a columnaris-resistant strain34.
Protocol
This experiment was conducted at the Fish Genetics Research Unit, E. W. Shell Fisheries Research Center, Auburn University, AL. All experimental protocols used in this experiment were approved by the Auburn University Institutional Animal Care and Use Committee (AU-IACUC) before the experiment was initiated. A list of the equipment and supplies used in this experiment can be found in the Materials Table. The following are the steps and procedures for preparation and microinjection of channel catfish one-cell embryos as illustrated in Figure 1.
1. Brood Stock Selection and Spawning
- Choose healthy channel catfish brood stock from the strain or genetic line of interest. Channel catfish males and females should exhibit good secondary sex characteristics.
- Select males that have a broad muscular head that is wider than the rest of their bodies with well-developed genital papilla. Dark color, scarring and wounds from territorial fighting are also signs of sexual readiness. Select females that have heads that are narrower than the rest of their bodies, and have soft, palpable, well-rounded abdomen. For accuracy and to avoid stressing the fish during initial handling, stop feeding the females 2-3 days before examining their readiness for spawning.
- Perform fish handling quickly, but carefully. To reduce handling stress, perform all procedures that require handling females while holding fish in spawning bags (32 mm size mesh).
- Just before hormone injection to induce ovulation, measure the body weight of the females.
- Hang the spawning bags with females inside in a flow through tank with continuous water flow and adequate aeration.
- Aseptically, load the implanter that has a 14 G needle with 85 µg/kg of luteinizing hormone releasing hormone analog (LHRHa) implant. Rinse the injection site (dorsal musculature) with 0.9% sterile saline solution before the injection. Insert the implanter at a 45° angle and release the implant. Withdraw the needle and swipe the injection site with a cotton ball previously soaked in 70% ethanol.
- Place the spawning bag in the holding tank so that the fish is completely submerged in water 15-20 cm below the water surface. Adequate aeration (above 5 ppm dissolved oxygen) and good water quality are important for ovulation of high quality eggs.
- Predict the ovulation time based on the water temperature using the degree-hour (h) relationship35. Degree-h response time = time between hormone injection and ovulation (h) * water temperature (°C). For example, for a degree-h response time of 900-1,000, a female is expected to spawn at 36-40 h from hormone injection time at 25 °C. Usually, the first check for eggs is done at about 36 h after hormone injection and then at 4 h interval.
- To check for ovulation, gently lift the spawning bag above the water surface while you are looking for the eggs that are attached inside the spawning bag. Seeing at least 10 eggs indicates this female is ovulating and ready for hand stripping.
2. Sperm Preparation
NOTE: Sperm can be prepared a few h before the expected ovulation time.
- Euthanize males by a percussive blow to the head without anesthesia, since anesthesia might have a negative effect on sperm motility.
- Collect the testes in a small weighing pan, remove any tissue, and wash them with 50-mL of 0.9% saline to remove blood, if necessary. Well-developed testes are white with many villiform projections ( Figure 2).
- Determine the weight of the testes.
- To prepare the sperm solution for egg fertilization, transfer the testes to the center of a 100 cm2 100 µm mesh using forceps. Fold the mesh with the testes inside, crush the testes and filter the sperm into a 50 mL collection tube. This can be done by applying pressure to testes inside the mesh against the rim of the collection tube via the thumb. Use 0.9% saline solution to wash the sperm from the mesh into the collection tube. Saline solution can be added up to 10 mL/g of testes.
- Determine the concentration of the sperm, check the motility and viability.
NOTE: Concentration can be determined by counting the sperm cells in a known diluted volume of milt using a hemocytometer. Alternatively, the sperm density can be estimated by evaluating the optical density of milt using a spectrophotometer. In this method, the absorption at 505 nm wavelength is compared to a control chart that has been previously prepared for the absorption of different concentrations of sperm36. Sperm motility can be checked under the microscope as the time from activation of the sperm until cessation of sperm movement (called motility duration)37. The viability of sperm can be determined by staining the sperm with selective dyes such as trypan blue, which will stain only non-viable sperm cells, while viable sperm will remain unstained. However, for these microinjection procedures, this is not necessary if the testes are well developed. - Store sperm in 0.9% saline from step 2.5 at 4 °C and use within 24 h after preparation.
3. Egg Collection and Fertilization
- Apply a very thin layer of vegetable shortening to a 20 cm diameter clean dry spawning pan.
- Place the female in the anesthetic solution containing 100 ppm of buffered tricaine methane sulfonate with sodium bicarbonate (pH of final solution should be 7.0) until the fish is completely anesthetized38.
CAUTION: Tricaine methane sulfonate may be irritating if inhaled, ingested or absorbed through the skin. - Carefully, remove the fish from the spawning bag and dip it in 0.9% saline to wash off the anesthetic solution. Dry the fish off completely using a clean towel, avoiding the removal of the mucus layer on the fish body surface.
- Apply vegetable shortening to the area around the vent, including the pelvic fins, to prevent attachment of the eggs during hand stripping.
- Hand strip the eggs into the greased spawning pan by applying gentle pressure to the ovaries. Good eggs should flow freely, be golden yellow in color, and have minimal or no clumps or blood clots (Figure 2). Avoid contact between eggs and water before fertilization, as water can stimulate micropyle closure.
- To fertilize the eggs for microinjection, transfer 200–300 eggs to a greased spawning pan. Greased plastic plant labels can be used as a spoon to transfer eggs to the greased spawning pan. Add 1–2 mL of the sperm solution (from step 2.5) to the eggs and mix gently. The ratio of sperm to egg should be approximately 11,000 sperm/egg.
- Add freshwater to the eggs to activate the sperm and eggs. Add enough water to slightly cover the eggs. Gently swirl the eggs for 30 s. Fertilization should occur in 1–2 min. It is important to fertilize eggs which are arranged in a single layer. Catfish eggs adhere to each other making it difficult to microinject multiple layers of embryos.
- Add more freshwater to the fertilized eggs and allow the eggs to harden for 10–15 min.
- Cover the spawning pan containing the remaining unfertilized eggs by a wet towel to prevent drying and fertilize over a few hours. Fertilization can be done in a staggered manner, for example, one batch of 200–300 eggs can be fertilized every 30–60 min to ensure a continuous supply of embryos at the one cell stage for microinjection and control non-injected embryos. Avoid contact between the wet towel and the eggs as the eggs may adhere to the wet towel making it difficult to remove them. In this protocol, eggs fertilized within 2–3 h after stripping were efficiently microinjected and had similar success as eggs fertilized immediately after stripping.
4. Needle Pulling and Loading
- Pull a 1.0 mm OD borosilicate glass capillary into 2 needles (Figure 3). Store the needles in a paper box with a piece of sponge in which several incisions have been made. To avoid breaking the needle, the diameter of the sponge should be smaller than the length of the needle stem.
- Open the needle tip by breaking a small piece of the needle's thinnest region using a sharp object. Alternatively, open the needle by pinching off the thinnest region of the tip with forceps under the microscope.
- Prepare the injection solution by mixing gRNA(s) with Cas9 protein. Other types of RNA or protein can also be injected with the same procedure. In this protocol, gRNAs were designed to target two of the channel catfish immune related genes, toll/interleukin 1 receptor domain-containing adapter molecule (TICAM1) gene and rhamnose binding lectin (RBL) gene and prepared according to Shah et al. with the use of a high fidelity Taq polymerase32,39. The genomic targets for gRNAs were 5' GGA GGT GAA GCA CGT CGA GGA 3' for TICAM 1 gene (Figure 4) and 5' GGA CTT TGA GTC GGA GAA GTG G 3' for exon 1 of RBL gene with protospacer adjacent motif (PAM) sequence underlined (Figure 6).
- BLAST guide RNA targets against the channel catfish genome sequence and select those with no potential off-target sites.
- Add phenol red to color the gRNA/Cas9 protein mix up to one third of the total volume.
- Adjust the final concentration of gRNA/Cas9 protein mix with nuclease free water so that each embryo is injected with 50 nL containing approximately 2.5 ng gRNA and 7.5 ng Cas9 protein. Incubate the mixture for 10 min on ice before use.
- With a microloader, load 5–10 µL of the gRNA/Cas9 mixture into the injection needle by inserting the microloader into the needle stem and expelling the mixture slowly while retracting the microloader tip (Figure 3). Avoid trapping air bubbles inside.
- Attach the needle to the micropipette holder and ensure a tight connection, and then attach the holder to the micromanipulator. Ensure free stable movement. Apply pressure for microinjection by opening the nitrogen cylinder and adjusting the pressure regulator.
NOTE: The volume of injection can be affected by the pressure, diameter of the needle aperture, and duration of injection. To determine the volume to inject, inject into a drop of mineral oil placed on a hemocytometer. If needed, the pressure, duration of injection, and needle diameter can be adjusted to increase or decrease the volume of the injection. Inject multiple times into the mineral oil to ensure consistent volume.
5. Microinjection of Catfish Embryos
- Apply a very thin layer of vegetable shortening to a 100 mm clean Petri dish.
- Using a greased plastic plant label, transfer 50–100 eggs from the fertilization pan to the Petri dish and cover them with Holtfreter's solution (Materials Table). Align the eggs against each other in a single layer. This alignment should hold the eggs in place during the microinjection process.
- Place the Petri dish with the eggs on the stage of the microscope and hold it with one hand. Lower the needle with the other hand (Figure 3) until it pierces the chorion and the yolk in a single smooth movement. The tip of the needle should be as close as possible to the blastodisc before delivering the injection material.
- Depress the pedal to deliver the injection material into the yolk. If the blastodisc is not clearly visible, then the injection material can be spread into different locations in the yolk. To do that, the needle is inserted to the far end of the yolk, then depressing the pedal and withdrawing the needle are done simultaneously to spread the injection material through different areas of the yolk. Up to 50 nanoliters can be injected into the yolk of channel catfish embryos, however, the amount of gRNA/Cas9 protein needs to be optimized for each gene to achieve the best results for mutation rate and embryo survival32.
- Retract the needle smoothly so as not to damage the egg. Move the Petri dish to inject another egg with the same procedure. Avoid movement of the Petri dish during the injection process as this may damage the egg or break the needle. Remove eggs that are ruptured or damaged due to microinjection. Injection can be started at 15–20 min after fertilization and continue as long as the embryos are still in the one cell stage (until about 60-90 min after fertilization).
- Place injected embryos back in Holtfreter's solution with 10 ppm of doxycycline and continuous gentle aeration for 6–7 days until hatching40. Change the solution daily and remove dead embryos.
6. Mutation Detection
- Randomly, collect several injected embryos at 72 h post fertilization, remove the yolk and extract genomic DNA. Include 3–5 non-injected embryos as a control.
NOTE: Genomic DNA can be extracted from embryos after removal of egg shells and yolk material using proteinase K digestion and ethanol precipitation32. - Amplify a DNA segment flanking the genomic targets for gRNA of each gene, where mutations are expected to occur, using a high fidelity Taq polymerase32. For TICAM 1, the primers 5'GCTGCTGAATGTCTGATTATG 3' (forward) and 5'GTCCTCCACACTCCTGAAG 3' (reverse) were used to amplify a 750 bp while for RBL, the primers 5'TCATTATTTCCTGGTACTCAGTA 3' (forward) and 5'AGAGATTAGTCACACATCATTATT 3' (reverse) were used to amplify a 375 bp segment from the RBL gene.
- Use the following PCR reaction: up to 20 µL PCR grade water; 1x high fidelity Taq enzyme reaction buffer with MgCl2, 0.2 mM dNTP mix, 0.4 µM for each of the forward and reverse primer of the same set, 100-300 ng genomic DNA and 1.25 units of high fidelity Taq enzyme blend. Perform PCR cycling conditions: initial denaturation at 94 °C for 3 min; 35 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 55 s for TICAM, and 40 s for RBL, extension at 72 °C for 1 min/kb; and final extension at 72 °C for 10 min.
- Detect the mutant embryos using a mutation detection method such as sequencing of purified PCR products, surveyor mutation detection assay, heteroduplex mobility assay, loss of restriction enzyme recognition site, or any other mutation detection method that is appropriate for the target. In this protocol, detect mutant embryos using surveyor mutation detection assay for TICAM 1 and loss of restriction enzyme SapI cut site as well as sequencing of purified PCR products for RBL gene.
- To identify mutant alleles, clone PCR products and sequence vectors from individual colonies. The mutant alleles presented in Figure 4 and Figure 6 came from injected embryos. PCR products from six microinjected and 3 control embryos for each gene were pooled in two separate tubes, one for microinjected embryos and the other for control embryos, rapidly cloned and 86 vectors for TICAM 1 and 48 vectors for RBL gene sequenced, including 8 sequencing reactions from 3 control non-injected embryos for each gene.
- To identify different mutant alleles, compare the sequences from mutant embryos to the wild type sequence using any DNA sequence alignment tool such as BLAST or T-COFFEE.
Representative Results
To demonstrate the efficiency of the microinjection protocol, gRNAs designed to target the channel catfish toll/interleukin 1 receptor domain-containing adapter molecule (TICAM1) gene and rhamnose binding lectin (RBL) gene were microinjected.
TICAM 1
DNA sequencing of vectors from individual colonies from pooled, cloned PCR products revealed the indel mutations induced in TICAM 1 gene. The mutation rate was 79% in the 24 individuals analyzed. Examples of mutations are illustrated in Figure 4 and Figure 5. Effects of indel mutations in the TICAM1 gene coding sequence of channel catfish on predicted protein length and sequence compared to the wild type sequence are illustrated in Table 1. Deletion mutations resulted in removal of a few to several amino acids from the predicted protein, leading to changing the downstream reading frame and prematurely terminating translation (Table 1).
In most cases, more than 80% of the predicted protein sequence was truncated due to a premature stop codon. In these cases, the predicted polypeptide length ranged from 91 to 106 amino acid residues (wild type TICAM 1 has 520 amino acid residues). Mutations with extremely truncated protein are expected to produce a non-functional protein. In the case of the 87 bp deletion mutation (Table 2, Figure 4), 29 amino acids were removed from the protein without changing the reading frame. The functional consequences of such mutations need to be experimentally evaluated.
RBL
DNA sequencing of vectors from individual colonies from pooled, cloned PCR products confirmed the presence of indel mutations (Figure 6). The mutation rate was 88% in the 40 individuals analyzed. Deletions ranged from 5 bp to 183 bp, while up to 20 bp were inserted. Interestingly, the 183 bp deletions completely removed intron 1, exon 2, and 19 bp from intron 2. Theoretically, this should influence the splicing of the RBL gene since the splice sites have been mutated.
Indel mutations had variable effects on the predicted protein sequence when compared to the wild type protein sequence (308 amino acid residues) (Table 2). More than 70% of indels resulted in a predicted truncated protein that is 10% or less than the length of the wild type protein. In some indels (mutation number 4, 5, and 6), the predicted polypeptide was less than 10 amino acids. Only 2 cases (number 2 and 3) resulted in deletion of 2 and 4 amino acids in the predicted protein without changing the reading frame which may or may not affect the protein function.
Bi-allelic mutations were induced with two other gRNAs (Elaswad et al., unpublished) where both chromosomes were mutated in some embryos and no wild type alleles were detected. With gel electrophoresis of PCR products, these embryos had only one band that was shorter than the wild type band (more than 200 bp deletion). DNA sequencing of cloned PCR products confirmed the presence of only one mutant allele in all vectors sequenced from one embryo (homozygous biallelic mutation) while two mutant alleles occurred in other embryos (heterozygous biallelic mutation). In some other embryos that were mosaic for the mutation, both mutant (up to 4 alleles) and wild type alleles were detected by DNA sequencing of cloned PCR products.
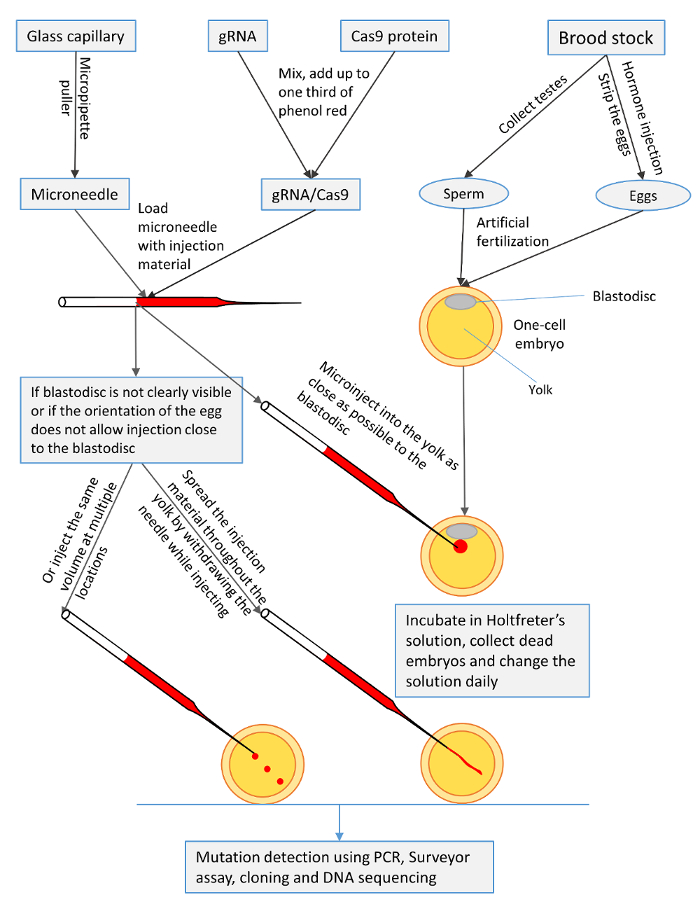
Figure 1: A summary of the microinjection procedures for one-cell channel catfish (Ictalurus punctatus) embryos with gRNA/Cas9 protein to knock out toll/interleukin 1 receptor domain-containing adapter molecule (TICAM1) gene and rhamnose binding lectin (RBL) gene. Please click here to view a larger version of this figure.
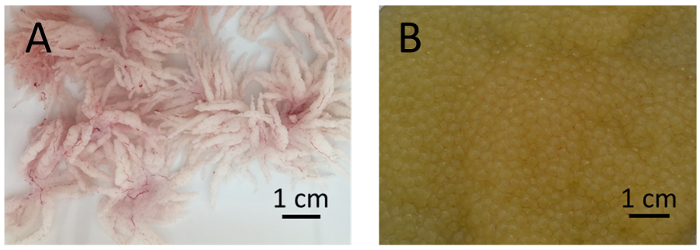
Figure 2: Testes and eggs of channel catfish, Ictalurus punctatus. (A) Well-developed testes of channel catfish males are white with many villiform projections. (B) Good eggs from channel catfish females are golden-yellow in color with minimal or no blood clots. Please click here to view a larger version of this figure.
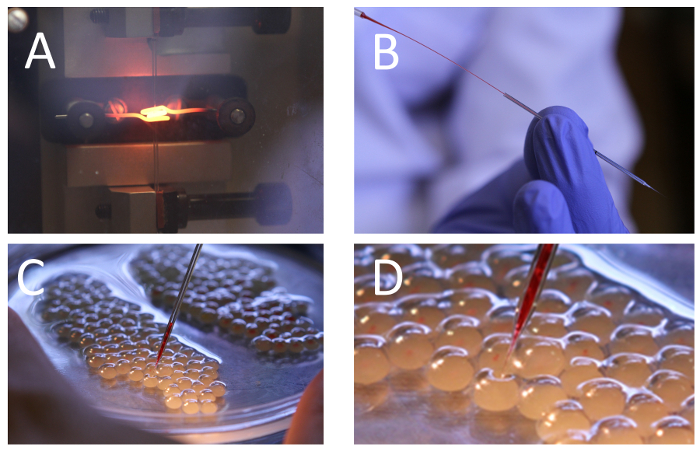
Figure 3: Microneedle pulling, loading and microinjection of one-cell channel catfish, Ictalurus punctatus, embryos.(A) Needle pulling with a vertical needle puller. Needle structure and diameter are affected by the heat of the heater filament and the solenoid force. (B) Microneedle loading with the CRISPR/Cas9 mix using a microloader tip. (C) Arrangement of channel catfish embryos in a 100-mm Petri dish containing Holtfreter's solution. Microneedle is lowered until it touches and pierces the egg. (D) Magnified section of panel C showing the process of piercing the egg. Note the needle tip is pushing the chorionic membrane inward but has not penetrated it yet. Injected embryos have the red injection material inside. Please click here to view a larger version of this figure.
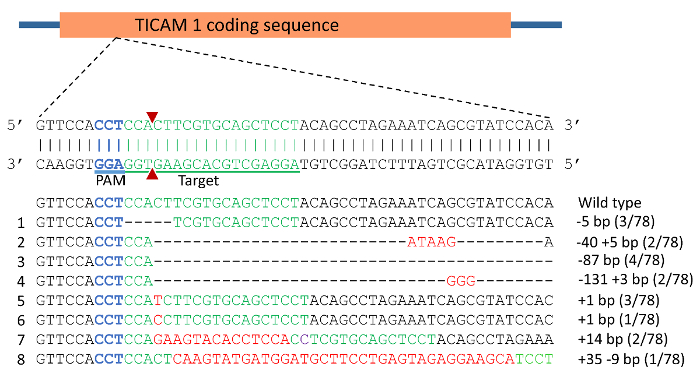
Figure 4: CRISPR/Cas9 induced mutations in toll/interleukin 1 receptor domain-containing adapter molecule (TICAM1) gene coding sequence of channel catfish, Ictalurus punctatus. Blue sequence represents the protospacer adjacent motif (PAM) while the green sequence represents the target for gRNA. Double strand break induced by Cas9 protein was expected to occur at the site of the 2 red triangles. Mutations are assigned numbers 1 through 8. Deletion mutations are represented by a dashed line in which each dash corresponds to a nucleotide that has been deleted. Red sequences are insertions while purple represents substitution mutation. Fractions of 78 represent the number of mutated alleles in 78 sequencing reactions (e.g., 3/78 means that an allele was detected in 3 sequencing reactions from a total of 78 reactions). Please click here to view a larger version of this figure.
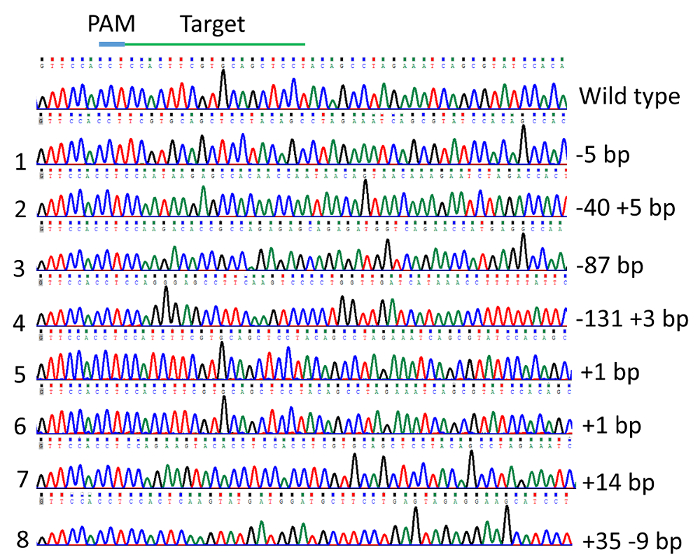
Figure 5: Indel mutations in the channel catfish, Ictalurus punctatus, toll/interleukin 1 receptor domain-containing adapter molecule (TICAM1) gene coding sequence induced by microinjection of gRNA/Cas9 protein into channel catfish one-cell embryos as revealed by DNA sequencing chromatogram. Please click here to view a larger version of this figure.
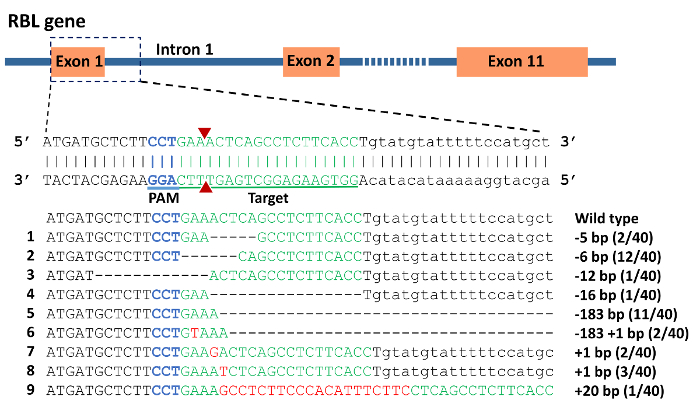
Figure 6: CRISPR/Cas9 induced mutations in rhamnose binding lectin (RBL) gene of channel catfish, Ictalurus punctatus. Exonic sequences are uppercase while intronic sequences are lowercase letters. Blue sequence represents the protospacer adjacent motif (PAM) while the green sequence represents the target for gRNA. A double strand break induced by Cas9 protein was expected to occur at the site of the 2 red triangles. Mutations are assigned numbers 1 through 9. Deletion mutations are represented by a dashed line in which each dash corresponds to a nucleotide that has been deleted. Red sequences are insertions. Fractions of 40 represent the number of mutated alleles in 40 sequencing reactions e.g. 2/40 means that an allele was detected in 2 sequencing reactions from a total of 40 reactions. Please click here to view a larger version of this figure.
| Mutation | Insertion | Deletion | Net change (Δ) | Protein length (amino acid) | Frameshift changed | Premature stop codon | Predicted protein sequence | |||
| WT | 0 | 0 | 0 | 520 | No | No | MAEETELVDEKKPTELCVNRNTVVNSPLERSISG SRSTENISGEHTAAECLIMGASLQPRRESTEAFK VQKEPPKRDDSYPSSLRSTSTSCSSYSLEISVST ATTNNSNKESRPLPLKTPPESRDGQNHEAKPSS PLVDHKPFYSVTKSYKSSDPTPLQERAQLEKFKF RQYPDKHDTSEIVTPKSPNIESGLEKTFLSIPSGG GKVGAQPVSTSKPPEASSTQEVGKHDSFSTEKQ ASQEEEDMFYAFVILHAEEDSEEAVRLKSRLESIS STIGATFSEDFAVPGQSTFRSVEDAIENSAYVMLL LTPNFNTHLNETNADSALMNSIEKPHKHNTVIPL LPRANGLTRNQMPFILRTKNPLVETRDRDTFEKM AKKVLDLRNIQRQKSMWTEAQLVKKQREKQQW LQEKKRYCKDFIQESPRVRELEEQIQQLKMQQQ HLQPPYAQQTNSHQGFPGRPQSSGPMPFRSPS PMPSYYSGNMWPQLPSNIHIQNAKCIMIGNNSTM TVGGGVDSGDEDNF (GenBank: NP_001187154.1 |
|||
| 1 | 0 | 5 | -5 | 104 | Yes | Yes | MAEETELVDEKKPTELCVNRNTVVNSPLERSISG SRSTENISGEHTAAECLIMGASLQPRRESTEAFK VQKEPPKRDDSYPSSLRSTFVQLLQPRNQRIHS HNQ |
|||
| 2 | 5 | 40 | -35 | 94 | Yes | Yes | MAEETELVDEKKPTELCVNRNTVVNSPLERSIS GSRSTENISGEHTAAECLIMGASLQPRRESTEAF KVQKEPPKRDDSYPSSLRSTSNKSHNQ |
|||
| 3 | 0 | 87 | -87 | 491 | No | Yes | MAEETELVDEKKPTELCVNRNTVVNSPLERSISG SRSTENISGEHTAAECLIMGASLQPRRESTEAFK VQKEPPKRDDSYPSSLRSTSKTPPESRDGQNHE AKPSSPLVDHKPFYSVTKSYKSSDPTPLQERAQ LEKFKFRQYPDKHDTSEIVTPKSPNIESGLEKTFL SIPSGGGKVGAQPVSTSKPPEASSTQEVGKHDSF STEKQASQEEEDMFYAFVILHAEEDSEEAVRLKS RLESISSTIGATFSEDFAVPGQSTFRSVEDAIENSA YVMLLLTPNFNTHLNETNADSALMNSIEKPHKHN TVIPLLPRANGLTRNQMPFILRTKNPLVETRDRDT FEKMAKKVLDLRNIQRQKSMWTEAQLVKKQREK QQWLQEKKRYCKDFIQESPRVRELEEQIQQLKMQ QQHLQPPYAQQTNSHQGFPGRPQSSGPMPFRSP SPMPSYYSGNMWPQLPSNIHIQNAKCIMIGNNST MTVGGGVDSGDEDNF |
|||
| 4 | 3 | 131 | -128 | 95 | Yes | Yes | MAEETELVDEKKPTELCVNRNTVVNSPLERSISG SRSTENISGEHTAAECLIMGASLQPRRESTEAFK VQKEPPKRDDSYPSSLRSTSRAFKSPG |
|||
| 5 | 1 | 0 | 1 | 106 | Yes | Yes | MAEETELVDEKKPTELCVNRNTVVNSPLERSISG SRSTENISGEHTAAECLIMGASLQPRRESTEAFK VQKEPPKRDDSYPSSLRSTSIFVQLLQPRNQRIH SHNQ |
|||
| 6 | 1 | 0 | 1 | 106 | Yes | Yes | MAEETELVDEKKPTELCVNRNTVVNSPLERSISG SRSTENISGEHTAAECLIMGASLQPRRESTEAFK VQKEPPKRDDSYPSSLRSTSTFVQLLQPRNQRIH SHNQ |
|||
| 7 | 14 | 0 | 14 | 100 | Yes | Yes | MAEETELVDEKKPTELCVNRNTVVNSPLERSISG SRSTENISGEHTAAECLIMGASLQPRRESTEAFK VQKEPPKRDDSYPSSLRSTSRSTPPPRAAPTA |
|||
| 8 | 35 | 9 | 26 | 91 | Yes | Yes | MAEETELVDEKKPTELCVNRNTVVNSPLERSISG SRSTENISGEHTAAECLIMGASLQPRRESTEAFK VQKEPPKRDDSYPSSLRSTSTQV |
|||
Table 1: Effects of indel mutations in the toll/interleukin 1 receptor domain-containing adapter molecule (TICAM1) gene coding sequence of channel catfish, Ictalurus punctatus, on predicted protein length (amino acids) and sequence compared to the wild type sequence (WT).
| Mutation | Insertion | Deletion | Net change (Δ) | Protein length (amino acid) | Frameshift changed | Premature stop codon | Predicted protein sequence | |||
| WT | 0 | 0 | 0 | 308 | No | No | MMLFLKLSLFTLIISAPGLMVSGENMITCYSDVQR LTCETGLIKVKSTVYGRTNSITCNTNRPFSEVTFT NCALRITTIADRCNGLKECELKTDLLGNPDPCFG TYKYYNTTYDCINGHRVVICEQGYSTLDCGSDSIE IINANYGRGNSRTCSNGILSSQTQNTNCYAPNTL SIVAAMCKGKKTCTVEASNTIFNDPCVGTVKYLT VSYICTREIVTCESSTATLNCGAHRIKIISANYGRT DSTTCSSGRPASQTSNKNCYTPDALNKIAARCE EQSSCEVPATNVVFSDPCFGTYKYLTIVYSCV (GenBank: AHJ14694.1) 53 |
|||
| 1 | 0 | 5 | -5 | 29 | Yes | Yes | MMLFLKPLHLNYFSSWLNGFWREYDYLLQ | |||
| 2 | 0 | 6 | -6 | 306 | No | No | MMLFLSLFTLIISAPGLMVSGENMITCYSDVQRLT CETGLIKVKSTVYGRTNSITCNTNRPFSEVTFTN CALRITTIADRCNGLKECELKTDLLGNPDPCFG TYKYYNTTYDCINGHRVVICEQGYSTLDCGSDSI EIINANYGRGNSRTCSNGILSSQTQNTNCYAPNT LSIVAAMCKGKKTCTVEASNTIFNDPCVGTVKYL TVSYICTREIVTCESSTATLNCGAHRIKIISANYGR TDSTTCSSGRPASQTSNKNCYTPDALNKIAARC EEQSSCEVPATNVVFSDPCFGTYKYLTIVYSCV |
|||
| 3 | 0 | 12 | -12 | 304 | No | No | MILSLFTLIISAPGLMVSGENMITCYSDVQRLTCE TGLIKVKSTVYGRTNSITCNTNRPFSEVTFTNCA LRITTIADRCNGLKECELKTDLLGNPDPCFGTYK YYNTTYDCINGHRVVICEQGYSTLDCGSDSIEIINA NYGRGNSRTCSNGILSSQTQNTNCYAPNTLSIVA AMCKGKKTCTVEASNTIFNDPCVGTVKYLTVSYI CTREIVTCESSTATLNCGAHRIKIISANYGRTDST TCSSGRPASQTSNKNCYTPDALNKIAARCEEQS SCEVPATNVVFSDPCFGTYKYLTIVYSCV |
|||
| 4 | 0 | 16 | -16 | 6 | Yes | Yes | MMLFLN | |||
| 5 | 0 | 183 | -183 | 8 | No | Yes | MMLFLKRI (Assuming splicing is not altered) | |||
| 6 | 1 | 183 | -182 | 5 | Yes | Yes | MMLFL (Assuming splicing is not altered) | |||
| 7 | 1 | 0 | 1 | 31 | Yes | Yes | MMLFLKTQPLHLNYFSSWLNGFWREYDYLLQ | |||
| 8 | 1 | 0 | 1 | 31 | Yes | Yes | MMLFLKSQPLHLNYFSSWLNGFWREYDYLLQ | |||
| 9 | 20 | 0 | 20 | 18 | Yes | Yes | MMLFLKASSHISSSASSP | |||
Table 2: Effects of indel mutations in the rhamnose binding lectin (RBL) gene of channel catfish, Ictalurus punctatus, on predicted protein length (amino acids) and sequence compared to the wild type sequence (WT).
Discussion
A detailed protocol for microinjection of channel catfish embryos to achieve gene knockout was presented. Injection of CRISPR/Cas9 protein in the yolk is much simpler, saves time, and does not require extensive training when compared to microinjecting the blastodisc of the one-cell embryo, which is the common technique for most gene transfer and gene editing with fish30,42. The current protocol proved to be successful in inducing indels in channel catfish TICAM 1 and RBL genes. These reliable and effective procedures lay the foundation for generating future gene knockouts in channel catfish. The protocol can be modified to target other genes in the channel catfish genome using the CRISPR/Cas9 system, or for adapting other gene technologies to catfish, such as tol2 mediated transgenesis. It is also worth evaluation in other catfish species such as the blue catfish (I. furcatus), which are commercially important and their eggs seem to be more sensitive to handling than channel catfish eggs. However, if other gRNAs or biologically active substances such as DNA or mRNA are to be injected, the protocol may need to be optimized to determine the best conditions.
In protocols for microinjection of fish embryos, microinjection procedures could be divided into two main categories: injection into the embryonic cells and injection into the yolk. In medaka, morpholinos were injected into the cytoplasm of one-cell embryos to study various developmental processes in vivo41. In channel catfish, CRISPR/Cas9 mRNA targeting the GnRH gene were microinjected into the blastodisc of one-cell embryos30. In three-spined stickleback (Gasterosteus aculeatus), blastomeres were microinjected to generate transgenics and gene knockouts42. On the other hand, one-cell zebrafish embryos were yolk-injected with morpholinos to manipulate the protein Heart of Glass (Heg), which proved to be effective43. These 2 types of approach have their advantages and disadvantages, but if both prove to have the same effectiveness for the same species, yolk microinjection of one-cell embryos would be preferred for several reasons.
Yolk microinjection is less invasive to the embryos, since the microinjection needles do not pierce the embryonic cells. In addition, many embryos can be injected in a short period while the embryos are still at the first cell stage. There is no need to align the embryos in a certain position. This also would save time and reduce the handling stress of embryos. In seasonally spawning fish, there is a limited spawning time in which all the procedures need to be done to achieve successful knockout. In channel catfish, the spawning season lasts for about 2 months44. Therefore, developing a rapid and effective microinjection protocol will allow microinjection of many embryos to target many genes in this short time period. Fortunately, channel catfish can be artificially spawned to supply a great enough number of embryos for microinjection to overcome the high mortality due to microinjection procedures45. In addition, the time of fertilization can be controlled to ensure the embryos are in the one-cell stage when needed.
Microinjection of CRISPR/Cas9 induced indels in different fish species including zebrafish, Atlantic salmon (Salmo salar L.), Nile tilapia (Oreochromis niloticus), channel catfish, Atlantic killifish (Fundulus heteroclitus)46, and common carp5,30,46,47,48,49,50. In addition, the CRISPR/Cas9 system was used to disrupt genes in Labeo rohita carp via homologous recombination-mediated insertion of foreign DNA into targeted genes51. In this protocol, successful indels were induced in the coding sequences with dramatic effects on the predicted protein sequence, including frameshift mutations resulting in premature stop codon. The phenotypic effects of these mutations still need to be investigated. Truncated mRNAs are most likely degraded through the nonsense-mediated mRNA decay (NMD) pathway, resulting in the reduction or inhibition of expression, and if expressed, truncated proteins will likely be nonfunctional52,53.
In our experiment with the RBL gene, two types of mutations (number 5 and 6) resulted in deletion of 183 bp completely removing half of exon 1, intron 1, exon 2, and part of intron 2. Theoretically, this type of mutation may alter the splicing of RBL pre-mRNA. Mutations at the splice sites inhibit the ability of the spliceosome to recognize the exon54.
In conclusion, the development of a reliable efficient protocol for targeted gene editing in channel catfish is crucial for studying functional genomics, especially after the genomic resources have been enriched with the recent sequencing of the channel catfish genome. The procedures described here are simple, rapid but efficient and are proven to successfully achieve gene knockout. Two genes, TICAM 1 and RBL, were targeted to demonstrate the efficiency of CRISPR/Cas9 knockout using microinjection. Depending on what is being injected, the protocol can be modified to include other biologically active substances. It can be also modified to microinject other catfish species or other fish species that have similar egg structure and composition. This protocol lay the foundations for gene knockout in channel catfish.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This research was funded by USDA-NIFA award 2015-67015-23488 to Roger Cone. The authors thank Dr. Ronald Phelps for the description of brood stock selection criteria in the video. Ahmed Elaswad and Karim Khalil would like to thank the Egyptian Cultural and Educational Bureau in Washington DC for funding their Ph.D. research.
Materials
| Reproboost implant | Center of Marine Biotechnology | Luteinizing hormone releasing hormone analog (LHRHa) for induction of ovulation in channel catfish females | |
| TRICAINE-S | Western Chemical. Inc. | For sedation of brood stock fish during hormone injection and egg stripping. | |
| Phenol red | Sigma-Aldrich | P0290 | 0.5%, sterile filtered |
| Stereo microscope | Olympus | 213709 | For visualizing the eggs during microinjection |
| Microinjector | ASI-Applied Scientific Instrumentation | Model MPPI-3 | For the delivery of the injection material into the embryos |
| Micromanipulator | ASI-Applied Scientific Instrumentation | Model MM33 | For holding and controlling the movement of the injection needle. |
| Eppendorf Microloader | Eppendorf | 5242956.003 | For loading injection solution into microinjection needles. |
| Vertical needle puller | David Kopf Instruments | Model 720 | For pulling microinjection needles |
| Cas9 protein | PNA Bio Inc. | CP01 | Recombinant Cas9 protein from Streptococcus pyrogenes. |
| Expand High FidelityPLUS PCR System | Roche Diagnostics, USA | For PCR and amplification of DNA templates to be used in gRNA preparation | |
| Borosilicate glass capillaries | Fisher Scientific | 1 mm outer diameter (OD), for making microinjection needles. | |
| Petri dish | VWR | 25384-302 | For holding the embryos during the microinjection. |
| Crisco | The J.M. Smucker Company | Vegetable shortening for coating spawning pans and petri dishes. | |
| Holtfreter`s solution | Home Made | 59 mM NaCl, 0.67 mM KCl, 2.4 mM NaHCO3, 0.76 mM CaCl2, 1.67 mM MgSO4 to incubate the microinjected embryos till hatch. | |
| Doxycycline hyclate USP (monohydrate) | Letco Medical | 690904 | Antibiotic added to Holtfreter's solution to 10 ppm to prevent bacterial infections. |
Riferimenti
- Hammer, R. E., et al. Production of transgenic rabbits, sheep and pigs by microinjection. Nature. 315, 680-683 (1985).
- Komarova, Y., Peloquin, J., Borisy, G. . Components of a microinjection system. , 935-939 (2011).
- Du, S. J., et al. Growth enhancement in transgenic Atlantic salmon by the use of an "all fish" chimeric growth hormone gene construct. Nat. Biotechnol. 10 (2), 176-181 (1992).
- Davis, B., Brown, D., Prokopishyn, N., Yannariello-Brown, J. Micro-injection-mediated hematopoietic stem cell gene therapy. Curr. Opin. Mol. Ther. 2 (4), 412-419 (2000).
- Jao, L. E., Wente, S. R., Chen, W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. U.S.A. 110 (34), 13904-13909 (2013).
- Liu, Z., et al. The channel catfish genome sequence provides insights into the evolution of scale formation in teleosts. Nat. Commun. 7, (2016).
- Abdelrahman, H., et al. Aquaculture genomics, genetics and breeding in the United States: current status, challenges, and priorities for future research. BMC Genomics. 18 (1), 191 (2017).
- Dunham, R. A. . Aquaculture and Fisheries Biotechnology : Genetic Approaches. , (2011).
- Miller, J. C., et al. An improved zinc-finger nuclease architecture for highly specific genome editing. Nat. Biotechnol. 25 (7), 778-785 (2007).
- Doyon, Y., et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat. Biotechnol. 26 (6), 702-708 (2008).
- Miller, J. C., et al. A TALE nuclease architecture for efficient genome editing. Nat. Biotechnol. 29 (2), 143-148 (2011).
- Ran, F. A., et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8 (11), 2281-2308 (2013).
- Hwang, W. Y., et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31 (3), 227-229 (2013).
- Cong, L., et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 339 (6121), 819-823 (2013).
- Cradick, T. J., Fine, E. J., Antico, C. J., Bao, G. CRISPR/Cas9 systems targeting β-globin and CCR5 genes have substantial off-target activity. Nucleic Acids Res. 41 (20), 9584-9592 (2013).
- Hruscha, A., et al. Efficient CRISPR/Cas9 genome editing with low off-target effects in zebrafish. Development. 140 (24), 4982-4987 (2013).
- Varshney, G. K., et al. High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome Res. 25 (7), 1030-1042 (2015).
- Li, M., Zhao, L., Page-McCaw, P. S., Chen, W. Zebrafish genome engineering using the CRISPR-Cas9 system. Trends Genet. 32 (12), 815-827 (2016).
- Neumann, E., Schaefer-Ridder, M., Wang, Y., Hofschneider, P. Gene transfer into mouse lyoma cells by electroporation in high electric fields. The EMBO Journal. 1 (7), 841 (1982).
- Powers, D. A., et al. Electroporation: a method for transferring genes into the gametes of zebrafish (Brachydanio rerio), channel catfish (Ictalurus punctatus), and common carp (Cyprinus carpio). Mol. Mar. Biol. Biotechnol. 1 (4-5), 301-308 (1991).
- Inoue, K., et al. Electroporation as a new technique for producing transgenic fish. Cell Differ. Dev. 29 (2), 123-128 (1990).
- Buono, R., Linser, P. Transient expression of RSVCAT in transgenic zebrafish made by electroporation. Mol. Mar. Biol. Biotechnol. 1 (4-5), 271-275 (1991).
- Sin, F., et al. Gene transfer in chinook salmon (Oncorhynchus tshawytscha) by electroporating sperm in the presence of pRSV-lacZ DNA. Aquaculture. 117 (1-2), 57-69 (1993).
- Dunham, R. A., et al. Enhanced bacterial disease resistance of transgenic channel catfish Ictalurus punctatus possessing cecropin genes. Mar. Biotechnol. 4 (3), 338-344 (2002).
- Lu, J. K., Fu, B. H., Wu, J. L., Chen, T. T. Production of transgenic silver sea bream (Sparus sarba) by different gene transfer methods. Mar. Biotechnol. 4 (3), 328-337 (2002).
- Cheng, Q., et al. Interaction of diet and the masou salmon Δ5-desaturase transgene on Δ6-desaturase and stearoyl-CoA desaturase gene expression and N-3 fatty acid level in common carp (Cyprinus carpio). Transgenic Res. 23 (5), 729-742 (2014).
- Liang, X., et al. Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. J. Biotechnol. 208, 44-53 (2015).
- Treen, N., et al. Tissue-specific and ubiquitous gene knockouts by TALEN electroporation provide new approaches to investigating gene function in Ciona. Development. 141 (2), 481-487 (2014).
- Qin, Z., et al. Editing of the luteinizing hormone gene to sterilize channel catfish, Ictalurus punctatus, using a modified zinc finger nuclease technology with electroporation. Mar. Biotechnol. 18 (2), 255-263 (2016).
- Qin, Z. . Gene editing of luteinizing hormone, follicle-stimulating hormone and gonadotropin-releasing hormone genes to sterilize channel catfish, Ictalurus punctatus, using zinc finger nuclease, transcription activator-like effector nuclease and clustered regularly interspaced short palindromic repeats/Cas9 technologies. , (2015).
- Dunham, R. A., Winn, R. N., Pinkert, C. A. . Transgenic Animal Technology: A Laboratory Handbook. , 308-336 (2014).
- Elaswad, A. . Genetic technologies for disease resistance research and enhancement in catfish. , (2016).
- Baoprasertkul, P., Peatman, E., Somridhivej, B., Liu, Z. Toll-like receptor 3 and TICAM genes in catfish: species-specific expression profiles following infection with Edwardsiella ictaluri. Immunogenetics. 58 (10), 817-830 (2006).
- Thongda, W., Li, C., Luo, Y., Beck, B. H., Peatman, E. L-rhamnose-binding lectins (RBLs) in channel catfish, Ictalurus punctatus: Characterization and expression profiling in mucosal tissues. Dev. Comp. Immunol. 44 (2), 320-331 (2014).
- Phelps, R. P., et al. Effects of temperature on the induced spawning of channel catfish and the production of channel× blue catfish hybrid fry. Aquaculture. 273 (1), 80-86 (2007).
- Poole, W., Dillane, M. Estimation of sperm concentration of wild and reconditioned brown trout, Salmo trutta L. Aquacult. Res. 29 (6), 439-445 (1998).
- Billard, R. Spermatogenesis and spermatology of some teleost fish species. Reprod. Nutr. Dev. 26 (4), 877-920 (1986).
- Coyle, S. D., Durborow, R. M., Tidwell, J. H. . Anesthetics in aquaculture. , (2004).
- Shah, A. N., Davey, C. F., Whitebirch, A. C., Miller, A. C., Moens, C. B. Rapid reverse genetic screening using CRISPR in zebrafish. Nat. Methods. 12 (6), 535-540 (2015).
- Armstrong, J., Duhon, S., Malacinski, G. . Developmental Biology of the Axolotl. , 220-227 (1989).
- Porazinski, S. R., Wang, H., Furutani-Seiki, M. Microinjection of medaka embryos for use as a model genetic organism. J. Vis. Exp. (46), (2010).
- Erickson, P. A., Ellis, N. A., Miller, C. T. Microinjection for transgenesis and genome editing in threespine sticklebacks. J. Vis. Exp. (111), e54055 (2016).
- Rosen, J. N., Sweeney, M. F., Mably, J. D. Microinjection of zebrafish embryos to analyze gene function. J. Vis. Exp. (25), e1115 (2009).
- Silverstein, J. T., Small, B. C., Tucker, C. S., Hargreaves, J. A. . Biology and Culture of Channel Catfish. 34, 69-94 (2004).
- Su, B., et al. Relative effectiveness of carp pituitary extract, luteininzing hormone releasing hormone analog (LHRHa) injections and LHRHa implants for producing hybrid catfish fry. Aquaculture. 372, 133-136 (2013).
- Aluru, N., et al. Targeted mutagenesis of aryl hydrocarbon receptor 2a and 2b genes in Atlantic killifish (Fundulus heteroclitus). Aquat. Toxicol. 158, 192-201 (2015).
- Chang, N., et al. Genome editing with RNA-guided Cas9 nuclease in zebrafish embryos. Cell Res. 23 (4), 465-472 (2013).
- Edvardsen, R. B., Leininger, S., Kleppe, L., Skaftnesmo, K. O., Wargelius, A. Targeted mutagenesis in Atlantic salmon (Salmo salar L.) using the CRISPR/Cas9 system induces complete knockout individuals in the F0 generation. PLoS ONE. 9 (9), e108622 (2014).
- Li, M., et al. Efficient and heritable gene targeting in tilapia by CRISPR/Cas9. Genetica. 197 (2), 591-599 (2014).
- Zhong, Z., et al. Targeted disruption of sp7 and myostatin with CRISPR-Cas9 results in severe bone defects and more muscular cells in common carp. Sci. Rep. 6, (2016).
- Chakrapani, V., et al. Establishing targeted carp TLR22 gene disruption via homologous recombination using CRISPR/Cas9. Dev. Comp. Immunol. 61, 242-247 (2016).
- Lim, J., Ghadessy, F. J., Yong, E. A novel splice site mutation in the androgen receptor gene results in exon skipping and a non-functional truncated protein. Mol. Cell. Endocrinol. 131 (2), 205-210 (1997).
- Gatfield, D., Unterholzner, L., Ciccarelli, F. D., Bork, P., Izaurralde, E. Nonsense-mediated mRNA decay in Drosophila: at the intersection of the yeast and mammalian pathways. The EMBO Journal. 22 (15), 3960-3970 (2003).
- Talerico, M., Berget, S. M. Effect of 5’splice site mutations on splicing of the preceding intron. Mol. Cell. Biol. 10 (12), 6299-6305 (1990).

