Performing Microscope-Mounted Y-Shaped Cutting Tests
Summary
Y-shaped cutting measures fracture-relevant length scales and energies in soft materials. Previous apparatuses were designed for benchtop measurements. This protocol describes the fabrication and use of an apparatus that orients the setup horizontally and provides the fine positioning capabilities necessary for in situ viewing, plus failure quantification, via an optical microscope.
Abstract
Y-shaped cutting has recently been shown to be a promising method by which to understand the threshold length scale and failure energy of a material, as well as its failure response in the presence of excess deformation energy. The experimental apparatus used in these studies was vertically oriented and required cumbersome steps to adjust the angle between the Y-shaped legs. The vertical orientation prohibits visualization in standard optical microscopes. This protocol presents a Y-shaped cutting apparatus that mounts horizontally over an existing inverted microscope stage, can be adjusted in three dimensions (X-Y-Z) to fall within the objective's field of view, and allows easy modification of the angle between the legs. The latter two features are new for this experimental technique. The presented apparatus measures the cutting force within 1 mN accuracy. When testing polydimethylsiloxane (PDMS), the reference material for this technique, a cutting energy of 132.96 J/m2 was measured (32° leg angle, 75 g preload) and found to fall within the error of previous measurements taken with a vertical setup (132.9 J/m2 ± 3.4 J/m2). The approach applies to soft synthetic materials, tissues, or bio-membranes and may provide new insights into their behavior during failure. The list of parts, CAD files, and detailed instructions in this work provide a roadmap for the easy implementation of this powerful technique.
Introduction
Nonlinear continuum mechanics has provided a critical lens through which to understand the concentration of energy that leads to failure in soft solids1. However, the accurate prediction of this failure also requires descriptions of the microstructural characteristics that contribute to new surface creation at the crack tip2,3. One method to approach such descriptions is through in situ visualization of the crack tip during failure4,5. However, crack blunting in typical far-field fracture tests makes the acquisition of in situ data challenging by spreading out the highly deformed material, potentially outside the microscope's field of view6. Y-shaped cutting offers a unique alternative for microstructural visualization because it concentrates the region of large deformation at the tip of a blade7. Furthermore, previous work from our group demonstrates that this unique experimental approach can provide insight into the differences in failure response between far-field tearing and contact-mediated loading conditions7.
The Y-shaped cutting method used in the apparatus presented here was first described decades ago as a cutting method for natural rubber8. The method consists of a fixed blade push-cutting through a preloaded Y-shaped test piece. At the intersection of the "Y" is the crack tip, which is created prior to testing by splitting a portion of a rectangular piece into two equal "legs" (Figure 1B and Figure 2D). The primary advantages of this cutting method include the reduction of frictional contributions to the measured cutting energy, the variable blade geometry (i.e., constraint of the crack tip geometry), the control of the failure rate (via the sample displacement rate), and the separate tuning of the cutting, C, and tearing, T, energy contributions to the total energy Gcut (i.e., altering the failure energy in excess of a cutting threshold)8. The latter contributions are expressed in a simple, closed-form expression for the cutting energy9
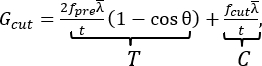 Eqn (1)
Eqn (1)
which uses experimentally selected parameters, including sample thickness, t, average leg strain, 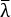 , preload force, fpre, and the angle between the legs and the cutting axis, θ. The cutting force, fcut, is measured with the apparatus as detailed in Zhang et al.9. Notably, the apparatus presented here includes a new, simple, and accurate mechanism for tuning the leg angle, θ, and ensuring the sample is centered. While both features are critical for a microscope-mounted setup, the mechanism may benefit future vertical implementations of the Y-shaped cutting test as well by increasing the ease of use.
, preload force, fpre, and the angle between the legs and the cutting axis, θ. The cutting force, fcut, is measured with the apparatus as detailed in Zhang et al.9. Notably, the apparatus presented here includes a new, simple, and accurate mechanism for tuning the leg angle, θ, and ensuring the sample is centered. While both features are critical for a microscope-mounted setup, the mechanism may benefit future vertical implementations of the Y-shaped cutting test as well by increasing the ease of use.
Progress in determining the appropriate failure criteria for soft solids has been ongoing since the early success of sample-independent fracture geometries introduced by Rivlin and Thomas10. Critical energy release rates10, cohesive zone laws11, and various forms of stress- or energy-at-a-distance approaches12,13,14 have been used. Recently, Zhang and Hutchens leveraged the latter approach, demonstrating that Y-shaped cutting with sufficiently small radius blades could yield threshold failure conditions for soft fracture7: a threshold failure energy and a threshold length scale for failure that ranges from tens to hundreds of nanometers in homogeneous, highly-elastic polydimethylsiloxane (PDMS). These results were combined with continuum modeling and scaling theory to develop a relationship between cutting and tearing in these materials, thus demonstrating the utility of Y-shaped cutting for providing insights into all modes of soft failure. However, the behavior of many material classes, including dissipative and composite materials, remains unexplored. It is anticipated that many of these will exhibit microstructure-governed effects at length scales above the wavelength of visible light. Therefore, an apparatus was designed in this study that allows for the close visual characterization of these effects during Y-shaped cutting for the first time (e.g., in composites, including soft tissues, or of dissipative processes, anticipated on the micrometer to millimeter length scales15).
Protocol
1. Adjustment and manufacturing of modifiable and consumable parts
- Use a laser cutter or 3D printer to manufacture disposable ABS or acrylic tabs that fit within the width of the sample legs, B1 and B2 (7.5 mm x 7.5 mm for a 1.5 cm x 7 cm x 3 mm sample) (Figure 1B and Figure 2D). Two tabs are needed for each test, one for each leg.
- Razor blade clip
NOTE: The exact dimensions of the required razor blade clip depend on the depth of the razor blade used.- Modify the CAD design (see Table of Materials) file Blade clip.SLDPRT (Supplemental Coding File 1) by changing the width of the clip base such that the distance from the tip of the selected razor blade to the back of the clip is 30.35 mm (Figure 1D). This adjustment keeps the tip of the blade directly under the pivot point (Figure 1E) of the angle adjustment mechanism (Figure 1A and Figure 2A) used to adjust the angle between the legs.
NOTE: The apparatus can hold blades with a depth of 8-20 mm. - Using fine settings, 3D-print the razor blade clip (Figure 1D). Due to 3D printing errors, the razor blade clip dovetail may not fit as printed. To fix this, use sandpaper or a fine file to remove material from the back of the razor blade clip until it can be inserted and removed from its slot on the blade clip mount by hand but is still tight during the cutting.
- Modify the CAD design (see Table of Materials) file Blade clip.SLDPRT (Supplemental Coding File 1) by changing the width of the clip base such that the distance from the tip of the selected razor blade to the back of the clip is 30.35 mm (Figure 1D). This adjustment keeps the tip of the blade directly under the pivot point (Figure 1E) of the angle adjustment mechanism (Figure 1A and Figure 2A) used to adjust the angle between the legs.
- Modify the sample holder dimensions (Figure 1C) using the CAD design file Sample holder.SLDPRT (Supplemental Coding File 2) to fit the opening of the specific microscope stage (Figure 2B). To ensure the apparatus can use its full range of motion, it is important that the inner cavity of the holder stays as large as possible.
- Load cell holder
NOTE: Bending type load cells come in many geometries. The location upon which to mount the load sensor (the inner slide, Figure 1E) will require adjustment depending on the load cell selected.- Adjust the following dimensions on the inner slide (Figure 1E) to accommodate the specific load cell: 1) the location of the mounting holes (currently two M3 holes with a 6 mm center-to-center distance); 2) the distance between the load cell beam and the inner slide plane, depending on the maximum deflection of the load cell beam (currently at 3 mm); and 3) the height and width to accommodate load cell geometry (currently 35 mm and 12.1 mm, respectively).
NOTE: The load cell length range that can be used without interfering with the vertical adjust system (Figure 1E and Figure 2A) is 10-63 mm. If the load cell size is out of this range, an alternative is to remove the height adjust system or redesign/lengthen the pulley arms (Figure 1A).
- Adjust the following dimensions on the inner slide (Figure 1E) to accommodate the specific load cell: 1) the location of the mounting holes (currently two M3 holes with a 6 mm center-to-center distance); 2) the distance between the load cell beam and the inner slide plane, depending on the maximum deflection of the load cell beam (currently at 3 mm); and 3) the height and width to accommodate load cell geometry (currently 35 mm and 12.1 mm, respectively).
- Redesign, using the appropriate CAD files, the mounting platform and frame arms (Figure 1A) to fit the specific microscope/microscope stage used. Specifically, the frame arms (frame arm.SLDPRT, Supplemental Coding File 3) may need to be modified to facilitate attachment. The height of the pulley arms (Figure 1A) (pulley arm.SLDPRT, Supplemental Coding File 4, and pulley arm_Mirror.SLDPRT, Supplemental Coding File 5) may also need to be modified depending on the heights of the plane of the microscope mounting holes and the top plane of the microscope's XY stage.
2. Mechanical assembly
- Once all the microscope, load-cell, razor blade, and sample components have been appropriately modified, manufacture all the components and construct the apparatus (Figure 2A). The components include 3D-printed, laser-cut, and commercial off-the-shelf parts. A detailed list of parts is given in the Table of Materials. Computer assembly drawings of all the parts and apparatus assembly are available in Supplemental Coding Files 1-17.
- To mount the load cell, first attach the blade clip mount to the load cell (Figure 1E). Attach this assembly to the inner slide of the vertical adjust system (Figure 1E and Figure 2A). Attach the combined system of the blade clip mount, load cell, and inner slide of the vertical adjust system into the outer slide of the vertical adjust system (Figure 1E) that is mounted to the bottom of the angle adjustment mechanism (Figure 1A and Figure 2A).
NOTE: Micro load cells are fragile. Use caution when handling the load cell to minimize any forces applied to it outside of testing, especially forces in the direction of load measurement.
3. Electrical assembly
- Set up the load cell and data acquisition system. Build an amplification circuit following the schematic (Figure 1F, Amplification circuit schematic.SchDoc [Supplemental Coding File 18], and Amplification circuit PCB.PcbDoc [Supplemental Coding File 19]). Connect the output signal directly to a data acquisition system with a 0-5 V input range. Connect the elements of the circuit according to Figure 1G.
- Calibrate the load cell by placing a weight of known quantity on the deflection beam and recording the voltage output in the calibration code (calibrate_ni_daq.mlapp, Supplemental Coding File 20). Repeat this process at least 5x for different weights of known quantity.
- Calculate the load cell calibration constant by fitting the known weight versus voltage data to a line. Input this calibration value into the data collection code (collect_data.mlapp, Supplemental Coding File 21).
NOTE: The approach to data acquisition will depend on the type of load cell selected. In this study, a deflection load cell with a maximum rated capacity of 0.5 N, 0.05% rated output (R.O.) maximum repeatability, and 0.03% R.O. hysteresis was used. The ~10 mV output signal is amplified to enable the use of a commercial data acquisition (DAQ) system (−5 to 5 V input range, 16-bit resolution). As a result, a force resolution finer than 1 mN was obtained while collecting data at a rate of 20 Hz after applying a rolling median filter.
4. Apparatus mounting
- After the apparatus has been constructed and the load cell and data acquisition system have been set up, replace the original, stage-mounted slide holder with the custom sample holder.
- Attach the assembly to the microscope. Use mounting holes on the top surface of the microscope if available.
- Set the angle of the cut by loosening the angle adjust thumb screw and then moving the linear slide (Figure 1A). Set the angle after measuring it with a protractor (Figure 2A) and tighten the angle adjust thumb screw. The angle between a leg and the sample midplane, θ, can be adjusted from 8°-45° (Figure 1B).
- Set up two vertical pulleys behind the apparatus.
5. Sample preparation
- Sample dimensions: Prepare a thin rectangular sample (e.g., 1.5 cm x 7 cm x 3 mm) of PDMS (see Table of Materials) by either cutting it from a larger sheet or using a mold of the correct dimensions. The dimensions may vary, but a width of 1.5 cm or less for a sample with a thickness of 3 mm or less is recommended to start.
- Cutting the legs: Using a razor blade, cut the sample 3 cm lengthwise along the centerline to create the Y-shaped sample (Figure 1B). This length may vary, but the legs should be long enough to accommodate the tabs yet short enough to leave uncut sample for measurement.
- Strain measurement marking: Using a marker or ink, place two marks, centered and separated by approximately 1 cm, on each of the thin legs (Figure 2D) and the body of the sample (six in total) to enable the measurement of the applied stretch in each of the three sample legs under load.
- Attaching the tabs: Use adhesive-like cyanoacrylate glue to attach a 3D-printed or laser-cut tab (step 1.1) to the end of each leg (Figure 1B and Figure 2D).
- Prepare the tension Line: Measure and cut two lengths of thin fishing line. Approximately 30 cm of line is needed for internal routing through the mechanism; add more as needed to route the line to the external set of pulleys (step 4.4). Attach 5 g weighing plates to the end of the lines passing through the external pulleys and tie the other end to the tab on each leg.
6. Sample mounting
NOTE: Take caution during this step to ensure that the sample does not touch the microscope objective to avoid damaging it. It may help to adjust the objective and microscope stage to create as much space as possible for sample mounting.
- Clamp the base of the sample using the sample holder thumb screw (Figure 1C).
- Route the line for each leg through each side of the pulley system (Figure 1A and Figure 2A). Take a picture of the sample from the top while the sample is under negligible weight by holding a camera against the underside of the angle adjustment mechanism. Make sure the camera is parallel to the sample plane to minimize perspective effects.
- Add the desired preload weight of 75 g to both ends of the fishing line near the external pulleys. Increase this quantity to 150 g or decrease it to 50 g to change the tearing contribution if desired for this example material and geometry. Take a second picture of the sample after the weight is added, again making sure that the camera is parallel to the sample plane.
NOTE: The example weights provided here apply specifically to the PDMS sample used in this study. - Align the fishing line from the lowest pulley with the Z plane of the sample legs using the Z component of the three-way micro-adjustment stage (Figure 1A). Approximately position the anticipated blade tip close to the objective's field of view (Figure 2B).
7. Blade mounting
- Place the razor blade into its corresponding blade clip (step 1.2) and secure the blade in place with a set screw. Seat the blade firmly into the blade clip (Figure 1D and Figure 2C) to ensure that it is square. Slide this clipped razor blade into the blade clip mount attached to the load cell (Figure 1E).
NOTE: The blade should always be placed after the sample is mounted. If the blade is in place before the sample, it presents a safety risk to the user.
8. Apparatus alignment
- Select the 2.5x microscope objective, or as high as 20x if closer images are desired.
- Use the transmitted light setting, augmenting the light behind the sample if needed.
- With the blade in place, focus the microscope on the bottom of it, using the blade's vertical adjust system if necessary to bring the tip to the appropriate working distance for the objective (Figure 1E and Figure 2A). Carefully align the razor blade within the microscope's field of view using only the X and Y directions of the three-way micro-adjustment stage (Figure 1A).
- Next, focus the microscope on the sample. Align the crack tip with the razor blade (Figure 2B) by translating the microscope XY stage (Figure 1A) to ensure that the midplane of the sample aligns with the midplane of the angle adjustment mechanism.
9. Testing
- Open the code used for the load cell data collection (collect_data.mlapp, Supplemental Coding File 21).
- Start recording the load cell data by clicking the Start Recording button.
- Translate the sample through the razor blade for 1 cm or more at a constant velocity using microscope stage control. Simultaneously gather images using the microscope's imaging interface.
- When the microscope XY stage stops (Figure 1A), click the Stop Recording button to stop recording data and automatically save a *.txt file of the load and time response.
Representative Results
The parameters used during step 4 and step 6 and the data gathered during step 6 and step 9 combine to yield the cutting energy of the sample. According to Eqn. 1, the determination of the cutting energy requires the following parameters: sample thickness, t, preload force, fpre, and the angle between the legs and the cutting axis, θ. The following data are also required: the cutting force, fcut, and the average leg strain, 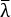 . The former comes from force-time data gathered via the computer code. The force-time data from a typical test (Figure 3A) illustrate a high initial force, as is typically required for cut initiation, followed by a constant force, indicating steady state cutting. The cutting force, fcut, is the maximum value of the force within this steady state regime9. The average strain in the legs,
. The former comes from force-time data gathered via the computer code. The force-time data from a typical test (Figure 3A) illustrate a high initial force, as is typically required for cut initiation, followed by a constant force, indicating steady state cutting. The cutting force, fcut, is the maximum value of the force within this steady state regime9. The average strain in the legs, 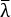 , is given by
, is given by
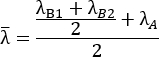 Eqn (2)
Eqn (2)
where images of the pre- and post-loaded sample prior to cutting (step 6.2 and step 6.3) are used as an optical strain gage to measure λB1, λB2, and λA. Finally, these values are combined to calculate the cutting energy using Eqn. 1.
For the representative results reported here: an ultrasharp blade (129 nm radius), a 32° leg angle, and a 75 g preload (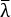 = 1.04), we measured a cutting energy of 132.96 J/m2 for PDMS. This value aligns well with the previously obtained cutting energy under these conditions of 132.9 J/m2 ± 3.4 J/m2, thus validating the mechanical portion of the test setup demonstrated here9. If desired, the force-time data can be converted approximately to force-displacement data using the microscope stage motion protocol (e.g., constant velocity).
= 1.04), we measured a cutting energy of 132.96 J/m2 for PDMS. This value aligns well with the previously obtained cutting energy under these conditions of 132.9 J/m2 ± 3.4 J/m2, thus validating the mechanical portion of the test setup demonstrated here9. If desired, the force-time data can be converted approximately to force-displacement data using the microscope stage motion protocol (e.g., constant velocity).
The viability of the setup for simultaneously gathering microscope images is illustrated in Figure 3B. These images are gathered using a 2.5x objective 1) from the start of the test, 2) past the cut initiation, and 3) throughout the steady state in a speckle-patterned PDMS sample mixed at the manufacturer's ratio of 10:1. We maintained focus throughout the test and demonstrated one-to-one correspondence between the mechanical and optical data. We note that the quality and magnification of the microscope images obtained will depend on the system/objective/stage /program combination used.
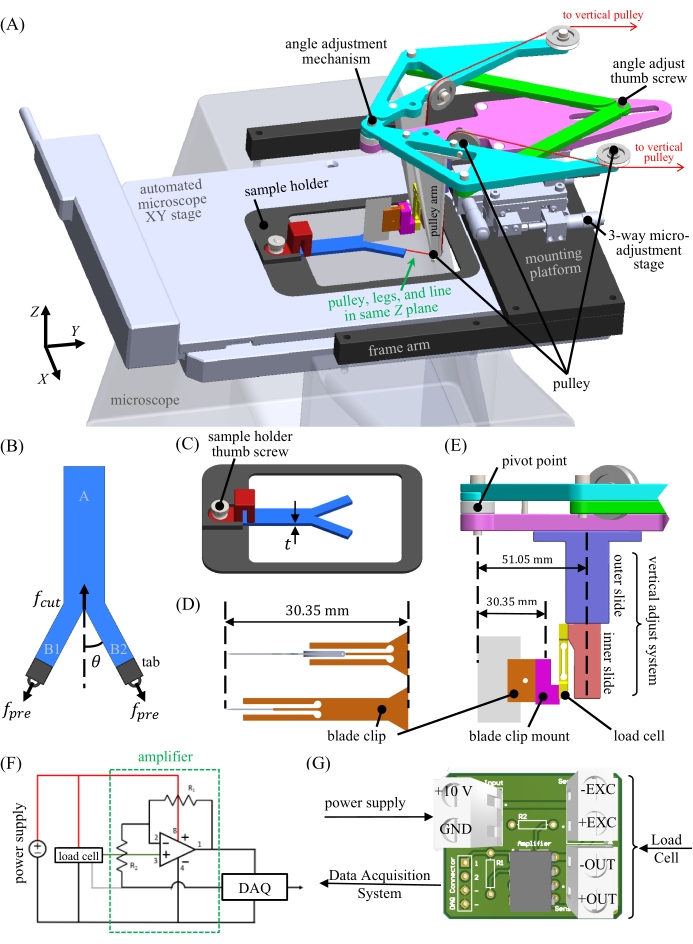
Figure 1: CAD images of the microscope-mounted Y-shaped cutting device. (A) The full cutting apparatus mounted above an inverted microscope with an automated XY stage. Not shown are the vertical pulleys behind the system from which dead weights are hung to create preload forces, fpre, on the sample. (B) The sample consists of a single leg, "A", from which two equal legs are cut, "B1" and "B2", to create a "Y" shape with leg angle θ. (C) The sample holder holds the sample in place within a slot in the microscope stage. (D) The top view of the customizable blade clips shows how their redesign accommodates blades of different heights while maintaining the 30.35 mm spacing that aligns the top with the pivot point of the angle adjustment mechanism. (E) A close-up side view of the vertical adjust system, load cell, and blade clip mounting parts. (F) The signal from the load cell is mediated by an amplification circuit used to convert the load cell output (0-10 mV) to the 0-5 V range of the data acquisition system. (G) This circuit is implemented by connecting it to the power supply, load cell, and data acquisition system using a printed circuit board. Please click here to view a larger version of this figure.
Figure 2: Photographs of the microscope-mounted Y-shaped cutting device. (A) A photograph of the operational Y-shaped cutting device with false-colored regions added to indicate the key design features. (B) A forward view of the device illustrating the approximate alignment of the load cell and sample midplane and indicating the region to be cut that falls within the microscope objective's field of view. (Blade and blade clip not mounted.) (C) Examples of mounted blades and clips with an equal overall height of 30.35 mm. (D) A PDMS Y-shaped sample prior to mounting, with the tabs and fishing line attached. Fiducial markers have been added to the legs "B1" and "B2" to measure the average stretch upon preload application. Please click here to view a larger version of this figure.
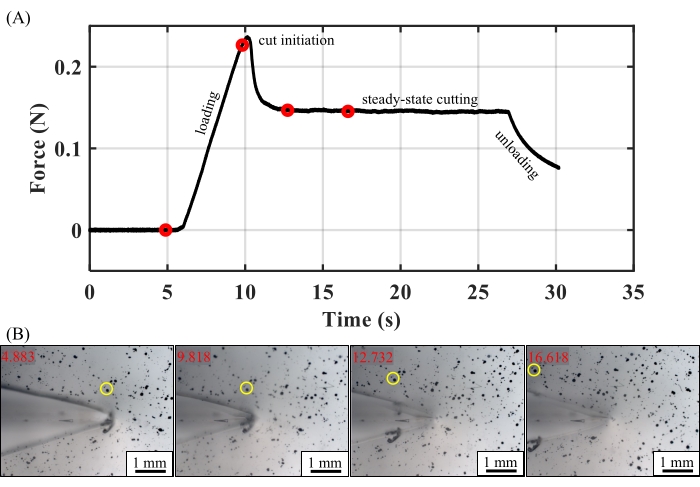
Figure 3: Representative in situ cutting results. (A) A force-time curve for PDMS (10:1) using an ultrasharp blade (129 nm radius), 32° leg angle, and 75 g preload (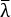 = 1.04). The elastic loading, cut initiation, steady state cutting, and unloading regions of the curve are labeled. (B) Red circles that correspond to the images obtained by the microscope are shown. A yellow circle has been added to facilitate observation of the speckle-pattern motion. Scale bar = 1 mm. Time stamps, in seconds, are included in the upper left-hand corner of each image. Please click here to view a larger version of this figure.
= 1.04). The elastic loading, cut initiation, steady state cutting, and unloading regions of the curve are labeled. (B) Red circles that correspond to the images obtained by the microscope are shown. A yellow circle has been added to facilitate observation of the speckle-pattern motion. Scale bar = 1 mm. Time stamps, in seconds, are included in the upper left-hand corner of each image. Please click here to view a larger version of this figure.
Supplemental Coding File 1. Please click here to download this File.
Supplemental Coding File 2. Please click here to download this File.
Supplemental Coding File 3. Please click here to download this File.
Supplemental Coding File 4. Please click here to download this File.
Supplemental Coding File 5. Please click here to download this File.
Supplemental Coding File 6. Please click here to download this File.
Supplemental Coding File 7. Please click here to download this File.
Supplemental Coding File 8. Please click here to download this File.
Supplemental Coding File 9. Please click here to download this File.
Supplemental Coding File 10. Please click here to download this File.
Supplemental Coding File 11. Please click here to download this File.
Supplemental Coding File 12. Please click here to download this File.
Supplemental Coding File 13. Please click here to download this File.
Supplemental Coding File 14. Please click here to download this File.
Supplemental Coding File 15. Please click here to download this File.
Supplemental Coding File 16. Please click here to download this File.
Supplemental Coding File 17. Please click here to download this File.
Supplemental Coding File 18. Please click here to download this File.
Supplemental Coding File 19. Please click here to download this File.
Supplemental Coding File 20. Please click here to download this File.
Supplemental Coding File 21. Please click here to download this File.
Discussion
The horizontal, Y-shaped cutting apparatus reported here enables in situ imaging capabilities along with improved ease-of-use for this failure technique. The apparatus includes a modular/portable design for quick mounting/unmounting from a microscope and continuous, pre-aligned leg angle adjustment. All the CAD files, required materials, and procedures have been included to facilitate the implementation of this method. In many instances (blade holders, sample holder, load-cell mount, mounting frame), the 3D-printed parts can be easily modified for a given material/blade or specific load cell/microscope. However, the following tips apply to all the parameters and usages of this apparatus.
The weight used to hold each leg in tension is critical to a successful measurement. A sufficiently low weight ensures that the test does not fail immediately (it can be helpful to apply weight slowly and incrementally). However, loading the legs with too little force will result in sample buckling, leading the sample to fold under or in front of the blade instead of or while being cut. An “apparent” cutting force may be measured under these conditions, but it will not be the cutting force of the material.
The sample legs must be of an appropriate length for the sample holder and desired travel. Legs that are too long will run into the pulley system before a long enough cut has been made. The legs must be long enough to accommodate the tabs. For the sample holder geometry reported here, a 7 cm total sample length with 3 cm legs provides a good starting point. The load cell should be calibrated before each use. Abrupt movement of the apparatus can cause the load cell to become uncalibrated or even damaged.
The key modifications fall into two categories: accommodation of available equipment/components and material/imaging requirements. In terms of the first category, the apparatus mounting frame may be adjusted for implementation on different microscopes. The load-cell mount, the vertical adjust, or the arms supporting the first set of pulleys may all be modified to accommodate load cells of different lengths. The blade clips may require adjustment depending on the blade depth, as detailed in step 2.2 of the protocol. In terms of the second category, the sample holder may be modified to adapt to the objective working distance or sample environment limitations. For example, in the case of testing hydrated materials, a Petri dish or slide may be incorporated beneath the sample to protect the microscope and maintain hydration.
As with vertical Y-shaped cutting, this approach applies primarily to soft, reasonably robust solids. Stiff materials prefer to twist rather than bend outward and maintain a planar sample when a Y-inducing load is applied16. When samples are extremely brittle, low leg angles are required to achieve a sufficiently low tearing contribution (Eqn. 1), at which point friction can become a problem. Hydrated samples, typically possessing very low friction, may be the exception for tests at such low leg angles. From experience, leg angles >35° generally avoid frictional effects in relatively “sticky” silicone7,9. Changes in the sample geometry, environment, or blade angle may overcome many of these barriers, in time. Limitations in the cutting speed and control will vary with the automated XY microscope stage used. Specifically, some stage/software combinations provide only a few standard options for constant velocity. At higher cutting speeds, image acquisition may be insufficient to avoid blurring. All such limitations are dependent on the microscope and stage manufacturers but may be overcome by applying this apparatus to a custom microscope.
Y-shaped cutting facilitates the determination of the threshold failure properties of soft solids and provides insight into the fundamental failure responses of these materials under highly controlled conditions. With the modification provided by the apparatus detailed here, these mechanical measurements can now be combined with existing optical characterization techniques such as, but not limited to, the following: mechanophore activation5, second harmonic generation (SHG)17, and digital image correlation18. This combination is expected to yield new, quantifiable observations of the intimate relationship between microstructure and stress concentration in soft failure.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We would like to thank Dr. James Phillips, Dr. Amy Wagoner-Johnson, Alexandra Spitzer, and Amir Ostadi for their advice on this work. Funding came from the start-up grant provided by the Department of Mechanical Science and Engineering at the University of Illinois Urbana-Champaign. M. Guerena, J. C. Peng, M. Schmid, and C. Walsh all received senior design credit for their work on this project.
Materials
| Buy Parts | |||
| 1" OD Pulley | McMaster Carr | 3434T75 | Pulley for Wire Rope (Larger) |
| 100 g Micro Load Cell | RobotShop | RB-Phi-203 | |
| 1K Resistor | Digi-Key | CMF1.00KFGCT-ND | 1 kOhms ±1% 1 W Through Hole Resistor Axial Flame Retardant Coating, Moisture Resistant, Safety Metal Film |
| 1M Resistor | Digi-Key | RNF14FAD1M00 | 1 MOhms ±1% 0.25 W, 1/4 W Through Hole Resistor Axial Flame Retardant Coating, Safety Metal Film |
| 3/8" OD Pulley | McMaster Carr | 3434T31 | Pulley for Wire Rope |
| 4" Clear Protractor with Easy Read Markings | S&S Worldwide | LR3023 | |
| Breadboard | ECEB | N/A | |
| IC OPAMP ZERO-DRIFT 2 CIRC 8DIP | Digi-Key | LTC1051CN8#PBF-ND | |
| M2 x 0.4 mm Nut | McMaster Carr | 90592A075 | Steel Hex Nut |
| M2 x 0.4 mm x 25 mm | McMaster Carr | 91292A032 | 18-8 Stainless Steel Socket Head Screw |
| M2 x 0.4 mm x 8 mm | McMaster Carr | 91292A832 | 18-8 Stainless Steel Socket Head Screw |
| M3 x 0.5 mm x 15 mm | McMaster Carr | 91290A572 | Black-Oxide Alloy Steel Socket Head Screw |
| M3 x 0.5 mm x 16 mm | McMaster Carr | 91294A134 | Black-Oxide Alloy Steel Hex Drive Flat Head Screw |
| M3 x 0.5 mm, 4 mm High | McMaster Carr | 90576A102 | Medium-Strength Steel Nylon-Insert Locknut |
| M4 x 0.7 mm Nut | McMaster Carr | 90592A090 | Steel Hex Nut |
| M4 x 0.7 mm x 15 mm | McMaster Carr | 91290A306 | Black-Oxide Alloy Steel Socket Head Screw |
| M4 x 0.7 mm x 16 mm | McMaster Carr | 91294A194 | Black-Oxide Alloy Steel Hex Drive Flat Head Screw |
| M4 x 0.7 mm x 18 mm | McMaster Carr | 91290A164 | Black-Oxide Alloy Steel Socket Head Screw |
| M4 x 0.7 mm x 20 mm | McMaster Carr | 91290A168 | Black-Oxide Alloy Steel Socket Head Screw |
| M4 x 0.7 mm x 20 mm | McMaster Carr | 92581A270 | Stell Raised Knurled-Head Thumb Screw |
| M4 x 0.7 mm x 30 mm | McMaster Carr | 91290A172 | Black-Oxide Alloy Steel Socket Head Screw |
| M4 x 0.7 mm x 50 mm | McMaster Carr | 91290A193 | Black-Oxide Alloy Steel Socket Head Screw |
| M4 x 0.7 mm, 5 mm High | McMaster Carr | 94645A101 | High-Strength Steel Nylon-Insert Locknut |
| M5 x 0.8 mm Nut | McMaster Carr | 90592A095 | Steel Hex Nut |
| M5 x 0.8 mm x 16 mm | McMaster Carr | 91310A123 | High-Strength Class 10.9 Steel Hex Head Screw |
| M5 x 0.8 mm x 35 mm | McMaster Carr | 91290A195 | Black-Oxide Alloy Steel Socket Head Screw |
| M5 x 0.8 mm, 13 mm Head Diameter | McMaster Carr | 96445A360 | Flanged Knurled-Head Thumb Nut |
| M5 x 0.8 mm, 5 mm High | McMaster Carr | 90576A104 | Medium-Strength Steel Nylon-Insert Locknut |
| Solidworks | Dassault Systemes | CAD software | |
| Wiring Kit | ECEB | N/A | |
| XYZ Axis Manual Precision Linear Stage 60 mm x 60 mm Trimming Bearing Tuning Platform Sliding Table | OpticsFocus | N/A | |
| Make Parts | |||
| Angle adjustment system- arm | 3D Printing | solidworks: arms_arm_single.SLDPRT QTY: 2 Setting: Fast/0.2 mm layer height |
|
| Angle adjustment system- arms stationary | 3D Printing | solidworks: arms_stationary.SLDPRT QTY: 1 Setting: Fast/0.2 mm layer height |
|
| Angle adjustment system- link | 3D Printing | solidworks: arms_arm_link.SLDPRT QTY: 2 Setting: Fast/0.2 mm layer height |
|
| Angle adjustment system- slider | 3D Printing | solidworks: arms_slider.SLDPRT QTY: 1 Setting: Fast/0.2 mm layer height |
|
| Angle adjustment system- spacer | 3D Printing | solidworks: arms_front_spacer.SLDPRT QTY: 1 Setting: Fast/0.2 mm layer height |
|
| Clip- Blade clip | 3D Printing | solidworks: Blade clip.SLDPRT QTY: 1 Setting: Fine/0.1 mm layer height |
|
| Clip- Blade clip mount | 3D Printing | solidworks: Blade clip mount.SLDPRT QTY: 1 Setting: Fine/0.1 mm layer height |
|
| Frame arm | 3D Printing | solidworks: frame arm.SLDPRT QTY: 2 Setting: Fast/0.2 mm layer height |
|
| Mounting platform | Laser Cut Acrylic | solidworks: mounting platform.SLDPRT QTY: 1 |
|
| Pulley arm (left) | 3D Printing | solidworks: pulley arm_Mirror.SLDPRT QTY: 1 Setting: Fast/0.2 mm layer height |
|
| Pulley arm (right) | 3D Printing | solidworks: pulley arm.SLDPRT QTY: 1 Setting: Fast/0.2 mm layer height |
|
| Sample holder and tab- Clamp | 3D Printing | solidworks: Clamp.SLDPRT QTY: 1 Setting: Fast/0.2 mm layer height |
|
| Sample holder and tab- Sample holder | 3D Printing | solidworks: Sample holder.SLDPRT QTY: 1 Setting: Fast/0.2 mm layer height |
|
| Sample holder and tab- Tab | 3D Printing | solidworks: Tab.SLDPRT QTY: 2 per test Setting: Fine/0.1 mm layer height, no brim |
|
| Vertical adjust system- Inner slide | 3D Printing | solidworks: Inner slide.SLDPRT QTY: 1 Setting: Fast/0.2 mm layer height |
|
| Vertical adjust system- Outer slide | 3D Printing | solidworks: Outer slide.SLDPRT QTY: 1 Setting: Fast/0.2 mm layer height |
References
- Long, R., Hui, C. -. Y. Crack tip fields in soft elastic solids subjected to large quasi-static deformation – A review. Extreme Mechanics Letters. 4, 131-155 (2015).
- Slootman, J., et al. Quantifying rate-and temperature-dependent molecular damage in elastomer fracture. Physical Review X. 10, 041045 (2020).
- Zhao, X., et al. Soft materials by design: Unconventional polymer networks give extreme properties. Chemical Review. 121 (8), 4309-4372 (2021).
- Mzabi, S., Berghezan, D., Roux, S., Hild, F., Creton, C. A critical local energy release rate criterion for fatigue fracture of elastomers. Journal of Polymer Science Part B: Polymer Physics. 49 (21), 1518-1524 (2011).
- Chen, Y., Mellot, G., Van Luijk, D., Creton, C., Sijbesma, R. P. Mechanochemical tools for polymer materials. Chemical Society Reviews. 50, 4100-4140 (2021).
- Hui, C. -. Y., Jagota, A., Bennison, S. J., Londono, J. D. Crack blunting and the strength of soft elastic solids. Proceedings of the Royal Society A Mathematical, Physical and Engineering Science. 459 (2034), 1489-1516 (2003).
- Zhang, B., Hutchens, S. B. On the relationship between cutting and tearing in soft elastic solids. Soft Matter. 17, 6728-6741 (2021).
- Lake, G. J., Yeoh, O. H. Measurement of rubber cutting resistance in the absence of friction. International Journal of Fracture. 14, 509-526 (1978).
- Zhang, B., Shiang, C. -. S., Yang, S. J., Hutchens, S. B. Y-shaped cutting for the systematic characterization of cutting and tearing. Experimental Mechanics. 59, 517-529 (2019).
- Rivlin, R. S., Thomas, A. G. Rupture of rubber. I. Characteristic energy for tearing. Journal of Polymer Science. 10 (3), 291-318 (1953).
- Elices, M., Guinea, G. V., Gómez, J., Planas, J. The cohesive zone model: Advantages, limitations and challenges. Engineering Fracture Mechanics. 69 (2), 137-163 (2002).
- Taylor, D. . The Theory of Critical Distances. , (2007).
- Williams, J. G. Stress at a distance fracture criteria and crack self-blunting in rubber. International Journal of Non-Linear Mechanics. 68, 33-36 (2015).
- Talamini, B., Mao, Y., Anand, L. Progressive damage and rupture in polymers. Journal of the Mechanics and Physics of Solids. 111, 434-457 (2018).
- Long, R., Hui, C. -. Y., Gong, J. P., Bouchbinder, E. The fracture of highly deformable soft materials: A tale of two length scales. Annual Review of Condensed Matter Physics. 12, 71-94 (2021).
- Gent, A. N., Wang, C. Cutting resistance of polyethylene. Journal of Polymer Science: Part B: Polymer Physics. 34 (13), 2231-2237 (1996).
- Chen, X., Nadiarynkh, O., Plotnikov, S., Campagnola, P. J. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nature Protocols. 7, 654-669 (2015).
- Pan, B., Qian, K., Xie, H., Asundi, A. Two-dimensional digital image correlation for in-plane displacement and strain measurement: A review. Measurements Science and Technology. 20 (6), 062001 (2009).

