In Vitro Stimulation and Visualization of Extracellular Trap Release in Differentiated Human Monocyte-derived Macrophages
Summary
Presented here is a protocol to detect macrophage extracellular trap (MET) production in live cell culture using microscopy and fluorescence staining. This protocol can be further extended to examine specific MET protein markers by immunofluorescence staining.
Abstract
The release of extracellular traps (ETs) by neutrophils has been identified as a contributing factor to the development of diseases related to chronic inflammation. Neutrophil ETs (NETs) consist of a mesh of DNA, histone proteins, and various granule proteins (i.e., myeloperoxidase, elastase, and cathepsin G). Other immune cells, including macrophages, can also produce ETs; however, to what extent this occurs in vivo and whether macrophage extracellular traps (METs) play a role in pathological mechanisms has not been examined in detail. To better understand the role of METs in inflammatory pathologies, a protocol was developed for visualizing MET release from primary human macrophages in vitro, which can also be exploited in immunofluorescence experiments. This allows further characterization of these structures and their comparison to ETs released from neutrophils. Human monocyte-derived macrophages (HMDM) produce METs upon exposure to different inflammatory stimuli following differentiation to the M1 pro-inflammatory phenotype. The release of METs can be visualized by microscopy using a green fluorescent nucleic acid stain that is impermeant to live cells (e.g., SYTOX green). Use of freshly isolated primary macrophages, such as HMDM, is advantageous in modeling in vivo inflammatory events that are relevant to potential clinical applications. This protocol can also be used to study MET release from human monocyte cell lines (e.g., THP-1) following differentiation into macrophages with phorbol myristate acetate or other macrophage cell lines (e.g., the murine macrophage-like J774A.1 cells).
Introduction
The release of ETs from neutrophils was first identified as an innate immune response triggered by bacterial infection1. They consist of a DNA backbone to which various granule proteins with anti-bacterial properties are bound, including neutrophil elastase and myeloperoxidase2. The primary role of neutrophil ETs (NETs) is to capture pathogens and facilitate their elimination3. However, in addition to the protective role of ETs in immune defense, an increasing number of studies have also discovered a role in disease pathogenesis, particularly during the development of inflammation-driven diseases (i.e., rheumatoid arthritis and atherosclerosis4). The release of ETs can be triggered by various pro-inflammatory cytokines including interleukin 8 (IL-8) and tumor necrosis factor alpha (TNFα)5,6, and the localized accumulation of ETs can increase tissue damage and evoke a pro-inflammatory response7. For example, ETs have been implicated as playing a causal role in the development of atherosclerosis8, promoting thrombosis9, and predicting cardiovascular risk10.
It is now recognized that in addition to neutrophils, other immune cells (i.e., mast cells, eosinophils, and macrophages) can also release ETs on exposure to the microbial or pro- inflammatory stimulation11,12. This may be particularly significant in the case of macrophages, considering their key role in the development, regulation, and resolution of chronic inflammatory diseases. Therefore, it is important to gain a greater understanding of the potential relationship between ET release from macrophages and inflammation-related disease development. Recent studies have shown the presence of METs and NETs in intact human atherosclerotic plaques and organized thrombi13. Similarly, METs have been implicated in driving kidney injury through the regulation of inflammatory responses14. However, in contrast to neutrophils, there are limited data on the mechanisms of MET formation from macrophages. Recent studies using human in vitro models of MET formation show some differences in the pathways involved in each cell type (i.e., regarding the absence of histone citrullination with macrophages)6. However, some have shown that NET release can also occur in the absence of histone citrullination15.
The overall goal of this protocol is to provide a simple and direct method to assess MET release in a clinically relevant macrophage model. There are a number of different in vitro macrophage cell models that have been used to study METs (i.e., the THP-1 human monocyte cell line and various murine macrophage cell lines)16. There are some limitations associated with these models. For example, the differentiation of THP-1 monocytes to macrophages usually requires a priming step, such as the addition of phorbol myristate acetate (PMA), which itself activates protein kinase C (PKC)-dependent pathways. This process is known to trigger ET release4 and results in a low basal MET release from THP-1 cells. Other studies have highlighted some differences in bioactivity and inflammatory responses mounted by macrophages in vivo compared to PMA-treated THP-1 cells17.
Similarly, the behavior and inflammatory responses of different murine macrophage-like cell lines do not completely represent the response spectrum of primary human macrophages18. Therefore, for the purpose of investigating macrophage ET formation in the clinical setting, primary human monocyte-derived macrophages (HMDMs) are believed to be a more relevant model rather than monocytic or murine macrophage-like cell lines.
ET release from M1 polarized HMDMs has been demonstrated following exposure of these cells to a number of different inflammatory stimuli, including the myeloperoxidase-derived oxidant hypochlorous acid (HOCl), PMA, TNFα, and IL-86. Described here is a protocol to polarize HMDMs to the M1 phenotype and visualize subsequent MET release upon exposure to these inflammatory stimuli. PMA is used as a stimulus of MET release to facilitate comparisons to previous studies that have used neutrophils. Importantly, HOCl, IL-8, and TNFα are also used to stimulate MET release, which are believed to be better models of the inflammatory environment in vivo. The microscopic method for visualization of ET release involves staining the extracellular DNA in live cell cultures using SYTOX green, an impermeable fluorescent green nucleic acid stain that has been successfully applied in previous neutrophil studies. This method allows for rapid and qualitative assessment of ET release, but it is not appropriate as a stand-alone method for the quantification of ET release extent. Alternative methodology should be used if quantification is required to compare the extent of ET release resulting from different treatment conditions or interventions.
Protocol
The HMDM were isolated from human buffy coat preparations supplied by the blood bank with ethics approval from the Sydney Local Health District.
1. HMDM Culture
- Isolate the monocytes from buffy coat preparations prepared from the peripheral blood of healthy human donors using a commercially available preparation to isolate lymphocytes, followed by countercurrent centrifugal elutriation19,20.
- Confirm the presence of monocytes by cytospinning and staining with modified Giemsa stain for monocyte characterization19.
- Under sterile conditions, adjust the density of monocytes to 1 x 106 cells/mL using RPMI-1640 media without serum. Add 1 mL of this cell suspension to each well of a 12 well tissue culture plate. Culture in a cell incubator at 37 °C in the presence of 5% CO2 for 2 h to promote adherence to the tissue culture plate.
- Under sterile conditions, remove the cell media and replace with complete RPMI-1640 culture media containing 10% (v/v) pooled human serum and 20 mM L-glutamine.
- Culture the cells at 37 °C in the presence of 5% CO2 in a cell incubator for 8 days, changing the media every 2 days.
2. Polarization of HMDM
- Under sterile conditions, prepare the M1 priming media by adding interferon γ (IFNγ; 20 ng/mL) and lipopolysaccharide (LPS; 1 μg/mL) to the complete RPMI-1640 culture media. Prepare the M2 priming media by adding interleukin 4 (IL-4; 20 ng/mL) to the complete RPMI-1640 culture media.
- Under sterile conditions, aspirate media from the tissue culture plate wells that contain the HMDM, which have been seeded and cultured as described in section 1.
- Carefully wash the wells containing the cells 3x with sterile PBS (pre-warmed to 37 °C), using 1 mL aliquots of PBS.
- Add 1 mL of either the M1 or M2 priming media to each well containing the HMDM (whichever is appropriate for the experiment).
- Incubate the cells for 48 h at 37 °C in the presence of 5% CO2 in a cell incubator.
3. Stimulation of HMDM to induce MET Release
- Under sterile conditions, prepare the culture media containing different stimulators of MET release (whichever is appropriate for the experiment) to the complete RPMI-1640 media: PMA (25 nM), human recombinant TNFα (25 ng/mL), or human recombinant IL-8 (50 ng/mL).
- For experiments with HOCl stimulation, prepare HOCl (200 μM) in HBSS (pre-warmed to 37 °C), immediately before the addition to the cells. Ensure that the HOCl is not prepared in complete cell media.
NOTE: The concentration of the stock solution of HOCl is quantified by measuring the UV absorbance of the solution at 292 nm and pH = 116 and using an extinction coefficient of 350 M-1cm-1 21. - After the polarization treatment described in section 2, aspirate the cell media from each well and carefully wash the cells 3x with 1 mL aliquots of either: sterile PBS (for PMA, TNFα and IL-8) or HBSS (for HOCl), which have been pre-warmed to 37 °C.
- For experiments with PMA, TNFα, or IL-8: add 1 mL of the complete media containing PMA, TNFα, or IL-8 after removing the PBS in the final washing step.
- For experiments with TNFα, incubate the cells for 6 h at 37 °C in the presence of 5% CO2 in a cell incubator. For experiments with PMA and IL-8, incubate the cells for 24 h at 37 °C in the presence of 5% CO2 in a cell incubator.
- For experiments with HOCl, add 1 mL of HOCl in HBSS after removing the HBSS in the final washing step. Then, incubate the cells for 15 min at 37 °C in the presence of 5% CO2 in a cell incubator.
- Carefully aspirate the cell supernatant and wash the cells 3x with 1 mL aliquots of HBSS as described in step 3.3.
- After removing the HBSS from the final wash step, add 1 mL of complete RPMI-1640 culture media. Then, incubate the cells for 24 h at 37 °C in the presence of 5% CO2 in a cell incubator.
4. Visualization of MET in Live Cell Culture
- Prepare SYTOX green dye in HBSS at a concentration of 40 μM.
- At the end of treatments described in section 3 to induce MET release, directly add 25 μL of 40 μM of the dye to each well containing HMDM.
- Incubate cells at room temperature (RT) for 5 min in the dark.
- Place the HMDM in tissue culture wells on the microscope stage of an inverted fluorescent microscope for imaging.
- Microscope procedures
- Turn on a broad-spectrum fluorescent light source, brightfield light source, and inverted microscope installed with a high-resolution color digital camera (see Table of Materials).
- Rotate the filter wheel to the “number 2” position for green fluorescence (excitation = 504 nm, emission = 523 nm) for imaging of the green stained samples contained within the tissue culture wells.
- Using the 5x objective, focus the image with the coarse focus, then the fine focus knobs on the microscope, until the image appears sharp, clear, and focused when viewed through the microscope eyepiece.
- Switch the microscope to the camera mode.
- Start the associated software.
- Select the Capture tab on the software.
- Click the Play button to preview the image and adjust the fine focus knob on the microscope until the image appears sharp, clear, and focused in the software preview window.
- Click the Capture button.
NOTE: The captured image will automatically be displayed in the accompanying software. - Within the software, click File | Save as the required image file type.
- On the microscope, rotate the filter wheel to the “number 5” position for brightfield imaging and repeat steps 4.5.2–4.5.9 to obtain the corresponding brightfield image.
- Repeat the steps 4.5.2–4.5.10 as necessary for subsequent image acquisition.
Representative Results
Brightfield images showing the morphological changes of HMDM in response to stimuli for cell differentiation are shown in Figure 1. M1 polarized macrophages from experiments with HMDM exposed to IFNγ and LPS showed an elongated and spindle-like cell shape, as indicated by the black arrows in Figure 1 (middle panel). For comparison, the morphology of the M2 polarized macrophages after exposure of HMDM to IL-4 for 48 h were typically round and flat, as indicated by the black arrows in Figure 1 (far right panel).
The ability of differentiated HMDM phenotypes to release METs was visualized by live cell fluorescence imaging with SYTOX green, as presented in Figure 2. Figure 2A shows the control data obtained from each HMDM phenotype incubated for 24 h in the absence of any pro-inflammatory stimuli. In this case, there was very limited green staining, as was expected, given the cell impermeant nature of this stain. Figure 2B showed positive staining for METs, resulting from the exposure of M1 HMDMs to HOCl, PMA, IL-8, or TNFα. The METs are indicated by the white arrows, shown as green streaks, resulting from the strands of extracellular DNA. With HOCl, in addition to staining extracellular DNA, there was some green staining apparent in the cells. This cellular staining was also observed to some extent with the other stimuli and is believed to reflect loss of membrane integrity resulting from ET-independent cell death as a result of the treatment conditions.
Figure 2B also shows the corresponding experiments performed with M2 HMDMs, which were exposed to IL-4. In this case, there was no release of DNA from the cells, as indicated by the absence of the strands/streaks of extracellular DNA; though, there was some cellular uptake of green fluorescent dye with the HOCl and TNFα. For comparative purposes, Figure 3 shows representative data indicating MET release from THP-1 macrophages exposed to TNFα (50 ng/mL) for 4 h. In this case, it should be noted that a non-polarized population of cells was used, and the THP-1 monocytes were differentiated to macrophages by pre-treatment with PMA (50 ng/mL for 72 h) as described previously22.

Figure 1: Morphological changes of differentially polarized HMDMs. Representative brightfield images from non-differentiated and differentiated HMDMs (n ≥5). HMDMs were cultured with complete media containing human serum and glutamine for 8 days before priming to the M1 or M2 phenotype upon exposure to IFNγ and LPS or IL-4, respectively, for 48 h. Scale bar = 200 μm. Arrows indicate examples of cells showing morphological characteristics of M1 or M2 HMDMs. Please click here to view a larger version of this figure.
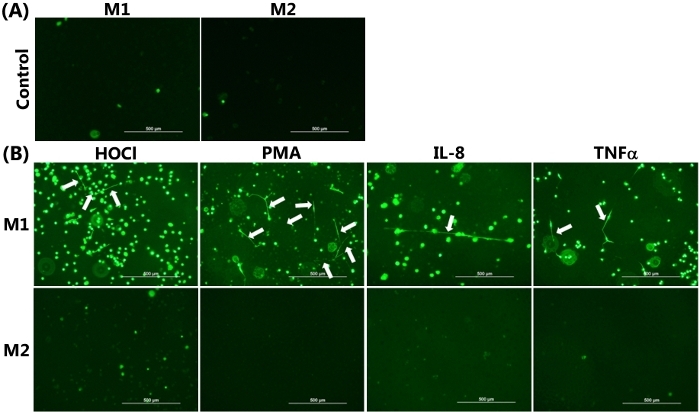
Figure 2: METs produced by M1 HMDMs following HOCl, PMA, IL-8, and TNFα stimulation. Representative images of SYTOX green stained HMDMs from (A) Non-stimulated M1 and M2 HMDMs incubated for 24 h in the absence of any inflammatory stimuli were used as the control, demonstrating the absence of MET release. (B) M1 and M2 HMDMs were treated with 1) HOCl (200 µM, 15 min), PMA (25 nM), or IL-8 (50 ng/mL) with incubation for 24 h or 2) TNFα (25 ng/mL) with incubation for 6 h to induce MET release. METs were visualized by the addition of SYTOX green, as indicated by white arrows in the upper panel from M1 HMDMs. No METS were seen in the corresponding experiments with M2 HMDMs. Data are representative of replicate culture wells from n ≥3 individual donors. Scale bar = 500 μm. Please click here to view a larger version of this figure.
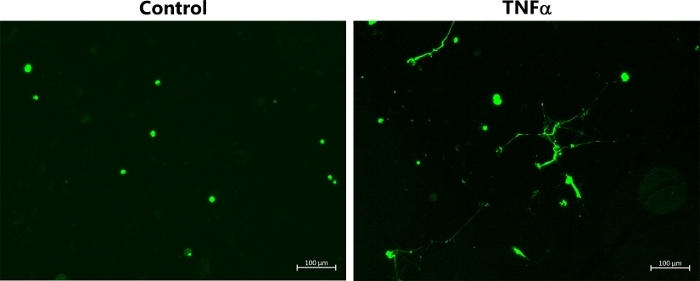
Figure 3: METs produced by non-polarized THP-1 macrophages following TNFα stimulation. Representative images of SYTOX green-stained TNP-1 macrophages, which were differentiated by pre-treatment with PMA (50 ng/mL for 72 h) before further incubation for 4 h in the absence or presence of TNFα (50 ng/mL) to induce MET release. METs were visualized by addition of SYTOX green. Data are representative of replicate culture wells from n ≥ 3 experiments. Scale bar = 100 μm. Please click here to view a larger version of this figure.
Discussion
The generation and visualization of MET formation using M1 differentiated HMDMs represents a new in vitro model that may be useful for investigating the potential pathological role of these macrophage structures, particularly under chronic inflammatory conditions. It provides a robust protocol for the stimulation of primary human macrophages to release METs, which can also be utilized in related studies with human monocyte or murine macrophage cell lines. The successful implementation of this protocol for the stimulation of MET formation by HMDM is dependent on careful cell culture and handling to maintain good cell viability. This will increase the extent and quality of METs observed. To provide better cell nutrition, the RPMI media used to maintain the HMDM should contain human serum, rather than fetal bovine serum, as well as L-glutamine. Each stock solution of this complete RPMI media should be used within 1 week. Correct storage of the media is also important. The media should be stored at 4 °C in the absence of light.
In addition, it is important to regularly observe and monitor the shape and morphology of the cells by microscopy during the culturing of HMDM. Any abnormal or unexpected changes in cell shape observed prior to the addition of IFNγ and LPS or IL-4 (to polarize the macrophages) may indicate a reduction in cell survival rate. HMDM should be adherent and do not proliferate. It is important, therefore, to monitor the cells for any changes in morphology, cell density, or loss of adherence during the normal 8 day culture period. A dramatic decrease in cell density during first 2 days post-isolation suggests that the resulting HMDMs may not be sufficiently viable for subsequent experiments. A limitation of using primary human cells is the variation between donors, which can markedly influence the extent of MET release. In addition, the quality of the buffy coat preparations received for the isolation of monocytes can vary. It is important to repeat experiments with at least three individual cell donors to ensure that data are representative of larger populations of cells.
It is important to note that the protocol described for HMDM differentiation has been optimized for cells isolated from human buffy coat preparations by countercurrent elutriation, which results in a high purity monocyte preparation. Validation of the polarization treatments (to confirm phenotype) by flow cytometry and assessment of the expression of M1 marker CD86 and M2 marker CD206 have been performed previously using this preparation of HMDM6. It is appreciated that this isolation method may be not be widely accessible, and that alternative isolation methods (i.e., magnetic bead sorting) may be preferable. If an alternative source of monocytes or different isolation protocols is used, additional flow cytometry experiments to validate phenotype change are recommended, and some optimization of treatment conditions to stimulate MET release may be required.
For optimal fluorescence images, exposure time should be carefully adjusted and kept as short as possible to avoid background fluorescence and staining artifacts from the plastic culture plates, which have adherent coatings. It is important to take multiple images from each well containing cells. It is advantageous if the samples are blinded before fluorescence microscopy analysis. In addition to staining extracellular strands of DNA, there is also evidence for the cellular uptake of SYTOX green. This is believed to reflect a loss in membrane integrity, which may be because of MET release, and it can also reveal alternative pathways of cell death (see also below). This highlights the importance of performing additional experiments to quantify and further characterize MET release.
For a quantitative comparison of the extent of MET release under different treatment conditions or following different interventions to modulate MET release, further analysis is required. This can be achieved by performing qPCR analysis of nuclear and mitochondrial DNA present in the cell supernatants, as described previously6. This method is usually combined with lactate dehydrogenase release assays to control for cell lysis unrelated to MET release6. The SYTOX green staining can also be quantified by a fluorescence plate reader following DNase treatment to partially digest the METs and release them from culture plates. This methodology has been used extensively in previous work with neutrophil NETs1,2,23.
The future applications of this protocol relate to providing opportunities for further characterization of METs using immunohistochemistry. It will be possible to probe the protein composition of METs using this approach with targeted antibodies (i.e., against citrullinated histones or MPO) to gain additional insight into the roles of these structures in more complex biological samples. These experiments will also be important for shedding more light on the delineation of MET release following other forms of cell death, such as pyroptosis15. It is worth noting that in our experience, METs from macrophages do not adhere to the plates as strongly as NETs from neutrophils, which can make immunohistochemistry analysis more challenging. Nonetheless, the data from these experiments will be of interest, given the fact that macrophages often play a dominant role in chronic inflammatory diseases. Further characterization of these structures is critical to assess their roles in vivo, which has thus far only been performed to a limited extent16. It is believed that the described method using HMDM to achieve this purpose may be more clinically relevant in comparison to other immortalized cell line-derived macrophage sources; although, these cell lines may have utility in providing a more stable model for MET characterization with less potential for donor variation.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by a Perpetual IMPACT Grant (IPAP201601422) and Novo Nordisk Foundation Biomedical Project Grant (NNF17OC0028990). YZ also acknowledges the receipt of an Australian Postgraduate Award from the University of Sydney. We would like to thank Mr. Pat Pisansarakit and Ms. Morgan Jones for assistance with the monocyte isolation and tissue culture.
Materials
| 120Q broad spectrum fluorescent light source | EXFO Photonic Solutions, Toronto, Canada | x-cite series | |
| Corning CellBIND Multiple Well Plate (12 wells) | Sigma-Aldrich | CLS3336 | For cell culture |
| Differential Quik Stain Kit (Modified Giemsa) | Polysciences Inc. | 24606 | Characterisation of monocytes |
| Hanks balanced salt solution (HBSS) | Thermo-Fisher | 14025050 | For washing steps and HOCl treatment |
| Hypochlorous acid (HOCl) | Sigma-Aldrich | 320331 | For MET stimulation |
| Interferon gamma | Thermo-Fisher | PMC4031 | For M1 priming |
| Interleukin 4 | Integrated Sciences | rhil-4 | For M2 priming |
| Interleukin 8 | Miltenyl Biotec | 130-093-943 | For MET stimulation |
| L-Glutamine | Sigma-Aldrich | 59202C | Added to culture media |
| Lipopolysaccharide | Integrated Sciences | tlrl-eblps | For M1 priming |
| Lymphoprep | Axis-Shield PoC AS | 1114544 | For isolation of monocytes |
| Olympus IX71 inverted microscope | Olympus, Tokyo, Japan | ||
| Phorbol 12- myristate 13-acetate (PMA) | Sigma-Aldrich | P8139 | For MET stimulation |
| Phosphate buffered saline (PBS) | Sigma-Aldrich | D5652 | For washing steps |
| RPMI-1640 media | Sigma-Aldrich | R8758 | For cell culture |
| SYTOX green | Life Technologies | S7020 | For MET visulaization |
| TH4-200 brightfield light source | Olympus, Tokyo, Japan | x-cite series | |
| Tumor necrosis factor alpha | Lonza | 300-01A-50 | For MET stimulation |
References
- Brinkmann, V., et al. Neutrophil extracellular traps kill bacteria. Science. 303 (5663), 1532-1535 (2004).
- Urban, C. F., et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathogens. 5 (10), 1000639 (2009).
- Brinkmann, V., Zychlinsky, A. Beneficial suicide: why neutrophils die to make NETs. Nature Reviews in Microbiology. 5 (8), 577-582 (2007).
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nature Reviews in Immunology. 18 (2), 134-147 (2018).
- Keshari, R. S., et al. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS One. 7 (10), 48111 (2012).
- Rayner, B. S., et al. Role of hypochlorous acid (HOCl) and other inflammatory mediators in the induction of macrophage extracellular trap formation. Free Radical Biology and Medicine. 129, 25-34 (2018).
- Gunzer, M. Traps and hyperinflammation – new ways that neutrophils promote or hinder survival. British Journal of Haematology. 164 (2), 189-199 (2014).
- Knight, J. S., et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circulation Research. 114 (6), 947-956 (2014).
- Megens, R. T., et al. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thrombosis and Haemostasis. 107 (3), 597-598 (2012).
- Doring, Y., Weber, C., Soehnlein, O. Footprints of neutrophil extracellular traps as predictors of cardiovascular risk. Arteriosclerosis Thrombosis and Vascular Biology. 33 (8), 1735-1736 (2013).
- Goldmann, O., Medina, E. The expanding world of extracellular traps: not only neutrophils but much more. Frontiers in Immunology. 3 (420), 1-10 (2012).
- Boe, D. M., Curtis, B. J., Chen, M. M., Ippolito, J. A., Kovacs, E. J. Extracellular traps and macrophages: new roles for the versatile phagocyte. Journal of Leukocyte Biology. 97 (6), 1023-1035 (2015).
- Pertiwi, K. R., et al. Extracellular traps derived from macrophages, mast cells, eosinophils and neutrophils are generated in a time-dependent manner during atherothrombosis. Journal of Pathology. 247 (4), 505-512 (2019).
- Okubo, K., et al. Macrophage extracellular trap formation promoted by platelet activation is a key mediator of rhabdomyolysis-induced acute kidney injury. Nature Medicine. 24 (2), 232-238 (2018).
- Boeltz, S., et al. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death and Differentiation. 26 (3), 395-408 (2019).
- Doster, R. S., Rogers, L. M., Gaddy, J. A., Aronoff, D. M. Macrophage Extracellular Traps: A Scoping Review. Journal of Innate Immunology. 10 (1), 3-13 (2018).
- Daigneault, M., Preston, J. A., Marriott, H. M., Whyte, M. K., Dockrell, D. H. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 5 (1), 8668 (2010).
- Mestas, J., Hughes, C. C. Of mice and not men: differences between mouse and human immunology. Journal of Immunology. 172 (5), 2731-2738 (2004).
- Brown, B. E., Rashid, I., van Reyk, D. M., Davies, M. J. Glycation of low-density lipoprotein results in the time-dependent accumulation of cholesteryl esters and apolipoprotein B-100 protein in primary human monocyte-derived macrophages. FEBS Journal. 274, 1530-1541 (2007).
- Garner, B., Dean, R. T., Jessup, W. Human macrophage-mediated oxidation of low-density lipoprotein is delayed and independant of superoxide production. Biochemical Journal. 301, 421-428 (1994).
- Morris, J. C. The acid ionization constant of HOCl from 5 °C to 35 °C. Journal of Phyical Chemistry. 70, 3798-3805 (1966).
- Pan, G. J., Rayner, B. S., Zhang, Y., van Reyk, D. M., Hawkins, C. L. A pivotal role for NF-kappaB in the macrophage inflammatory response to the myeloperoxidase oxidant hypothiocyanous acid. Archives of Biochemistry and Biophysics. 642, 23-30 (2018).
- Parker, H., Albrett, A. M., Kettle, A. J., Winterbourn, C. C. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. Journal of Leukocyte Biology. 91 (3), 369-376 (2012).

