The Three-Chamber Choice Behavioral Task using Zebrafish as a Model System
Summary
We present a behavioral chamber designed to assess cognitive performance. We provide data showing that once acquired, zebrafish remember the task 8 weeks later. We also show that hyperglycemic zebrafish have altered cognitive performance, indicating that this paradigm is applicable to studies assessing cognition and memory.
Abstract
Neurodegenerative diseases are age-dependent, debilitating, and incurable. Recent reports have also correlated hyperglycemia with changes in memory and/or cognitive impairment. We have modified and developed a three-chamber choice cognitive task similar to that used with rodents for use with hyperglycemic zebrafish. The testing chamber consists of a centrally located starting chamber and two choice compartments on either side, with a shoal of conspecifics used as the reward. We provide data showing that once acquired, zebrafish remember the task at least 8 weeks later. Our data indicate that zebrafish respond robustly to this reward, and we have identified cognitive deficits in hyperglycemic fish after 4 weeks of treatment. This behavioral assay may also be applicable to other studies related to cognition and memory.
Introduction
Neurodegenerative diseases are age-dependent, debilitating, and incurable. These diseases are increasing in prevalence, resulting in an urgent need to improve upon and develop new therapeutic strategies. The onset and presentation of each disease is unique, as some affect language, motor, and autonomic brain regions, while others cause learning deficits and memory loss1. Most notably, cognitive deficits and/or impairment are the most prevalent complications across all neurodegenerative diseases2. In hopes of shedding light on the underlying mechanisms involved in these neurodegenerative diseases, the use of many different model systems (including single-celled organisms to Drosophila to higher-order vertebrates such as rodents and humans) have been employed; however, the majority of neurodegenerative diseases remain incurable.
Learning and memory are highly conserved processes among organisms as constant changes to the environment require adaptation3. Impairment in both cognition and synaptic plasticity has been demonstrated in several rodent models. Specifically, well-established behavioral assays use associative learning to assess cognitive changes following various impairment-induced diseases and disorders4. Additionally, contrast discrimination reversal assesses cognitive deficits because it involves higher-order learning and memory functions, and reversal depends on inhibition of a previously learned association. The widely used three-chamber choice task elucidates possible deficits in learning and memory pathways of the central nervous system5,6. Recently, this field has expanded to include non-mammalian models, such as zebrafish (Danio rerio), as several paradigms have been developed for a range of ages from larvae to adults7,8.
Zebrafish provide a balance of complexity and simplicity that is advantageous for the assessment of cognitive impairments with behavioral techniques. First, zebrafish are amenable to high-throughput behavioral screening given their small size and prolific reproductive nature. Second, zebrafish possess a structure, the lateral pallium, which is analogous to the mammalian hippocampus as it has similar neuronal markers and cell types7. Zebrafish are also able to acquire and remember spatial information9 and, like humans, are diurnal10. Therefore, it is not surprising that zebrafish are being used as a model for neurodegenerative diseases with increasing frequency. However, the absence of appropriate behavioral assays has made it difficult to apply the zebrafish model for cognitive assessments. Published work using zebrafish-specific behavioral assays include associative learning tasks11, anxiety behavior12, memory13, object recognition14, and conditioned-place-preference15,16,17,18,19. Though there have been many developments with respect to zebrafish behavioral assays, counterparts for some tests of cognitive functions in rodents have yet to be developed for use with zebrafish18.
Building on previous studies from our lab, we modeled/developed a cognitive task in zebrafish based on the three-chamber choice task used with rodents using social interaction as a reward. Additionally, we expanded upon the associative learning aspect of the behavioral task and incorporated contrast discrimination reversal in hopes of further developing this behavioral task to assess cognitive impairment. This enabled us to examine both the initial acquisition of discrimination learning and the subsequent inhibition of that learning in the reversal phase. In the current study, we demonstrate that this procedure provided a reliable method for assessing cognitive functioning in zebrafish following glucose immersion for 4 or 8 weeks.
Protocol
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at American University (protocol # 1606, 19-02).
1. Animals
- Animal rearing and maintenance
- Obtain adult wild-type zebrafish (Danio rerio) aged 4–11 months as embryos and rear them in-house.
- Maintain the fish in an aquatic rack system at 28–29 °C on a 14-h light:10-h dark photoperiod.
- Feed the fish twice per day with commercial flakes and supplement with live Artemia.
- Choose fish randomly from these stock tanks for behavioral experiments.
- Upon experiment completion, anesthetize the animals by immersion in 0.02% tricaine for 2 min or until there is a lack of motor coordination and reduced respiration rate for later molecular and/or neurochemical analyses.
2. Three-chamber choice testing chamber
NOTE: This behavioral technique was modified from Ruhl et al.20.
- Chamber construction
- Modify the behavioral chamber26—a 40 L aquarium (50 x 30 x 30 cm3)— to have a central or starting chamber (10 x 30 x 30 cm3), separated from two side choice chambers (each 20 x 30 x 30 cm3, Figure 1A).
- Construct the three compartments using an aluminum “U-shaped” channel affixed to the interior glass walls with aquarium sealant, to separate the tank into three chambers.
- Construct opaque dividers, 10 cm high, out of grey PVC sheet fit into the aluminum channels on either side. Make each divider out of 2 pieces, each equal in size: a stationary bottom piece permanently mounted within the tank and a moveable top piece that moves up and down in the aluminum tracks.
- Glue extra-large binder clips to the top of the grey PVC sheets to act as handles.
- Using a permanent marker, draw small horizontal lines 10 cm above the adhered grey PVC pipe on the outside of the tank.
NOTE: This mark is the point to which the top grey PVC sheet will be opened to allow access to either side. - Add control (system) water to the tank to a level of 25 cm from bottom to top of the tank, or ~ 30 L. Place glass aquarium heaters into each section of the chamber for 24 h prior to testing to bring the temperature to 28.5 °C.
NOTE: Remove the heaters at the start of the behavior session and perform a full water exchange after two days of use.
- Discrimination setup
- For each discrimination task, individually place colored felt pieces (beige, black, or white) on the outer back, side, and bottom of the choice chambers using Velcro (Figure 1B–D).
NOTE: The central chamber should have no background color associated with it.
- For each discrimination task, individually place colored felt pieces (beige, black, or white) on the outer back, side, and bottom of the choice chambers using Velcro (Figure 1B–D).
- As a reward, create a group of conspecifics (shoal) by placing 4 adult zebrafish, who will not otherwise be used in the study, in a small, clear tank in the far back corner of each choice chamber (Figure 1B–D).
NOTE: Choose shoal fish randomly from stock tanks each day, with at least one male and one female of the same age and size as the experimental fish in each shoal tank.
3. Behavioral tasks
- Acclimation
NOTE: Acclimation to the behavior chamber consists of three days of training; two days of group acclimation followed by one day of individual training. - Group acclimation
- Attach the beige (neutral) felt background to the outside of both choice compartments, and submerge a live shoal tank in each of the choice compartments (Figure 1B).
- Add five-six zebrafish to the center starting chamber with both sliding doors open, and allow the fish to roam freely for 30 min.
NOTE: The experimental zebrafish should be able to interact and socialize with these shoal fish through the tank as a reward after crossing into either choice compartment during acclimation. A fish is considered to have entered one of the side chambers when its entire body enters the chamber. - Repeat this procedure with the same experimental fish for a second day (2 days of group acclimation).
NOTE: Do not keep the same groups of fish.
- Individual acclimation
- Chamber setup: Attach the beige (neutral) felt background to the outside of both choice compartments, and submerge a live shoal tank in both of the choice compartments, as in group acclimation (Figure 1B,E).
- Place an individual zebrafish in the central starting chamber for 2 min with the sliding doors closed, and after the 2-min period, open both doors simultaneously.
- Make sure that each fish swims from the central chamber through a door for a total of 10 times, regardless of which side. Reward the fish each time it enters one of the side chambers (1 day of individual acclimation).
NOTE: If a fish is unable to complete this task 10 times within a 30-min period or refuses to leave the starting chamber at all, exclude it from the study.
- Data acquisition: Record the number of times the fish swims into either side and the total time it takes to complete the task.
- Chamber setup: Attach the beige (neutral) felt background to the outside of both choice compartments, and submerge a live shoal tank in both of the choice compartments, as in group acclimation (Figure 1B,E).
- Acquisition
NOTE: After acclimation, zebrafish began a 3-day acquisition task.- Chamber setup: Attach a white felt piece to the outside of one choice compartment and a black felt piece to the outside of the other choice compartment (Figure 1C,F).
NOTE: Alternate the background color of each side daily using a pseudorandom schedule37.- For the duration of this phase of training, place a shoal-reward only placed in one of the choice compartments; this becomes the rewarded side.
- To begin acquisition, place a single experimental fish in the starting chamber for a 2-min period with the choice compartments closed off.
- After the 2-min acclimation, simultaneously open both doors, giving access to both choice compartments, and start the stopwatch to assess the choice latency.
- Using a biased design, randomly assign the fish either a black or white preference (i.e., either W+/B- or B+/W-), meaning that the shoal is placed in either the black (B+) or white (W+) choice compartment.
- Denoting the choice response
- Once the fish makes a choice by entering one of the side compartments, stop the timer.
- If the fish correctly chooses the preferred side, immediately close the door between the central chamber and that side to restrict the fish to the preferred side for 1 min, and allow it to be rewarded by interacting with the shoal tank (Figure 1C,F). Score this trial as “C” for “Correct” (rewarded).
- If the fish swims through the incorrect door, transfer it back to the central chamber, close both doors, and score the trial as “I” for “incorrect” (non-rewarded).
- If the fish does not make a decision within 2 min after the doors are opened, move the fish to the correct side for 1 min, and score the trial as “M” for “marked” (force-rewarded).
- When transferring/moving fish back into the starting chamber, gently guide the fish into the central chamber using a fish net as a shepherding tool.
NOTE: Do not scoop the fish out of the water and replace it into the starting chamber as this could affect the behavioral assay. - Once the fish returns to the central chamber, wait for 1 min before performing the task again. Ensure that each fish performs the task 8 times.
- Data acquisition
- For each experimental fish, record the time to first decision (or choice latency) and the individual scores (C, I, or M) for each of the 8 acquisition trials (section 3.4.2) in order.
- Report the results for these experiments were reported as group averages for each trial on each acquisition day.
- Once a fish has completed a trial, categorize it as either a “high performing” fish or a “low performing” fish.
NOTE: A fish was considered “high performing” if it successful chose the correct side of the tank in at least 6 of the 8 total trials for the day. Any fish that does not meet this criterion is a “low performer”. - Once identified, house high performing and low performing fish separately.
- Categorize the fish as “high” or “low” performers on each of the three days of acquisition, after a fish has completed the trials.
NOTE: At the end of the third day of acquisition, fish remain as either “high” or “low” performers for the duration of the study.
NOTE: Some fish that were initially in the ‘low performing’ group learn the task on acquisition day 2 or 3. When this happens, the initial ‘low performing’ fish may be moved into the ‘high performing’ group. Do not move the fish between groups in this manner after day 3 (the end of acquisition).
- Chamber setup: Attach a white felt piece to the outside of one choice compartment and a black felt piece to the outside of the other choice compartment (Figure 1C,F).
4. Experimental treatment
- After the acquisition period, when the fish demonstrate the ability to solve a simple discrimination task between the black and white background, start the treatment regimen for the experimental zebrafish.
NOTE: To show the applicability of this method, this study shows two experimental designs:- Longitudinal study
- Return the experimental fish to their holding tanks for 8 weeks. Maintain the fish in standard tanks with daily water changes, and feed them twice daily.
NOTE: Do not conduct any behavioral training during these 8 weeks in the holding tanks. - Perform reversal assessment after this period to assess whether the zebrafish can solve the reversal task after 8 weeks without training.
- Return the experimental fish to their holding tanks for 8 weeks. Maintain the fish in standard tanks with daily water changes, and feed them twice daily.
- Hyperglycemia: Expose the experimental groups to water (handling stress control), mannitol (1%–3%, osmotic control), or glucose (1%–3%) for 4 or 8 weeks22,23.
NOTE: Do not conduct any behavioral training during these 4 or 8 weeks.
- Longitudinal study
5. Reversal
NOTE: Following experimental manipulation (as in section 4.2), the fish are tested in the final part of the 3-chamber choice paradigm—reversal. To do this, the rewarded side is reversed (compared to acquisition) such that fish previously rewarded with a shoal on the white side are now rewarded with a shoal on the black side and vice versa. In this way, reversal assesses whether the fish have learned where the reward (shoal) is located, irrespective of the color of the background.
- Chamber setup
- Attach black felt to the outside of one of the choice chambers and white felt to the outside of the other, making sure that the black and white sides are the same sides as in the acquisition trials (section 3.4).
- Submerge the shoal tank in the far back corner of the side that is the opposite of the previously rewarded choice chamber (Figure 1D,G).
NOTE: In other words, fish previously rewarded on the white side are now rewarded on the black side and vice versa. - Test the fish individually as in section 3.5. Begin by placing a single experimental fish in the starting chamber for a 2-min period, and close off access to the choice compartments.
- Simultaneously open both sides of the chamber.
NOTE: Complete a total of 8 trials each day for three consecutive treatment days.
- Denoting the choice response
- If the fish correctly chooses the preferred color, immediately close the door to the central chamber for 1 min, allowing the fish to interact with the shoal reward. Score this trial as “C” for “Correct” (rewarded).
- If the fish swims through the incorrect door, transfer it back into the central chamber, close both doors, and score this trial as “I” for “incorrect” (non-rewarded).
- If the fish does not make a decision within 2 min after the doors are opened, move the fish to the correct side, and score the trial as “M” for “marked” (force-rewarded).
- Data acquisition
- For each experimental zebrafish, record the choice latency and the individual scores (C, I, M), in order, for each trial.
- Report the results for these experiments as group averages for each two-trial block on each of the 3 reversal days.
- Keep the data for high and low performing fish separate to determine whether they display the same level of performance during reversal as they did during acquisition.
Representative Results
Acclimation to the behavior chamber involves three days of training: 2 days of group acclimation followed by 1 day of individual acclimation. However, because we could not distinguish individual zebrafish from one another, we were only able to collect data during individual acclimation. At this time, experimental animals (n = 30), conditioned using a shoal-based reward, took an average of 125.11 s to reach their first decision (Figure 2A) and an average of 725.34 s (12 min) to complete the entire individual acclimation task (Figure 2B). There was no significant side preference during acclimation (Figure 2C). The number of excluded fish was minimal as compared to other reward types (food) we had previously assessed in our laboratory (Figure 2C).
After acclimation, zebrafish began the acquisition phase. As fish were tested individually, we collected data from each fish on each of the three acquisition days. The fish were classified as either ‘high or low performers’, with ‘high performers’ responding faster and more accurately, despite all fish having the same previous exposure to the testing chamber. Only fish that selected the rewarded choice compartment in at least 6 of the 8 trials were classified as ‘high performers.’ Fish that did not meet this criterion were ‘low performers.’ High and low performing fish were housed separately to distinguish their performance in all subsequent trials. Interestingly, we observed that some fish changed category (i.e., were initially ‘low performers’, but became ‘high performers’) during the course of acquisition. In fact, the number of high performing animals increased each day, with more high performing fish on acquisition day 3 compared to day 1 (Figure 3A). By day 3, >50% of the fish had become ‘high performers.’ Further, initial choice latency for all fish across the three acquisition days (A1–A3) decreased, indicating an improved performance with each day of acquisition (Figure 3B). The same trend was also seen when only the high performing fish group was considered: by day 3, the time to first decision improved (became faster) (Figure 3C).
A discrimination ratio (rewarded trials/(reward + nonrewarded trials) was calculated for each acquisition trial block (average of two trials/fish) across the three acquisition days (A1–A3) for all experimental animals (n = 30) to determine how accurately the fish were solving (acquiring) the discrimination task (i.e., going to the rewarded side of the tank). This ratio revealed that the percentage of fish moving to the rewarded side during each trial increased daily (i.e., across trial blocks on each individual day) and overall (i.e., across the three acquisition days) resulting in all fish performing above chance by the end of acquisition (dotted line represented on the graph; (Figure 4A) and indicating that the fish had learned the discrimination task.
Following the acquisition of discrimination learning, we tested how long zebrafish would remember the task. To do that, tested zebrafish remained in holding tanks for 8 weeks. After this time, fish were tested on a reversal task that lasted 3 days (R1–R3). We found that the fish demonstrated strong reversal behavior and increased discrimination over the three days of reversal (Figure 4B), indicating they were able to (1) remember the relationship between the color of the tank and the reward and (2) inhibit what they had previously learned during acquisition and learn the reversal/opposite paradigm. As shown in Figure 4B, zebrafish initially went to the non-rewarded side of the tank, as indicated by the discrimination ratio being below chance during initial trails on reversal day 1. However, by the end of R1, performance increased to greater than chance, a result that was maintained on R2 and R3, with the highest discrimination ratio scores observed on R3. Taken together, these data show that naïve experimental animals are capable of solving the discrimination task, even though the initial behavior was acquired 8 weeks earlier, without any additional training in between behavioral sessions.
The 3-chamber choice paradigm can also be applied to examination of disease complications. In our study with hyperglycemic zebrafish, acclimation and acquisition were as described, and reversal was tested following either 4 or 8 weeks of hyperglycemia. Hyperglycemia was induced with an alternate immersion protocol (McCarthy et al., 2020 – this issue), so that training occurred every other day, on days after zebrafish had been in test solutions for 24 h. During acquisition, there was a main effect of training day on discrimination ratio (F (2, 239) = 4.457, p = 0.012; Figure 5A), with the ratio on A1 being significantly lower than on A3 (p = 0.010), indicating that the fish improved their choice accuracy over time. During reversal, there was a significant main effect of treatment (F (2, 326) = 3.057, p = 0.048), but no other significant main effects or interactions (training day: (F (2, 326) = 1.602, p = 0.203); training day x treatment: (F (4, 326) = 0.661, p = 0.620); Figure 5A). The response of glucose-treated animals was significantly reduced compared to the water-treated animals (p = 0.037), but there were no other significant differences (control v. mannitol: p = 0.387; mannitol v. glucose: p = 0.524), suggesting a glucose-specific effect. After 8 weeks of hyperglycemia, no statistical differences were noted in discrimination ratios across acquisition training (F (2,263) = 2.909, p = 0.056; Figure 5B). However, there were significant main effects of both training day (F (2, 189) = 4.721, p = 0.010) and treatment (F (2, 189) = 7.940, p = 0.000) on reversal, but no significant interaction (training day * treatment = F (4, 189) = 0.869, p = 0.484). Subsequent least significant difference (LSD) pairwise comparisons identified significant differences between R1 and R3 (p = 0.022) and between R2 and R3 (p = 0.003). LSD pairwise comparisons also revealed significant differences between the water treatment group and both glucose and mannitol treatment groups (water v. mannitol: p = 0.008; water v. glucose: 0.000); however, the glucose and mannitol groups were not significantly different from one another (p = 0.265), suggesting that these differences in discrimination ratio may be due to osmotic effects.
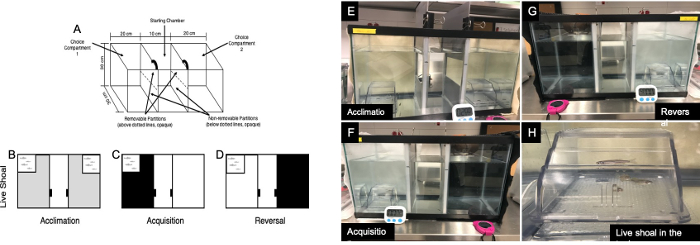
Figure 1: Three-chamber choice testing chamber and behavior setup. (A) 3-chamber schematic. Experimental animals were restricted to the central starting chamber for 2 min and then allowed access to either side of the tank at the start of a trial. To do so, the top half of each of the two partitions were raised to create a 10 cm space for fish to cross into either choice compartment. (B,E) Acclimation was performed using a beige background and shoal of conspecifics as the reward. (C,F) Acquisition was performed using black and white backgrounds on the choice chambers; reward was only located on one side of the chamber. (G) Reversal was performed using black and white backgrounds on the choice compartments; reward was only available on the opposite side of the chamber (vs. acquisition). (H) Up-close image of shoal tank submerged in one of the choice compartments. Please click here to view a larger version of this figure.
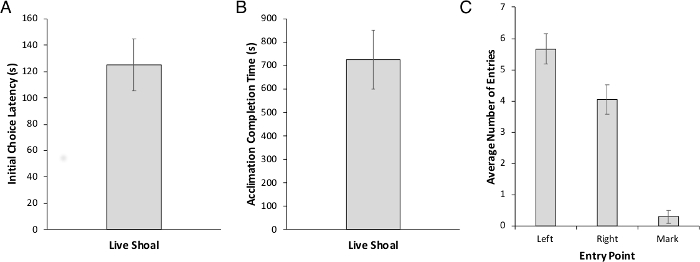
Figure 2: Latency and number of marked trials during individual acclimation. (A) Choice latency of first decision. (B) The total amount of time to complete individual acclimation. (C) The number of entries to the left and right side are not different, indicating no inherent side preference prior to the start of acquisition. We also report the total number of mark trials during individual acclimation. Values are reported as Mean ± SEM. Please click here to view a larger version of this figure.
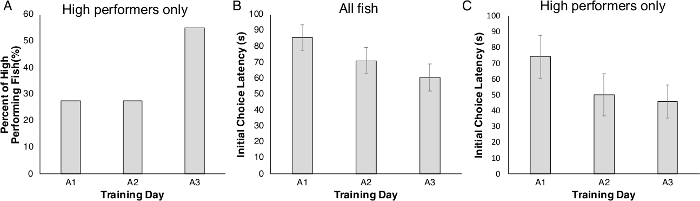
Figure 3: The percentage of high performing fish and the initial choice latency among all fish and high performing fish across the three acquisition days. (A) High performing fish moved from the central chamber to the rewarded side of the chamber in at least 6 out of 8 trials each acquisition day (A1–A3). (B) Across three days of acquisition training (A1–A3), overall initial choice latency decreased; a trend also evident in high performing fish (C). Values are reported as Mean ± SEM. Please click here to view a larger version of this figure.
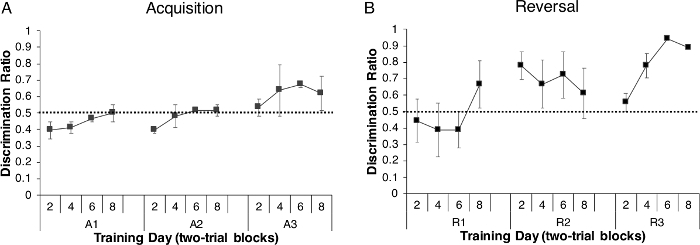
Figure 4: Discrimination performance during acquisition and reversal trials. (A) Discrimination ratio (rewarded trials/(reward + nonrewarded trials) of fish across acquisition days (A1–A3) and (B) during reversal learning 8 weeks later. Reversal was also assessed for 3 days (R1–R3). For both tasks, each fish had to complete 8 trials, and results are presented in two-trial blocks (2, 4, 6, 8). Correct responses during both acquisition and reversal increased with time, with a faster response observed during reversal, indicating the fish learned and remembered the task. Values are reported as Mean ± SEM. The dotted line represents chance. Please click here to view a larger version of this figure.
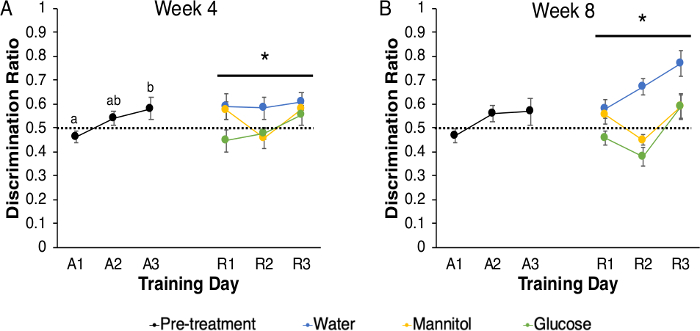
Figure 5: Acquisition and Reversal of hyperglycemic zebrafish using the 3-chamber choice behavioral task. (A) Prior to treatment, naïve zebrafish acquired the 3-chamber choice behavioral task across three days of behavioral training (acquisition, A1–A3). There was a significant difference between discrimination ratios on A1 and A3 indicating learning took place (p = 0.012). Following 4 weeks of treatment (colored symbols) there was a significant effect of treatment (p = 0.048), with glucose-treated animals displaying significantly reduced discrimination ratios compared to water-treated animals (p = 0.037). (B) In a separate experiment, behavior before and after 8 weeks of hyperglycemia was assessed. Despite the steady increase in performance across each acquisition day, there were no significant differences discrimination ratio across A1–A3. However, after 8 weeks of treatment (colored symbols), there was a main effect of treatment (p < 0.001) and an individual main effect of training day (p = 0.010). Post-hoc analyses revealed a significant difference between the water-treated group and both mannitol- and glucose-treated groups, suggesting an osmotic effect (water v. mannitol: p = 0.008; water v. glucose: p < 0.001). * denotes a significant main effect. Data points represent group mean ± SEM, and data points with different letters are significantly different from one another. The dotted line represents chance. Please click here to view a larger version of this figure.
Discussion
Although there has been tremendous growth in the amount and variety of neuroscience research performed using zebrafish in the past 15 years24, behavioral assays are lacking in this species compared to mammalian model systems11,25,26. Here, we show that a three-chamber choice task developed for use with rodents can be adapted to assess the acquisition and reversal of a visual discrimination learning in zebrafish. Using a live shoal as the reward, this task provided a robust assay that can be applied to a variety of studies examining behaviorally linked diseases such as hyperglycemic complications of diabetes, Alzheimer’s, and dementia.
It has been previously established that zebrafish are capable of learning and storing information as it is required to make ecologically relevant decisions and necessary for survival in the wild3. Our acquisition and reversal data across an 8-week longitudinal study supports prior evidence that zebrafish, while small, are able to learn and remember a simple discrimination task, and that zebrafish can also inhibit previously acquired responses. In the longitudinal 8-week study, the number of forced rewards decreased, and the discrimination ratio increased, indicating that the fish get better at choosing the correct, rewarded side and have learned the task. While these changes were non-significant, we did see an overall downward trend in the number of force-rewarded trials during acquisition and an increase in discrimination ratio. Further, the results of the three-chamber choice behavioral task with hyperglycemic fish revealed the applicability of the test to studies examining hyperglycemic conditions and indicates that this paradigm could be used in combination with other experimental manipulations, such as drug exposures or mutant lines, to assess potential effects on cognition.
An important limitation of this study is that we cannot identify individual fish over time and therefore must rely on group averages to assess the data. Developing a way to individually track fish in the different treatment groups, as in rodents, could address these issues. In an attempt to resolve these differences, we sorted the fish during the acquisition phase based on their performance, which turned out to be an unexpected benefit of our methodology. ‘High performing fish’ scored ≥ 6/8 on each day of training, whereas fish with lower scores were ‘low performing fish’. When counted each day, the number of ‘high performers’ increased in the shoal rewarded group so that by day 3, there were significantly more fish in this category compared to the fish-rewarded treatment. Choice latency trends observed across all (‘high + low performers’) fish are similar to those observed only in the ‘high performing’ group, suggesting that the strong responses of this group were driving overall responses.
In summary, these findings indicate that shoal-based discrimination learning in zebrafish provide a feasible cost-effective model for the study of normal and impaired cognitive functioning.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We thank Sabrina Jones for her assistance adapting a rodent three-chamber choice paradigm to the zebrafish model and Jeremy Popowitz and Allison Murk for their help on behavior collection days, assistance with running trials, animal care, and tank set-up. Special thanks also to James M. Forbes (Mechanical Engineer) for his assistance with the 3-chamber choice tank design and construction.
Funding: VPC and TLD received a joint Faculty Research Support grant (FRSG) from American University College of Arts and Sciences. CJR received support from American University College of Arts and Sciences Graduate Student Support.
Materials
| Champion Sports Stopwatch Timer Set: Waterproof, Handheld Digital Clock Sport Stopwatches with Large Display for Kids or Coach – Bright Colored 6 Pack | Amazon | N/A | https://www.amazon.com/Champion-Sports-910SET-Stopwatch-Timer/dp/B001CD9LJK/ref=sr_1_17?dchild=1&keywords=stopwatch+for+sports&qid=1597081570&sr=8-17 Recommend two of different colors; one for choice latency and one for time to completion |
| Coofficer Extra Large Binder Clips 2-Inch (24 Pack), Big Paper Clamps for Office Supplies, Black | Amazon | N/A | https://www.amazon.com/Coofficer-Binder-2-Inch-Clamps-Supplies/dp/B07C94YCR5/ref=sr_1_3_sspa?dchild=1&keywords=large+binder+clips&qid=1597081521&sr=8-3-spons&psc=1&spLa=ZW5jcnlwdGVkUXVhbGlmaWVyPUExUENWUTRZVjlIWEVPJmVuY3J5cHRlZElkPUEwNDQ5NDU0MlpSREkwTFlLSThVQiZlbmNyeXB0ZWRBZElkPUEwMTg5NDI3MllRV1EzOUdWTVpSOCZ3aWRnZXROYW1lPXNwX2F0ZiZhY3Rpb249Y2xpY2tSZWRpcmVjdCZkb05vdExvZ0NsaWNrPXRydWU= |
| Marineland® Silicone Aquarium Sealant | Petsmart | Item #2431002 | |
| PVC (Polyvinyl Chloride) Sheet, Opaque Gray, Standard Tolerance, UL 94/ASTM D1784, 0.125" Thickness, 12" Width, 24" Length | Amazon | N/A | https://www.amazon.com/Polyvinyl-Chloride-Standard-Tolerance-Thickness/dp/B000MAMGEQ/ref=sr_1_2?dchild=1&keywords=grey+PVC+sheet&qid=1597081440&sr=8-2 |
| Steelworks 1/4-in W x 8-ft L Mill Finished Aluminum Weldable Trim U-shaped Channel | Lowes | Item #55979Model #11377 | https://www.lowes.com/pd/Steelworks-1-4-in-W-x-8-ft-L-Mill-Finished-Aluminum-Weldable-Trim-Channel/3058181 |
| Tetra 10 Gallon Fish tank | Petsmart | Item #5271256 | |
| Top Fin Fine Mesh Fish Net (3 in) | Petsmart | Item #5175115 |
Referencias
- Gitler, A. D., Dhillon, P., Shorter, J. Neurodegenerative disease: models, mechanisms, and a new hope. Disease Models & Mechanisms. 10, 499-502 (2017).
- Perry, R. J., Watson, P., Hodges, J. R. The nature and staging of attention dysfunction in early (minimal and mild) Alzheimer’s disease: relationship to episodic and semantic memory impairment. Neuropsychologia. 38, 252-271 (2000).
- Gerlai, R. Learning and memory in zebrafish (Danio rerio). Methods in Cell Biology. 134, (2016).
- Davidson, T. L., et al. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiology and Behavior. 107, 26-33 (2012).
- Yang, M., Silverman, J. L., Crawley, J. N. Automated three-chambered social approach task for mice. Current Protocols in Neuroscience. 56 (1), (2011).
- Remmelink, E., Smit, A. B., Verhage, M., Loos, M. Measuring discrimination- and reversal learning in mouse models within 4 days and without prior food deprivation. Learning and Memory. 23, 660-667 (2016).
- Salas, C., et al. Neuropsychology of learning and memory in teleost fish. Zebrafish. 3, 157-171 (2006).
- Kalueff, A. V., et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish. 10, 70-86 (2013).
- Luchiaria, A. C., Salajanb, D. C., Gerlai, R. Acute and chronic alcohol administration: Effects on performance of zebrafish in a latent learning task. Behavior Brain Research. 282, 76-83 (2015).
- Fadool, J., Dowling, J. Zebrafish: A model system for the study of eye genetics. Progress in Retinal and Eye Research. 27, 89-110 (2008).
- Fernandes, Y. M., Rampersad, M., Luchiari, A. C., Gerlai, R. Associative learning in the multichamber tank: A new learning paradigm for zebrafish. Behavioural Brain Research. 312, 279-284 (2016).
- Reider, M., Connaughton, V. P. Developmental exposure to methimazole increases anxiety behavior in zebrafish. Behavioral Neuroscience. , (2015).
- Capiotti, K. M., et al. Hyperglycemia induces memory impairment linked to increased acetylcholinesterase activity in zebrafish (Danio rerio). Behavioural Brain Research. 274, 319-325 (2014).
- May, Z., et al. Object recognition memory in zebrafish. Behavioural Brain Research. 296, 199-210 (2016).
- Mathur, P., Lau, B., Guo, S. Conditioned place preference behavior in zebrafish. Nature Protocols. 6, 338-345 (2011).
- Guo, S. Linking genes to brain, behavior and neurological diseases: What can we learn from zebrafish. Genes, Brain and Behavior. 3, 63-74 (2004).
- Kily, L. J. M., et al. Gene expression changes in a zebrafish model of drug dependency suggest conservation of neuro-adaptation pathways. Journal of Experimental Biology. 211, 1623-1634 (2008).
- Webb, K. J., et al. Zebrafish reward mutants reveal novel transcripts mediating the behavioral effects of amphetamine. Genome Biology. 10, (2009).
- Clayman, C. L., Malloy, E. J., Kearns, D. N., Connaughton, V. P. Differential behavioral effects of ethanol pre-exposure in male and female zebrafish (Danio rerio). Behavioural Brain Research. 335, 174-184 (2017).
- Ruhl, T., et al. Acute administration of THC impairs spatial but not associative memory function in zebrafish. Psychopharmacology. 231, 3829-3842 (2014).
- Gellermann, L. W. Chance orders of alternating stimuli in visual discrimination experiments. The Pedagogical Seminary and Journal of Genetic Psychology. 42, 206-208 (1933).
- Gleeson, M., Connaughton, V., Arneson, L. S. Induction of hyperglycaemia in zebrafish (Danio rerio) leads to morphological changes in the retina. Acta Diabetologica. 44, 157-163 (2007).
- Connaughton, V. P., Baker, C., Fonde, L., Gerardi, E., Slack, C. Alternate immersion in an external glucose solution differentially affects blood sugar values in older versus younger zebrafish adults. Zebrafish. 13, 87-94 (2016).
- Goldsmith, J. R., Jobin, C. Think small: Zebrafish as a model system of human pathology. Journal of Biomedicine and Biotechnology. 2012, 817341 (2012).
- Kalueff, A. V., Stewart, A. M., Gerlai, R., Court, P. Zebrafish as an emerging model for studying complex brain disorders. Trends in Pharmacological Sciences. 35, 63-75 (2014).
- Gerlai, R. Associative learning in zebrafish (Danio rerio). Methods in cell biology. 101, 249-270 (2011).

