Expansion and Enrichment of Gamma-Delta (γδ) T Cells from Apheresed Human Product
Summary
Presented is a protocol for the expansion of Gamma-Delta (γδ) T cell drug product. Lymphocytes are isolated by elutriation and γδ-enriched with zoledronic acid and interleukin-2. Alpha-beta T cells are depleted using a clinical-grade magnetic separation device. The γδ cells are co-cultured with K562-derived, artificial antigen-presenting cells and expanded.
Abstract
Although Vγ9Vδ2 T cells are a minor subset of T lymphocytes, this population is sought after for its ability to recognize antigens in a major histocompatibility complex (MHC)-independent manner and develop strong cytolytic effector function that makes it an ideal candidate for cancer immunotherapy. Due to the low frequency of Gamma-Delta (γδ) T cells in the peripheral blood, we developed an effective protocol to greatly expand a highly pure γδ T cells drug product for first-in-human use of allogeneic γδ T cells in patients with acute myeloid leukemia (AML). Using healthy donor apheresis as an allogenic cell source, the lymphocytes are isolated using a validated device for a counterflow centrifugation method of separating cells by size and density.
The lymphocyte-rich fraction is utilized, and the γδ T cells are preferentially activated with zoledronic acid (FDA-approved) and interleukin (IL)-2 for 7 days. Following the preferential expansion of γδ T cells, a clinical-grade magnetic cell-separation device and TCRαβ beads are used to deplete contaminating T-cell receptor (TCR)αβ T cells. The highly enriched γδ T cells then undergo a second expansion using engineered artificial antigen-presenting cells (aAPCs) derived from K562 cells-genetically engineered to express single-chain variable fragment (scFv) for CD3 and CD28, 41BBL (CD137L) and IL15-RA-together with zoledronic acid and IL-2. Seeding all day-7 enriched γδ T cells in co-culture with the aAPCs facilitates the manufacture of highly pure γδ T cells with an average fold expansion of >229,000-fold from healthy donor blood.
Introduction
Leukemia relapse is the leading cause of mortality after hematopoietic cell transplantation (HCT) in patients with AML1,2,3. Better leukemia-free survival was reported with the increased recovery of blood γδ T cells after HCT without increased risk of Graft-versus-Host Disease (GVHD)4. The ability of γδ T cells to recognize antigens in an MHC-independent manner and develop strong cytolytic and Th1-like effector functions make this minor subpopulation of T cells ideal for the treatment of AML patients undergoing allogeneic transplantation at risk for relapse5. Given that the Vγ9Vδ2 T cells are a minor subset of T lymphocytes ranging from 0.5% to 5% of T cells in the periphery6, we set out to establish a robust system to expand this rare population of blood cells to achieve potentially therapeutic doses for clinical trials.
Although others have successfully expanded γδ T cells using zoledronic acid and even aAPCs, we have developed a process that can potentially expand γδ T cells by 229,749-fold. The expansion is biphasic: first, lymphocytes are obtained by elutriation using the separation instrument. The equipment provides a closed system that allows the separation of cells based on their size, shape, and density by counterflow centrifugation. After enriching for lymphocytes, selective expansion of Vγ9Vδ2 T cells is achieved by treatment with zoledronic acid and IL-2 for 7 days. Immediately following this treatment, TCR-αβ T cells are depleted using microbead technology, allowing for subsequent expansion of the γδ T cells with K562-derived aAPCs.
For process validation, only 5 × 106 zoledronic acid-expanded γδ T cells were used for the phase-2 co-culture expansion with aAPCs. In this second phase of expansion, γδ T cells are activated using a current Good Manufacturing Practices (cGMP)-compliant Working Cell Bank (WCB) of genetically engineered K562-derived aAPCs (K562VL6(scFv-CD3-41BBL;scFv-CD28-IL15-RA)) manufactured at Moffitt. The rationale of this biphasic expansion is based on the ability of zoledronic acid to inhibit farnesyl diphosphate synthase (FDPS) in monocytes, leading to the accumulation of isopentenyl pyrophosphate, which directly stimulates the Vγ2Vδ2 cells. In the second phase of expansion, the K562-derived aAPCs (K562VL6(scFv-CD3-41BBL;scFv-CD28-IL15-RA)) provide a robust stimulation to all T cells. However, the cell product has already been enriched for the γδ T cells, resulting in a robust expansion of the γδ T cells.
With the use of specific equipment and flasks, the process is a functionally closed system, thus decreasing the risk of contamination. In addition, the 1 L closed-system bioreactor facilitates maximal growth and expansion of cells in a total volume of 1 L of medium with minimal need for feeding. The advantage of the Moffitt method is that it provides a rapid, reproducible, and highly feasible GMP system to produce a highly pure, donor-derived γδ T cell product for allogeneic administration. This method can be applied to any clinical trial that aims at using human γδ T cells expressing Vγ2Vδ2 T cell receptors as adoptive immunotherapy to mediate immunity against microbes and tumors in cancer patients with partial and complete remission. In addition, it provides a robust platform for the development and production of γδ chimeric antigen receptor-positive (CAR+) T cells.
Protocol
NOTE: IRB approval was obtained, and informed consent was obtained from the donors.
1. Lymphocyte isolation
- Transfer the apheresis product to a clean room.
NOTE: Process validation was performed using normal donor apheresis from an external commercial vendor compliant with raw cellular material collection regulations. - Collect samples for sterility testing, cell count, and cell phenotyping.
- Elutriate on the counterflow centrifugation device using a primary medium of Hanks Balanced Salt Solution with 1% human serum albumin (HSA) and a secondary medium of saline solution (0.9% sodium chloride Injection USP) or Dulbecco's Phosphate-Buffered Saline (DPBS). Set the elutriation centrifugation speed at 900 × g and collect fractions based on flow rate and time.
- Collect samples from fraction 2 and perform the following tests: 2 mL for sterility testing; 0.5 mL for cell count and viability using acridine orange/propidium iodide (AO/PI); 5 × 106 cells for cell phenotyping by flow cytometry.
- Expand a pure lymphocyte fraction (fraction 2) of cells in culture at 10 × 106 cells/cm2 in a 1 L closed-system bioreactor with 5 µmol/L of zoledronic acid and 300 IU/mL of IL-2.
- Incubate for seven days in an incubator set at 37 °C with 5% CO2.
2. Alpha-beta (αβ) T cell depletion
- Harvest cells from the 1 L closed-system bioreactor flask. Sterile-weld a 1 L transfer pack to the red line of the closed-system bioreactor, and use the appropriate pharmaceutical pump to transfer the cells into the transfer pack.
- Take the following samples: 10 mL for spent medium sterility; 0.5 mL of cells for cell count and viability using AO/PI; 5 × 106 cells for flow cytometry
- Resuspend the cells at ~5 × 108 cells/mL in phosphate-buffered saline (PBS) or (PBS/ethylenediaminetetraacetic acid (EDTA)) buffer + 0.5% HSA and biotinylated TCR αβ-specific antibody.
- Place the shaker in the refrigerator and incubate the cells at 2-8 °C for approximately 15 min with shaking.
- Wash the cells with a total of 600 mL of PBS/EDTA buffer + 0.5% HSA. Centrifuge to remove the unbound antibody at 200-500 × g for 15 min at 2-8 °C. Resuspend ~5 × 108 cells/mL in PBS/EDTA buffer + 0.5% HSA with anti-biotin-specific microbeads (7.5 mL/1 vial).
- Place the shaker in the refrigerator and incubate the cells at 2-8 °C for approximately 15 min with shaking. After incubation, centrifuge the cells at 200-500 × g for 15 min at 2-8 °C to remove the unbound microbeads. Resuspend ~6 × 107 cells/mL in PBS/EDTA buffer + 0.5% HSA and transfer them to a transfer pack bag.
- Install the tubing set in the clinical grade magnetic cell separation device by following the manufacturer's instructions, place the packs with the PBS/EDTA buffer and the transfer pack with the cell product in the instrument and spike it when instructed by the instrument.
- Select the Depletion 1.2 protocol for the depletion of the labeled αβ T cells.
- Centrifuge the target fraction (enriched γδ T cells) and resuspend the cells in medium supplemented with 10% human AB serum.
- Take a 0.5 mL sample and perform a cell count and viability with AO/PI stain. Bring cells to a final concentration of approximately 1 × 106 cells/mL. Take a sample of 5 × 106 cells of the product for flow cytometry phenotyping post-depletion.
3. Co-culture with aAPCs
- Irradiate 5 × 107 aAPCs/flask at 100 Gy on the X-ray generating instrument.
- Use the aAPCs in co-culture at a 10:1 ratio with the γδ T cells. Place the irradiated aAPCs (5 × 107 cells/flask) and γδ T cells (5 × 106 cells/flask) in 1 L closed-system bioreactor flasks with 1 L of culture medium supplemented with 10% human AB serum. Seed up to 10 flasks.
- Expand the cells in culture for 10 days in an incubator at 37 °C and 5% CO2.
- Monitor glucose and lactate levels every 3-4 days using strips, glucose, and a lactate meter.
- If glucose drops to 250 mg/dL, reduce the volume in the flask to 200 mL using a pharmaceutical pump by sterile-welding a 1 L transfer pack to the red line of the closed system bioreactor.
- Mix the cells in the remaining 200 mL, and take a 0.5 mL sample for cell counting and viability measurement by AO-PI staining. If the cell count is ≥109, split one flask into two flasks and fill each flask up to 1 L with AIM-V supplemented with 10% human AB serum. If the cell count is <109, feed the cells with a fresh liter of culture medium supplemented with 10% human AB serum.
- Repeat step 3.6 for all the flasks and return them to the incubator at 37 °C and 5% CO2. Repeat steps 3.4-3.7 every 3 to 4 days.
4. Cell harvest
- At the end of 10 days in co-culture, harvest all the bioreactor flasks. Harvest 1 bioreactor flask at a time, and pool all the cells into a transfer pack of appropriate size. Sterile-weld the transfer pack to the red line of the closed-system bioreactor, and use the pharmaceutical pump to transfer the cells into the transfer pack.
- Remove the following quality control samples: 1% of the drug product (DP) for sterility by blood culture and gram staining; 0.5 mL for cell counting and viability using AO/PI; 5-10 × 106 cells for flow cytometry (see Figure 1 for gating strategy); 0.5 mL for endotoxin; 0.5 mL for gram staining; 106 cells spiked into 10 mL of spent medium for Mycoplasma testing.
- Centrifuge the cells at 200-500 × g for 15 min at room temperature and discard the supernatant.
- Wash the cells in a solution of balanced crystalloid solution+ 0.5% HSA at 200-500 × g for 15 min at room temperature. Resuspend them in a target volume of 100-300 mL of balanced crystalloid solution + 0.5% HSA.
5. Release testing
- Perform quality control testing of the γδ T cell DP for the following: purity and Identity by flow cytometry (Live/Dead, CD45, CD3, TCR αβ, TCR γδ, CD20, CD56, CD16); viability by AO/PI staining; endotoxin; Mycoplasma testing by polymerase chain reaction (PCR); sterility by gram staining and aerobic and anaerobic blood cultures; residual K562 assay by flow cytometry (CD3–CD16–CD56–CD71+).
Representative Results
The γδ T cell process was characterized and optimized for the production of the γδ T cell drug product. Process optimization included 1) lymphocyte enrichment using elutriation, 2) γδ T cell drug substance (DS) cell-specific expansion with zoledronic acid, 3) γδ T cell DS depletion of TCRαβ, 4) secondary expansion of the γδ T cell DS using K562-derived aAPCs, and 5) final DP harvest and formulation of product for administration or cryopreservation. After process optimization, confirmation runs were performed at scale using the material derived from three healthy donors to confirm cell-processing suitability. All data were analyzed and are summarized in Table 1, Table 2, and Table 3. The cells separated from the post-counterflow centrifugation fraction 2 (F2) yielded a pure lymphocyte population with an average of 99.23% CD45+ cells (reported as the frequency of total live gate) and excellent average viability of 95.80% (Table 1).
The γδ T-cell-specific expansion with zoledronic acid depended on the initial percentage of natural killer (NK) cells present in the lymphocyte fraction (F2) after elutriation. The enrichment of γδ T cell DS with TCRαβ depletion was consistent (Table 2). The γδ T cell DP manufactured from three healthy donors had an average of 0.11% ± 0.05% CD20+ B cells and 0.00% ± 0.00% TCR αβ+ T cells, thus meeting the release criterion of ≤1% of TCR αβ+ T cells. The average percentage of NK cells in the final product is 17.06% ± 26.19% and meets the release criterion of <35%. Additionally, the average percentage of T cell and NK cell lineage-negative cells in the final product was 0.48% ± 0.42% (Table 3). Cell surface staining and flow cytometric analysis were utilized to characterize the identity, purity, and process impurities of the DS and DP, as shown in Figure 2A–D.
The secondary expansion, achieved from the co-culture of the aAPC (K562CL6(CD3-CD137L:CD28-IL-15RA)) WCB and γδ T cell DS at a ratio of 10:1, generated a γδ T cell DP that met all release criteria, as shown in Table 4. In addition, the cells were stained and assessed by flow cytometry at Day 0-counterflow centrifugation F2 cells, Day 7-zoledronic acid-expanded T cells, Day 7-TCR αβ T cell depletion, Day 17-final DP for the following biomarkers cluster of differentiation (CD)3, TCRαβ, TCR γδ, CD45RA, CD45RO, CC chemokine receptor 7 (CCR7), programmed cell death protein-1 (PD-1), cytotoxic T lymphocyte-associated protein 4 (CTLA4), lymphocyte-activating gene 3 (LAG3), and T cell immunoglobulin and mucin domain-containing protein 3 (TIM3). Data shown in Figure 3 are averaged from three independent runs and demonstrate that cells have not reached exhaustion. The Moffitt CTF also developed a residual K562 assay to determine the DP impurities related to the K562-derived aAPCs (Figure 4).
The flow cytometric gating strategy used to characterize the percentages of the cell types was as follows: 1) gating on T and NK cell lineage-negative population (CD3–CD56–CD16–); 2) gate on CD71+ (transferrin receptor expressed in erythroid lineage and AML allowing for the detection of residual K562 cells). This gating strategy allowed the evaluation of the CD3–CD16–CD56–CD71+ cells, which are the aAPC (K562CL6(scFv-CD3-CD137;scFv-CD28-IL15-RA)) WCB (termed "residual K562" in Figure 4). This gating allows the enumeration of residual K562 in the final DP by multiplying the frequency of residual K562 by the total viable count (TVC) count of the DP (%CD71+ × DP TVC = Residual K562 cells in the DP). All flow cytometric data are reported as the frequency of live cells. Table 5 and Figure 4 provide the percentages of T cell and NK cell lineage-negative as well as residual K562 cells. A two-tailed t-test was performed to determine the statistical significance of the differences between these populations and revealed that there was a significant difference between WCB and γδ T cell DP and between WCB and γδ T cells (t = 0.0019 for T cell and NK cell lineage-negative; t < 0.0001 for Residual K562 and t = 0.0314 for T cell and NK cell lineage-negative; t < 0.0001for Residual K562) (Table 5).
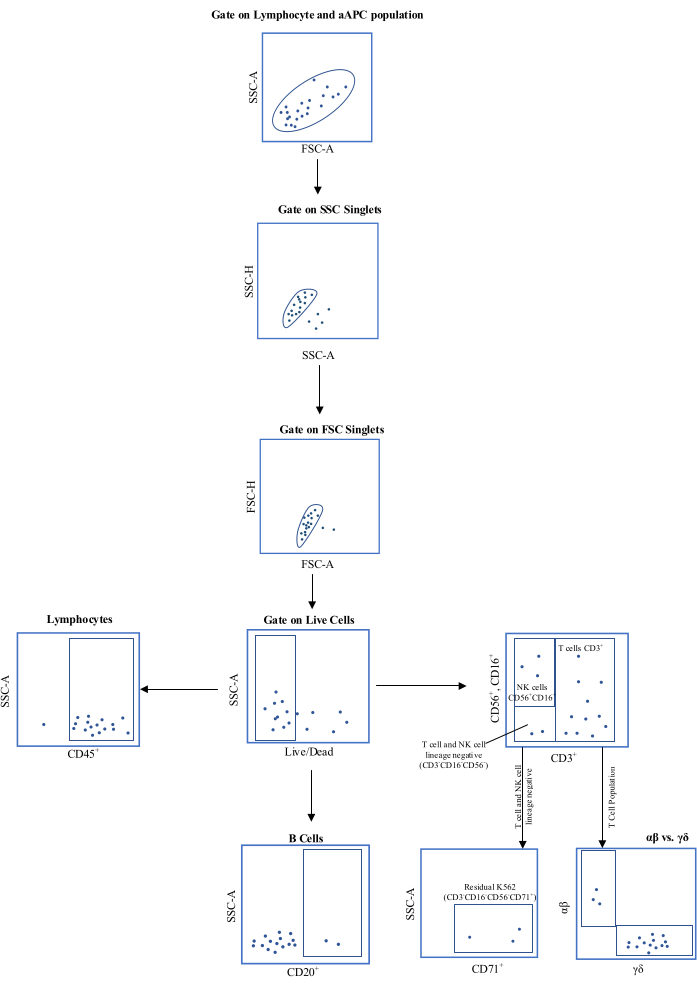
Figure 1: Schematic representation of the flow cytometric gating strategy. Abbreviations: aAPC = , artificial antigen-presenting cell; SSC-A = side scatter-area of peak; FSC-A = forward scatter-area of peak; SSC-H = side scatter-height of peak; FSC-H = forward scatter-height of peak; CD = cluster of differentiation. Please click here to view a larger version of this figure.
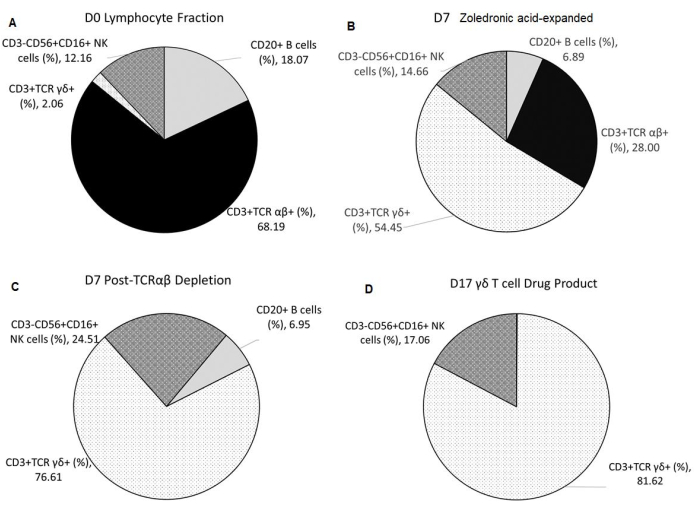
Figure 2: Composition of Starting Material, Intermediates, and Final Drug Product. All data shown are averaged from three independent runs. (A) Apheresis from healthy donors undergoes elutriation using the counterflow centrifugation device, resulting in F2 (lymphocyte-rich fraction), which is used as the starting material. (B) F2 undergoes Vγ9Vδ2 T cell-specific expansion for 7 days with 5 µmol/L of zoledronic acid and 300 IU/mL of IL-2 in 1 L of medium supplemented with 10% human AB serum. (C) TCR αβ T cell depletion is performed on the zoledronic acid-expanded product. (D) A highly pure γδ T cell Drug Product is harvested after a second 10-day expansion with irradiated aAPCs at a 1:10 ratio with 5 µmol/L of zoledronic acid and 300 IU/mL of IL-2 in 1 L of medium supplemented with 10% human AB serum. Abbreviations: NK = natural killer; CD = cluster of differentiation; TCR= T-cell receptor; IL = interleukin; aAPCs = artificial antigen-presenting cells. Please click here to view a larger version of this figure.
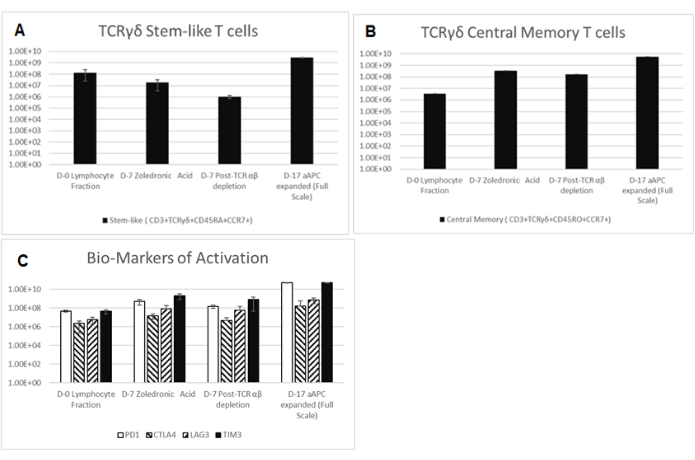
Figure 3: Biomarkers of Starting Material, Intermediates, and Final Drug Product. Cells are collected and stained for CD3, TCRαβ, TCR γδ, CD45RA, CD45RO, CCR7, PD-1, CTLA4, LAG3, and TIM3 at Day 0-Counterflow Centrifugation F2 cells, Day 7-zoledronic acid-expanded T cells, Day 7-TCR αβ T cell depletion, Day 17-Final Drug Product. All data shown are averaged from three independent runs. (A) Stem-like (CD3+, TCR γδ+, CD45RA+, CD45RO–, and CCR7+) shown as a total number of live cells. (B) Central memory (CD3+, TCR γδ+, CD45RA–, CD45RO+, and CCR7+) depicted as a percentage of CD3+ TCR γδ+ cells. Abbreviations: CD = cluster of differentiation; TCR= T-cell receptor; IL = interleukin; .CCR7 = CC chemokine receptor 7; PD-1 = programmed cell death protein-1; CTLA4 = cytotoxic T lymphocyte-associated protein 4; LAG3 = lymphocyte-activating gene 3; TIM3 = T cell immunoglobulin and mucin domain-containing protein 3. Please click here to view a larger version of this figure.
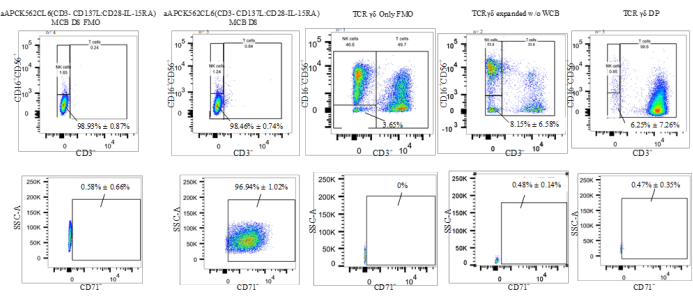
Figure 4: Representative data of a K562 residual assay(CD3–CD16–CD56–CD71+). Abbreviations: aAPC = artificial antigen-presenting cell; NK = natural killer cell; SSC-A = side scatter-area of peak; CD = cluster of differentiation; IL = interleukin; TCR = T-cell receptor; MCB =master cell bank ; FMO = fluorescence minus one; DP = drug product; WCB = working cell bank. Please click here to view a larger version of this figure.
| Process Steps | Parameters | Donors | Average | St. Dev. | |||
| Run 1 | Run 2 | Run 3 | |||||
| Post-Enrichment (Lymphocyte fraction F2) | TVC All Process Validations were seeded at 109 TVC | 1.00 X 109 | 1.00 X 109 | 1.00 X 109 | 1.00 X 109 | 0.00 | |
| Viability (%) | 98.6 | 96.6 | 92.2 | 95.8 | 3.27 | ||
| CD20+ B cells (%) | 15.6 | 23.4 | 15.2 | 18.07 | 4.62 | ||
| CD3+ T cell (%) | 80.04 | 66.7 | 76.1 | 74.28 | 6.85 | ||
| TCR αβ+ (%) | 77.59 | 58.03 | 68.96 | 68.19 | 9.8 | ||
| TCR γδ+ (%) | 2.48 | 1.59 | 2.1 | 2.06 | 0.45 | ||
| CD3–CD56+CD16+ NK cells (%) | 6.57 | 19.4 | 10.5 | 12.16 | 6.57 | ||
| T cell and NK cell lineage negative (%) | 13 | 13.7 | 13.4 | 13.37 | 0.35 | ||
Table 1: Summary of lymphocyte enrichment by elutriation reported as frequency of live cells. Abbreviations: TVC = total viable count; TCR = T cell receptor; CD = cluster of differentiation; NK = natural killer cell.
| Process Steps | Parameters | Donors | Average | St. Dev. | |||
| Run 1 | Run 2 | Run 3 | |||||
| 7-day Post Zoledronic acid Expansion (pre- TCRαβ depletion) | TVC | 3.69 X 109 | 1.79 X 109 | 1.42 X 109 | 2.3 X 109 | 1.22 X 109 | |
| Viability (%) | 99.2 | 82.6 | 89.8 | 90.53 | 8.32 | ||
| CD20+ B cells | 2.1 | 11.5 | 7.08 | 6.89 | 4.7 | ||
| CD3+ T cell (%) | 95.7 | 64.1 | 91.9 | 83.9 | 17.25 | ||
| TCR αβ+ (%) | 13.88 | 38.14 | 31.98 | 28 | 12.61 | ||
| TCR γδ+ (%) | 81.44 | 24.93 | 56.98 | 54.45 | 28.34 | ||
| CD3–CD56+CD16+ NK cells (%) | 3.59 | 33.1 | 7.28 | 14.66 | 16.08 | ||
| T cell and NK cell lineage negative (%) | 0.67 | 2.7 | 0.82 | 1.4 | 1.13 | ||
| Post- TCRαβ depletion | TVC | 1.81 x 109 | 4.95 X 108 | 3.80 X 108 | 8.95 X108 | 7.94 X 108 | |
| Cell viability (%) | 98.8 | 87.6 | 89.8 | 92.07 | 5.93 | ||
| CD20+ B cells | 2.26 | 12 | 6.59 | 6.95 | 4.88 | ||
| CD3+ T cell (%) | 95.8 | 45.3 | 89.7 | 76.93 | 27.56 | ||
| TCR αβ+ (%) | 0 | 0.001 | 0.001 | 0.001 | 0.001 | ||
| TCR γδ+ (%) | 95.61 | 45.07 | 89.16 | 76.61 | 27.51 | ||
| CD3–CD56+CD16+ NK cells (%) | 3.85 | 59.9 | 9.79 | 24.51 | 30.79 | ||
| T cell and NK cell lineage negative (%) | 0.34 | 1.72 | 0.45 | 0.84 | 0.77 | ||
| TCR αβ+ TVC | 0.00 | 4.95 x 103 | 3.8 X 103 | 2.92 X 103 | 2.59 X 103 | ||
| TCR γδ+ TVC | 1.73 X 109 | 2.23 X 108 | 3.39 X 108 | 7.64 X 108 | 8.39 X 108 | ||
| CD3–CD56+CD16+ NK TVC | 6.97 X 107 | 2.97 X 108 | 3.72E+07 | 1.35 X 108 | 1.42 X 108 | ||
Table 2: Summary of γδ T cell expansion with zoledronic acid and instrument enrichment reported as frequency of live cells. Abbreviations:TVC = total viable count; TCR = T cell receptor; CD = cluster of differentiation; NK = natural killer cell.
| Product Attributes | Parameters | Donors | Average | St. Dev. | |||
| Run 1 | Run 2 | Run 3 | |||||
| Day 0 γδ T Cells | 2.48 X 107 | 1.59 X 107 | 2.10 X 107 | 2.06 X 107 | 4.47 X 107 | ||
| Day 7 post enrichment γδ T Cells | 1.73 X 109 | 2.23 X 108 | 3.39 X 108 | 7.64 X 108 | 8.39 X 108 | ||
| Fold Expansion at Day 7 | 69.76 | 14.03 | 16.14 | 33.31 | 31.58 | ||
| TVC at harvest* | 8.14 X 1010 | 1.67 X 1010 | 6.84 X 1010 | 5.55 X 1010 | 3.42 X 1010 | ||
| Cell viability (%) | 92.8 | 85.5 | 87.3 | 88.53 | 3.80 | ||
| CD20+ B cells (%) | 0.12 | 0.06 | 0.15 | 0.11 | 0.05 | ||
| CD3+ T cell (%) | 97.8 | 52 | 97.5 | 82.43 | 26.36 | ||
| TCR αβ+ (%) | 0 | 0.001 | 0 | 0.00 | 0.00 | ||
| TCR γδ+ (%) | 97.51 | 50.13 | 97.21 | 81.62 | 27.27 | ||
| CD3–CD56+CD16+ NK cells (%) | 2.16 | 47.3 | 1.71 | 17.06 | 26.19 | ||
| T cell and NK cell lineage negative, CD71+ Residual K562 (%) | 0.018 | 0.61 | 0.82 | 0.48 | 0.42 | ||
| Total γδ T cells at Harvest | 7.93 X 1012 | 8.38 X 1011 | 6.65 X 1012 | 5.14 X 1012 | 3.78 X 1012 | ||
| Total Fold Expansion of γδ T cells (From day 0 to Harvest) | 3.20 X 105 | 5.27 X 104 | 3.17 X 105 | 2.30 X 105 | 1.53 X 105 | ||
| *Process Validation was scaled down to flask with 5 X 106 γδ T cell and 50 X106 irradiated aAPCs. Numbers reported are for a projected full scale run if 24 flasks are seeded from the D7 drug substance was used. | |||||||
Table 3: Summary of γδ+ T cell co-culture with aAPCs and expanded γδ+ T cell harvest reported as frequency of live cells. *Process validation was scaled down to one closed-system bioreactor (1 L capacity) with 5 × 106 γδ T cells and 50 × 106 irradiated aAPCs. Numbers reported are for a projected full-scale run if 24 flasks are seeded from the D7 drug substance. Abbreviations: aAPCs = artificial antigen-presenting cells; TVC = total viable count; TCR = T cell receptor; CD = cluster of differentiation; NK = natural killer cell.
| Test Parameter | Acceptance Criteria | Résultats | ||
| Validation 1 | Validation 2 | Validation 3 | ||
| Viability | ≥ 70% | 92.80% | 85.50% | 87.30% |
| Mycoplasma | Negative | Negative | Negative | Negative |
| Sterility | No Growth Final (14 days) | No Growth Final (14 days) | No Growth Final (14 days) | No Growth Final (14 days) |
| Gram stain | No organisms seen (NOS) | NOS | NOS | NOS |
| Endotoxin | ≤ 2 EU/mL | <0.50 EU/mL | <0.50 EU/mL | <0.50 EU/mL |
Table 4: Summary of quality control release testing results for the γδ T cells.
| Residual K562 Assay | K562 | WCB | γδ Only | γδ + WCB Product | ||||
| T and NK cell lineage neg. % | Residual K562 % | T and NK cell lineage neg. % | Residual K562 % | T and NK cell lineage neg. % | Residual K562 % | T and NK cell lineage neg. % | Residual K562 % | |
| 99.4 | 98.8 | 98.7 | 96.83 | 3.49 | 0.58 | 0.48 | 0.07 | |
| 99.2 | 98.31 | 99.5 | 98.21 | 12.8 | 0.38 | 3.87 | 0.71 | |
| 98.9 | 98.6 | 97.6 | 95.55 | N/A | N/A | 14.4 | 0.63 | |
| 99.2 | 98.9 | 97.9 | 96.53 | N/A | N/A | N/A | N/A | |
| 98.7 | 98.3 | 98.6 | 97.6 | N/A | N/A | N/A | N/A | |
| Average | 99.08 | 98.58 | 98.46 | 96.94 | 8.15 | 0.48 | 6.25 | 0.47 |
| St. Dev. | 0.28 | 0.27 | 0.74 | 1.02 | 6.58 | 0.14 | 7.26 | 0.35 |
Table 5: T cell and NK cell lineage-negative and residual K562 percentages reported as frequency of live cells. Abbreviations: NK = natural killer cell; WCB = working cell bank.
Discussion
The Moffit Cell Therapy Lab has developed a protocol with a biphasic expansion of highly pure γδ T cells for use as a DP in clinical trials. This protocol provides a manufacturing method under cGMP guidelines in a closed system that yields a highly pure γδ T cell DP that is successfully activated and expanded by zoledronic acid and the WCB aAPCs. This protocol has been approved by the FDA for the manufacture of an allogeneic γδ T cell DP for AML patients. Using healthy donors, we successfully expanded the small population of donor γδ T cells in just 7 days from 2.06 ± 0.45% to 54.45 ± 28.34%. After the 7-day expansion with zoledronic acid, it was observed that donor 2 had an increase in the NK population.
Zoledronic acid inhibits farnesyl diphosphate synthase (FDPS) in monocytes, which, in turn, leads to the accumulation of isopentenyl pyrophosphate (IPP), which has been correlated with a significant increase in the proliferation of T cells and natural NK cells7,8,9. This increased NK population hinders the 2nd phase of expansion with the aAPCs, as the aAPCs will only contribute to the further expansion of NK cells. For this reason, the donor criteria were modified to exclude donors with high NK populations. After depletion of the αβ T cells, the γδ T cells were further enriched to 76.61 ± 27.51%. This unique protocol includes a second expansion utilizing the Moffit-manufactured aAPCs to target the CD8, CD28, and CD127L receptors in the γδ T cells. This second expansion phase with the aAPCs yielded a DP with ≥65% for CD3+TCR γδ+ T cells, ≤1% TCRαβ T, and <35% CD3–CD16+CD56+ NK cells. Owing to the use of K562-derived aAPCs, it was necessary to demonstrate that these aAPCs comprised <1% of the final product.
The Moffit CTF developed a flow cytometric assay used for the release criteria to measure the percentage of the residual K562 cells in the final DP. This flow cytometric assay mitigates all the issues of utilizing cell surface antigens to identify the K562 cells. As activated T cells can express CD71, we devised a strategy to exclude all T cells and NK cells by gating on CD3– CD56– and CD16– populations and then examining the CD71+ cells, which would be exclusively K562 cells. This protocol demonstrates that the γδ T cell DP yields 0.48 ± 0.42% of residual K562 cells and meets all the release criteria of ≥70% viability, Mycoplasma negativity by PCR, no organisms seen by gram staining, ≤2 EU/mL of endotoxin, and no growth final (14 days) blood culture sterility.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We give thanks to the Cellular Immunotherapies-Investigator Initiated Trials Award Intramural Funding Opportunity from Moffitt Cancer Center for providing the funding for this protocol development. We also thank Dr. Claudio Anasetti for his invaluable help and guidance through this project. Finally, we thank Dr. Justin Boucher for his insights and review of the manuscript.
Materials
| Hanks Balanced Salt Solution | R&D | 285-GMP | |
| Human Albumin 25% | Grifolis | 65483-16-071 | |
| Plasmalyte A | Fisher | 2B2543Q | |
| Zoledronic Acid (Zometa) | Hos pira | 4215-04–8 | FDA approved drug |
| DMSO | WAK-CHEMIE MEDICAL GMBH | WAK-DMSO-10 | |
| CS10 | BIOLIFE SOLUTIONS | 210374 | |
| 3 mL syringe | BD | 309657 | |
| 10 mL syringe | BD | 309604 | |
| 20 mL syringe | BD | 302830 | |
| 50 mL syringe | BD | 309653 | |
| 100 mL syringe | JMS | 992861 | |
| 18g Needle | Fisher | 305198 | |
| Cryovials 1.8 mL | Fisher | 375418 | |
| 5 mL pipette | Fisher | 1367811D | |
| 50 mL pipette | Fisher | 1367610Q | |
| 10 mL pipette | Fisher | 1367811E | |
| 100 mL pipette | Fisher | 07-200-620 | |
| 15 mL conical | Fisher | 05-539-12 | |
| 50 mL conical | Fisher | 05-539-7 | |
| 250 mL conical | Fisher | 430776 | |
| 600 mL Transfer Pack | TERUMO BCT INC | 1BBT060CB71 | |
| 4" Plasma Transfer Set | INDEPENDENT MEDICSL ASSOCIATES | 03-220-90 | |
| Elutra Tubing Set | TerumoBCT | 70800 | |
| 100 MCS GREX | WILSON WOLF MFG CORP | 81100-CS | |
| Ashton Sterile Pumpmatic Liquid dispensing system | Fisher Scientific | 22-246660 | |
| Acacia Pump boot | MPS Medical In | 17789HP3MLL | |
| CliniMACS PBS/EDTA Buffer | Miltenyi Biotec Inc | 130-070-525 | |
| Dornase Alpha | Genentech, Inc | 50242-100-40/186-0055 | FDA approved drug |
| 1000 mL 0.22 um Filter | Fisher | 157-0020 | |
| Blood Filter 170um | B.Braun | V2500 | |
| CliniMACs Tubing set | Miltenyi Biotec Inc | 130-090-719 | |
| CliniMACS TCRα/β Kit | Miltenyi Biotec Inc | 130-021-301 | |
| Y-Type blood set | Fenwal | FWL4C2498H | |
| 75 mL Flask | Fisher | 430641U | |
| IL-2 | Prometheus | 65483-116-071 | FDA approved drug |
| AIM-V | Fisher | 0870112BK | |
| Human AB serum | Gemini Bio-Product | 100H41T | |
| 3 Liter Transfer pack | Independent Medical Associates | T3109 | |
| 1000 pipette tips | Fisher Scientific | 5991040 | |
| CF-250 | KOLBio | CF-250 | |
| Elutra | TERUMOBCT | ||
| CliniMACS | Miltenyi Biotec Inc | ||
| GatheRex Liquid Handling, Cell Harvest Pump | WILSON WOLF MFG CORP | ||
| HERAcell Vios CO2 Incubator | Thermo Scientific |
References
- Bejanyan, N., et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biology of Blood and Marrow Transplantation. 21 (3), 454-459 (2015).
- Bejanyan, N., et al. Clinical outcomes of AML patients relapsing after matched-related donor and umbilical cord blood transplantation. Bone Marrow Transplantation. 49 (8), 1029-1035 (2014).
- Schmid, C., et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 119 (6), 1599-1606 (2012).
- Siegers, G. M., et al. Anti-leukemia activity of in vitro-expanded human gamma delta T cells in a xenogeneic Ph+ leukemia model. PLoS One. 6 (2), 16700 (2011).
- Airoldi, I., et al. γδ T-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-αβ+/CD19+ lymphocytes. Blood. 125 (15), 2349-2358 (2015).
- Acuto, O., et al. The human T cell receptor: appearance in ontogeny and biochemical relationship of alpha and beta subunits on IL-2 dependent clones and T cell tumors. Cell. 34 (3), 717-726 (1983).
- Xiao, L., et al. Large-scale expansion of Vγ9Vδ2 T cells with engineered K562 feeder cells in G-Rex vessels and their use as chimeric antigen receptor-modified effector cells. Cytotherapy. 20 (3), 420-435 (2018).
- Peters, C., Kouakanou, L., Oberg, H. H., Wesch, D., Kabelitz, D. In vitro expansion of Vγ9Vδ2 T cells for immunotherapy. Methods in Enzymology. 631, 223-237 (2020).
- Xu, Y., et al. Allogeneic Vγ9Vδ2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer. Cellular & Molecular Immunology. 18 (2), 427-439 (2021).

