A System for Tracking the Dynamics of Social Preference Behavior in Small Rodents
Summary
Described here is a novel automated experimental system that offers an alternative to the three-chamber test and also solves several caveats. This system supplies multiple behavioral parameters that enable rigorous analysis of small rodent behavioral dynamics during the social preference and social novelty preference tests.
Abstract
Exploring the neurobiological mechanisms of social behavior requires behavioral tests that can be applied to animal models in an unbiased and observer-independent manner. Since the beginning of the millennium, the three-chamber test has been widely used as a standard paradigm to evaluate sociability (social preference) and social novelty preference in small rodents. However, this test suffers from multiple limitations, including its dependence on spatial navigation and negligence of behavioral dynamics. Presented and validated here is a novel experimental system that offers an alternative to the three-chamber test, while also solving some of its caveats. The system requires a simple and affordable experimental apparatus and publicly available open-source analysis system, which automatically measures and analyzes multiple behavioral parameters at individual and population levels. It allows detailed analysis of the behavioral dynamics of small rodents during any social discrimination test. We demonstrate the efficiency of the system in analyzing the dynamics of social behavior during the social preference and social novelty preference tests as performed by adult male mice and rats. Moreover, we validate the ability of the system to reveal modified dynamics of social behavior in rodents following manipulations such as whisker trimming. Thus, the system allows for rigorous investigation of social behavior and dynamics in small rodent models and supports more accurate comparisons between strains, conditions, and treatments.
Introduction
Revealing the biological mechanisms underlying neurodevelopmental disorders (NDDs) is one of the main challenges in the field of neuroscience1. Addressing this challenge requires behavioral paradigms and experimental systems that typify the behavior of rodents in a standard and unbiased manner. An influential study published more than a decade ago by Moy and colleagues2 presented the three-chamber test. Since then, this test has been widely used to investigate social behavior in rodent models of NDDs. This test evaluates two innate tendencies of rodents: 1) to stay in the proximity of a social stimulus over an object (sociability, also termed social preference [SP]), and 2) to prefer the proximity of a novel social stimulus over a familiar one (social novelty preference [SNP])3,4. Several subsequent studies suggested methods of automated analysis of the three-chamber test using computerized methods5,6.
This test still suffers from several caveats. First, it principally examines social place preference rather than the motivation of the subject to directly interact with a social stimulus, although some groups also measure olfactory investigation (sniffing) time, either manually7 or using commercial computerized systems8,9,10. Second, the three-chamber test is mostly used to measure the total time spent by the subject in each chamber, and it neglects behavioral dynamics. Finally, it relies on only one aspect of the social behavior, which is the time spent by the subject in each chamber (or sniffing time, if measured).
Here we present a novel and affordable experimental system that is an alternative to the three-chamber apparatus. It also allows performance of the same behavioral tests while solving the abovementioned caveats. The presented behavioral system automatically and directly measures the investigative behavior of a rodent towards two stimuli. Additionally, it analyzes the behavioral dynamics in an observer-independent manner. Moreover, this system measures multiple behavioral parameters and analyzes these at both individual and population levels; thus, it supports a rigorous analysis of social behavior and its dynamics during each test. Furthermore, random repositioning of the chambers in opposite corners of the arena during the various test stages neutralizes any effects of spatial memory or preference. This system may also be used for other discrimination tests, such as sex discrimination. The custom apparatus is easy to produce, and the analysis system is publicly accessible as an open-source code, thereby allowing its use in any laboratory. We demonstrate the ability of this system to measure multiple parameters of social behavior in rodent strains with distinct fur colors during the social preference and social novelty preference tests. We also validate the ability of the system to reveal modified dynamics of social behavior in rodents following manipulations, such as whisker trimming.
TrackRodent software: three algorithms were written in MATLAB (2014a-2019a) to track the experimental subject and its interactions with the stimuli. All algorithms were deposited in GitHub, found at <https://github.com/shainetser/TrackRodent>. The main aim of all four algorithms is to track the contours of the subject's body to detect any direct contact with the stimuli areas.
Body-based algorithm: this algorithm has three versions that track the contours of an unwired dark mouse on a white background (BlackMouseBodyBased), a white mouse on a dark background (WhiteMouseBodyBased), or a white rat on a dark background (WhiteRatBodyBased). The graphical user interface (GUI) of the software requires that the experimenter chooses an experiment using either mice or rats and then selects the correct code. For each version of the algorithm, there are two optional codes: one that presents the tracking process on the screen while it performs the analysis, and one that does not (hence, it runs faster and is termed "fast"). For example, the names of the relevant codes for the BlackMouseBodyBased algorithm are: "BlackMouseBodyBased23_7_14" and "BlackMouseBodyBased23_7_14_Fast". All algorithms ending with "fast" do not show the tracking online, and users must directly save the data to the results file (.mat file). All body-based algorithms require setting a single threshold ("low threshold" in the software's GUI) to detect the body of the subject.
Head-directionality based algorithm: the second algorithm, which is available only for black mice, is based on the body-based algorithm, in addition to determining head directionality. This algorithm detects the interactions of subject's head with the "stimuli" areas, thus avoiding false positives that can arise from random contacts of the subject' with these areas. For this algorithm, two detection thresholds of mouse body contours are defined: high threshold, which includes the brighter tail of black mice, and low threshold, which includes the body without tail. Thereafter, the algorithm fits an ellipsoid to the detected boundaries using the lower threshold and defines the location of the mouse head and tail (with no distinction between the two). The final discrimination between the tail and head is based on the boundaries defined by the higher threshold.
Wired animal algorithm: the third algorithm aims to minimize artifacts resulting from cables (i.e., electrical wire or optical fiber) connected to the animal, allowing analysis of the animal's behavior while connected to a cable. This algorithm has codes only for black mice and white rats. The code for rats requires the experimenter to define both low and high thresholds, while the mouse code requires only a low threshold.
Protocol
All methods described have been approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Haifa.
1. Experimental Set-up
- Arena
- Construct the experimental arena for mice (Figure 1A,D) by placing a white or black (depending on the animal's color) plexiglass box (37 cm x 22 cm x 35 cm) in the middle of an acoustic chamber (60 cm x 65 cm x 80 cm, made of 2 cm thick wood coated on the inside with 2 cm thick foam). For the light, remove a strip (2 cm wide, 10 cm below the ceiling of the chamber) of the foam around the acoustic chamber (besides the door), and attach an LED strip with either red or white light bulbs. Make sure the light is as uniform as possible around the arena to prevent reflections.
- Construct the arena for rats similarly to the one described above for mice, with different dimensions that appropriately fit the size of Sprague-Dawley (SD) rats (Figure 1G). Place a black plexiglass box (50 cm x 50 cm x 40 cm) in the middle of an acoustic chamber (90 cm x 60 cm x 85 cm, made of 2 cm thick wood coated on the inside with 2 cm thick foam).
- Chambers
- For mice, create two black or white (depending on the fur color) triangular chambers (12 cm isosceles, 35 cm height, with the floors closed) from 6 mm thick plexiglass. Locate them in two randomly selected opposite corners of the arena (Figure 1B,E). Stick a metal mesh (18 mm x 6 cm; 1 cm x 1 cm holes) in the lower part of each chamber using epoxy glue to allow direct interactions with the stimulus through the mesh (Figure 1C,F). Mark each chamber in a manner that allows discrimination from the others in a video without giving clues to the subjects (see Figure 1B,E for examples).
NOTE: Each chamber will eventually contain a social stimulus (mouse) or object (plastic toy, 5 cm x 5 cm x 5 cm, with a distinct shape and color; Figure 1C,I insets). Let the epoxy glue smell evaporate for at least a week before usage. - For rats, create two black triangular chambers (20.5 cm isosceles, 40 cm height, made of 6 mm thick plexiglass, with the floors closed) and place them in two randomly selected opposite corners of the arena (Figure 1H), each with a metal mesh (25 cm x 7 cm; 2.5 cm x 1 cm holes) covering its lower portion (Figure 1I).
- For mice, create two black or white (depending on the fur color) triangular chambers (12 cm isosceles, 35 cm height, with the floors closed) from 6 mm thick plexiglass. Locate them in two randomly selected opposite corners of the arena (Figure 1B,E). Stick a metal mesh (18 mm x 6 cm; 1 cm x 1 cm holes) in the lower part of each chamber using epoxy glue to allow direct interactions with the stimulus through the mesh (Figure 1C,F). Mark each chamber in a manner that allows discrimination from the others in a video without giving clues to the subjects (see Figure 1B,E for examples).
- Place a high quality monochromatic camera, equipped with a wide-angle lens, at the top of the acoustic chamber and connect it to a computer to enable clear viewing and recording of the subject's behavior using commercial software (see Table of Materials for suggestions).
2. Behavioral Paradigm
NOTE: Steps 2.1-2.7 describe the behavioral paradigm for mice. See section 2.8 for specific instructions involving rats.
- Make sure that the cages of all animals (subjects: 2-4 month-old male mice; stimuli: 21-30 day-old juvenile mice) remain in the experimental room for at least 1 h before beginning the behavioral experiment.
- Following the acclimation period, insert two empty chambers into the arena randomly at two opposite corners. Place the subject in the middle of the arena for 15 min of habituation. During that time, place the two social stimuli, each in a different chamber located out of the arena for habituation. Place an object (a plastic toy, 5 cm x 5 cm x 5 cm, with a distinct shape and color) in another chamber.
- To perform the social preference (SP) test, start video recording and keep recording until the end of the test.
- Remove the two empty chambers and immediately insert the object and one of the social stimuli, each in a distinct chamber. Locate these chambers randomly in the opposite corners of the arena that were empty during habituation. Allow the subject to interact with the stimuli for the 5 min of the SP test. At the end of the test, stop recording.
- Following the SP test, remove the stimuli-containing chambers from the arena and leave the subject in the empty arena for 15 min. Clean the chambers from outside with 10% ethanol wipes.
- To perform the social novelty preference (SNP) test, start video recording and insert two chambers into the arena: one containing the same social stimulus used for the SP test (familiar stimulus), and the other containing the novel social stimulus. Place these chambers randomly in two opposite corners of the arena, making sure these locations were not used for the SP test. Allow the subject to interact with the stimuli for the 5 min of the SNP test.
- At the end of the SNP test, stop video recording, remove the subject and chambers from the arena, and place the subject back in its home cage. Leave the stimuli in the chambers for the next experiment (with another subject) or return them to their home cages. Clean the arena and chambers with running water followed by 10% ethanol and let dry.
- Behavioral paradigm for rats
- For rats, repeat the behavioral paradigm described in steps 2.1-2.7, with two modifications: 1) handle the rat subjects and habituate the social stimuli to the chambers for 2 days (10 min every day) prior to the experiment; and 2) extend the SP test for 15 min to give the rats a longer period of exposure to social stimuli. Later, restrict the analysis of the SP test to the initial 5 min.
NOTE: At least one arena and five chambers are needed in order to run a single session.
- For rats, repeat the behavioral paradigm described in steps 2.1-2.7, with two modifications: 1) handle the rat subjects and habituate the social stimuli to the chambers for 2 days (10 min every day) prior to the experiment; and 2) extend the SP test for 15 min to give the rats a longer period of exposure to social stimuli. Later, restrict the analysis of the SP test to the initial 5 min.
3. Using the TrackRodent GUI for Behavioral Analysis
NOTE: See the upper panel of Figure 2A for the TrackRodent GUI.
- Open MATLAB (tested with 2014a-2019a) and choose the TrackRodent folder.
- Add all subfolders to the working path by right-clicking on each folder and selecting Add to Path | Selected Folders and Subfolders.
- Type TrackRodent in the command window and press Enter.
- Upload a single file or multiple video files (AVI or MP4 format) by selecting Load session file (AVI).
- A movie inspector, allowing the inspection of the video clip frame-by-frame, will immediately be opened for the first file in the list (Figure 2A). Use it to examine the video clip and define the first and last frames of the segment to be analyzed. Record the numbers of these frames, which will be required later. Close the window when done.
- For inspecting additional video files, open the video inspector at any time by pressing Inspect movie and selecting a specific video file.
- Select the species tested (mouse or rat; mouse is the default).
- Exclude all the areas that may interrupt the tracking, in accordance with the colors of the subject and arena (black or white).
- To exclude a given area, press Exclude area, and after the cursor changes to a cross shape, mark all corners of the area for exclusion. When done, right-click on the mouse, then double left-click the center of the marked area. The excluded area will become a shade of red on the screen. Repeat this procedure to exclude as many areas as needed.
- To remove an area from exclusion, press Remove excluded area, then (using the crossed cursor) click on the area to be removed from exclusion.
- To define each chamber as a 'stimulus' area, for automatic detection of its investigation by the subject, choose the shape of the 'stimulus' area to be either polygon or elliptical by checking the appropriate box, then pressing Stimulus X (where "X" represents 1, 2, or 3). Mark the 'stimulus' areas similarly to the excluded areas, which will then become yellow in color. For changing the location of a specific 'stimulus' area, press Stimulus X again and mark the new area location (this will automatically update the location).
NOTE: Choose the different stimuli number in a consistent manner for all files (i.e. object as stimulus 1 for all the SP test files). - To track the presence of the subject in a specific virtual compartment inside the arena, choose the shape of the 'compartment' area (polygon or elliptical), then press Compartment X (where "X" represents 1, 2, 3, 4, or 5). Mark the 'compartment' areas similarly to the excluded or stimuli areas, which will then become blue in color. For changing the location of a specific 'compartment' area, press Compartment X again and mark the new area location (this will automatically update the location).
- Choose the desired algorithm (BlackMouseBodyBased was used for the video) from the list (see available algorithms in Figure 2B).
- Write the numbers of starting and ending frames for the analysis in the corresponding edit boxes of the GUI.
- Choose a threshold for detecting the subject body.
NOTE: Most algorithms use the "Low" threshold only, while the head directionality-based algorithms use the "High" threshold, as well. For the "Low" threshold, choose a level that includes the mouse/rat body without the tail (as much as possible), while the "High" threshold should also include the tail. In the case of using head directionality-based algorithms, the software will determine the head location as being opposite to the tail location.
NOTE: The software will later on ignores small objects detected using the chosen threshold. - To evaluate automatic detection of the subject borders for a given threshold, insert a value to the relevant threshold field and press Enter on the keyboard.
- When choosing multiple files, move to the next file (using the Next button at the top) and select the appropriate definitions for each file. When finished, verify the parameters and area locations for all files by moving between each, using the Previous and Next buttons at the top of the GUI.
NOTE: The definitions of all areas and parameters are specific to a given file. - For starting behavioral analysis of all selected files, move to the first file and press Start.
- At the end of the analysis, a results file (.mat file) is saved for each movie in the same directory of the movie files.
NOTE: If the slow (non-fast) version of the algorithm is used, it will also save a version of the movie with a white cross of the center of body mass, which changes its color every frame that is detected as investigatory, unless the Save analyzed movie toggle button of the GUI is unchecked. This version of the movie (saved in the same directory, with the same name as the original movie, with the suffix 'analyzed movie') can be used offline to evaluate the quality of the automatic detection performed by the system.
4. Using the TrackRodent GUI for Results Presentation
NOTE: See the lower panel of Figure 2A for results presentation.
- To inspect the results of each movie file, press Load results file and choose the .mat files generated by the behavioral analysis.
- Move between the toggle buttons to examine onscreen any of the following analyses: Mouse location trace (Figure 2A); Compartments occupation along session (if 'compartments' were defined, not shown); Stimuli exploration along session (Figure 2C); Total time in compartments (if 'compartments' were defined, not shown); and Total stimuli exploration time (Figure 2D).
NOTE: 'Stimulus' areas are areas in which the software evaluates subject interaction, while 'Compartment' areas are areas in which the software tracks the presence of the subject. Stopping the analysis using the Stop analysis button will automatically save the results generated up to the last analyzed frame. For most computers, it should be possible to upload and analyze as many as 20 movies at once (depending on computer performance).
5. Using the TrackRodentPopulationSummary GUI for Population Analysis (Figure 2E)
- Open MATLAB (tested with 2014a-2019a) and choose the TrackRodent folder.
- Tipo TrackRodentPopulationSummary in the command window and press Enter.
- Upload multiple TrackRodent results files (.mat format) by pressing Choose results files.
- Fill in the numbers of Last frame for analysis, Test name, Stimulus 1 name, and Stimulus 2 name.
- Choose the desired analyses from the list of optional analyses by checking all appropriate boxes.
- Choose the Export results to a speadsheet by checking the appropriate box to extract all the results of the checked analyses as a single spreadsheet file.
- Press Start and wait until analysis complete.
NOTE: This concludes the analysis. The software can be used to analyze the results of as many movie files as desired, given that they were all behaviorally analyzed using the TrackRodent software. The analysis performed by the software assumes video recording at a frame rate of 30 Hz. In the case that a different frame rate was used, multiply the time by 30 and divide by the frame rate used for recording to convert it to the correct value (s).
Representative Results
Using the system for the social preference test in C57BL/6J mice
Figure 1 shows three versions of the experimental set-up. The first version (Figure 1A-C) is designed for mice with dark fur colors, such as C57BL/6J mice. The second (Figure 1D-F) is planned for mice with bright fur colors, such as BALB/c or ICR (CD-1) mice. The third is larger (Figure 1G-I) and designed for rats with bright fur colors, such as SD rats. The first subjects examined were naïve adult (8-12 weeks old), group-housed (two to five animals per cage) male C57BL/6J mice. Social stimuli were juvenile (21-30 days old), group-housed (two to six animals per cage), male C57BL/6J mice. Object stimuli were odorless plastic toys with distinct colors (insets in Figure 1C,I). Video files of the experiments were analyzed using the TrackRodent software (body-based algorithm, see exemplified screen picture and GUI in Figure 2A), which automatically and continuously tracked subject location based on 1) body center and 2) the contact of its body contours with the stimuli-containing chambers.
The analysis involved the following procedures. After uploading a movie file and graphically defining areas to be excluded from the analysis, the experimenter graphically defined two areas, each comprising a distinct chamber, as 'stimuli' (Figure 2A, yellowish areas). Users can also define up to five areas as virtual 'compartments'. Then, users choose the "BlackMouseBodyBased23_7_14_Fast" algorithm from of the list of possible algorithms (Figure 2B). After pressing the Start analysis button, the software tracked the animal location frame-by-frame and depicted the mouse location trace in the arena (Figure 2A, blue line). During this analysis, the software also tracked contacts between the 'stimuli' areas and body contour of the subject (Figure 2C). Such contact events were defined as investigatory and served to calculate the investigation time for each stimulus and the time during which no investigation behavior was displayed (Figure 2D). As apparent in Figure 2C,D showing the analyzed movie, higher investigation time for 'stimulus 2 was observed', which was the social stimulus, compared to the object, which in this case was defined as 'stimulus 1'.
Population analysis of the social preference test in C57BL/6J mice
After allowing the TrackRodent software to analyze all video files of the experimental group, a population analysis for this group was performed using the "TrackRodentPopulationSummary" GUI (Figure 2E), while choosing all possible analyses of this software. The distinct outputs of these analyses for the SP test performed with 58 C57BL/6J adult male mice are displayed in Figure 3, exactly as they appeared on the computer screen, with all error bars depicting SEM. Statistical analyses of these results were previously published11.
The mean total investigation time of the population of subjects towards each of the two stimuli (same-sex juvenile social stimulus and an object) is displayed in Figure 3A, which depicts a clear preference of the subject towards the social stimulus. Although the algorithm is simple, an excellent correlation was observed (r2 = 0.91, p < 10-6, Pearson's correlation) between the investigation time manually measured by a trained observer and the value calculated by the software. The dynamics of this preference can be seen in Figure 3B, in which the mean investigation time for each stimulus is plotted along time in 20 s bins. As shown, social preference is maintained by the subjects throughout the session, although it seemed slight stronger in early stages.
Figure 3C depicts the investigation time for each stimulus, categorized according to bout duration into short (≤6 s), medium (>6 s, ≤19 s), and long (>19 s) bouts, along with the total investigation time. The distribution of investigation time for each stimulus according to bout duration in 1 s bins is displayed in Figure 3D. As shown in Figure 3C,D, there was no difference between stimuli in the short bouts, while the medium and long bouts showed clear social preferences. These results suggest that in C57BL/6J mice, short bouts reflect curiosity, while long bouts reflect an interaction between the subject and stimuli. The relative duration of investigation (RDI, [social-object]/[social+object]) values for each category (short, medium, and long bouts), as well as for the total investigation time, are shown in Figure 3E. As shown, the highest RDI value is achieved with the long bouts, suggesting that they best reflect the social preferences of subjects.
To analyze the dynamics of investigation bout during the test, distributions over time for each category were separately plotted. The distributions over time of the short (Figure 3F) and medium (Figure 3G) investigation bouts suggest a gradual reduction of these bouts over time. In contrast, a similar analysis of the long bouts (Figure 3H) shows a gradual increase over the course of the test. This tendency is also reflected by the distribution of the mean bout duration (Figure 3I), showing a gradual increase over time. Thus, it seems as if the subjects exhibited curiosity mainly during the early stages of the test, while later they displayed more interactions with the stimuli. It should be noted that during the last minute, there is an inherent bias of the analysis towards short bouts, because long bouts were artificially terminated at the end of the session. Therefore, when considering the time course of the investigation bouts, the last minute should be ignored.
We also analyzed the intervals between successive investigation bouts towards the same stimulus (i.e., the time it took the subject to return to the same stimulus after the end of each investigation bout). This parameter reflects the urge of the subject to return to the same stimulus and investigate. Here, the intervals were also categorized into short (≤5 s), medium (>5 s, ≤20 s), and long (>20 s) bouts, which are shown together with total time intervals for each stimulus in Figure 3J. The histogram of the intervals in 1 s bins in displayed in Figure 3K. As shown in Figure 3J,K, the intervals between investigations of the social stimulus were much shorter than those between investigations of the object. Accordingly, the RDI values of intervals in the long range yielded the highest absolute value (Figure 3L); hence, they may be used as an investigative time-independent variable assessing social preference. The distributions over time of intervals plotted separately for each category (Figure 3M-O) suggest a gradual reduction in short and medium intervals and an opposite change in long intervals between investigation bouts, which most likely reflect the general lengthening of investigation bouts. Similar to the distribution of investigation bouts, the last minute of intervals is biased towards short values and should be ignored.
We also analyzed the transitions observed in subjects from one stimulus to another. This plot is displayed in Figure 3P, at which every timepoint the subject started to investigate a new stimulus is marked by a blue dot, every row represents a distinct subject, and the red line represents the mean transition rate. As apparent, the transition rate was highest (~1.5 transitions/min) during the first 2 min of the test and was gradually reduced to less than 50% of the peak. The same tendency is apparent from the distribution of mean transition rate over time (Figure 3Q).
Finally, heatmaps of investigation bouts towards the social stimulus (Figure 3R) and object (Figure 3S) during the course of the test for each subject are shown, with the colors marking bout duration (Figure 3S). Altogether, the results suggest that in C57Bl/6J mice, the SP test is roughly divided into an early exploratory phase (characterized by a high transition rate and short investigation bouts) and a late interaction phase (characterized by low transition rate and long investigation bouts).
Population analysis of the social novelty preference test in SD rats
Figure 4 shows the same analysis as above for the SNP test performed by 59 SD rats. Typically, the preference of novel vs. familiar social stimuli is weaker than the preference towards a novel social stimulus vs. an object, exhibited by mice in the SP test (Figure 3). Nevertheless, the social novelty preference is reflected clearly by all parameters. Notably, as concluded above from the SP test in mice, the long investigation bouts and long intervals are the parameters most clearly exhibiting differences between the stimuli, as reflected by their highest absolute RDI values (Figure 4E,L; it should be noted that RDI values for the SNP test are defined as [familiar-novel]/[familiar+novel]).
Loss of whiskers impairs social preference in both C57BL/6J mice and SD rats
To evaluate the effects of somatosensory manipulation on the dynamics of social behavior, analysis was performed of the SP test completed by 29 C57BL/6J mice and 33 SD rats following whisker trimming (conducted 3-7 days before testing). Then, these results were compared to control animals. As displayed in Figure 5A-F, the whisker-trimmed mice did not prefer the social stimulus over the object, as reflected by the lack of difference in investigation time between the two stimuli (compare Figure 5A a Figure 5D) and the loss of long investigation bouts towards to social stimulus (compare Figure 5B a Figure 5E). Interestingly, the whisker-trimmed mice also showed increased rates of transition compared to control mice (compare Figure 5C a Figure 5F).
Altogether, these data suggest that whisker-trimmed mice markedly reduced the duration of their interactions with the social stimulus. In contrast to mice, whisker-trimmed rats did not lose their social preferences (Figure 5G-L). However, they significantly changed their behavior during the first minute of the test, when they had fewer long investigation bouts (compare Figure 5H a Figure 5K) and many more transitions (compare Figure 5I a Figure 5L). Thus, whisker trimming significantly modified the behavior of both rats and mice in the SP test, but in a very different manner between the two species. These results suggest a distinct role for whisker-dependent somatosensory stimulation in social interactions of rats and mice.

Figure 1: Experimental set-up. (A) A schematic depiction of the experimental arena designed for mice with black fur. (B) A picture of the arena from above, using dim red light, showing a C57BL/6J subject mouse in the arena. (C) A picture showing the meshed area of a white chamber, through which the subject interacts with a stimulus (inset: a picture of an object stimulus used for mice). (D-F) As shown in panels A-C, an experimental arena and chambers designed for mice with bright fur. (G-I) As shown in panels A-C, an experimental arena and chambers designed for rats with bright fur (inset in [I]: picture of an object stimulus used for rats). Please click here to view a larger version of this figure.
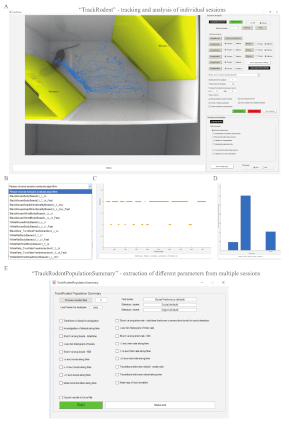
Figure 2: TrackRodent software. (A) A screen of a computer running the TrackRodent software. Left: the video image at the beginning of the analyzed movie (first frame chosen by the experimenter), showing the labeled 'stimuli' areas and tracked movement path of the C57BL/6J subject. Right: the software GUI. (B) The list of optional codes of the software to be used with the GUI. Each of these codes fits a distinct experimental condition. (C) The analysis of the specific experiment shown in panel A, when the option of Stimuli exploration along session was selected in the Results presentation section of the GUI. This plot shows (at each frame) whether the subject was in contact with 'stimulus 1' or 'stimulus 2'. In the presented case, a clear preference towards 'stimulus 2' is evident by the higher number and duration of investigation bouts detected towards this stimulus. (D) The analysis of the experiment shown in panels A and C, when the option of Total stimuli exploration time was selected in the Results presentation section of the GUI. Here too, a clear preference towards 'stimulus 2' is evident from the higher level of investigation time towards this stimulus. (E) The GUI of the TrackRodentPopulationSummary software that enables analysis and plotting of the results from a population of animals, each of which is analyzed using the TrackRodent software (see Figure 3 for the results of such analysis). Please click here to view a larger version of this figure.
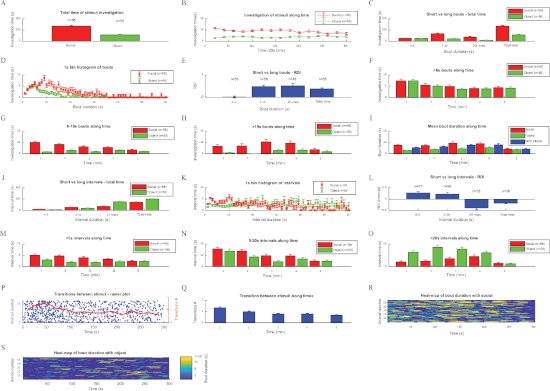
Figure 3: Analyzing the investigation behavior of C57BL/6J mice during SP test using the TrackRodent software. This figure shows the various plots of all optional analyses of the TrackRodentPopulationSummary software (Figure 2E) employed for a population of 58 C57BL/6J adult male mice performing the SP test. The various plots are displayed in a similar order and manner as they appeared on the computer screen (see the representative results section for a detailed explanation of each plot). (A) Total time of stimuli investigation, (B) Investigation of stimuli along time, (C) Short vs long bouts – total time, (D) 1s bin histogram of bouts, (E) Short vs long bouts – RDI, (F) <6 s bouts along time, (G) 6−19 s bouts along time, (H) >19 s bouts along time, (I) Mean bout duration along time, (J) Short vs long intervals – total time, (K) 1 s bin histogram of intervals, (L) Short vs long intervals – RDI, (M) <5 s intervals along time, (N) 5−20 s intervals along time, (O) >20 s intervals along time, (P) Transitions between stimuli – raster plot, (Q) Transition between stimuli along times, (R) Heat-map of bout duration with social, (S) Heat-map of bout duration with object. Please click here to view a larger version of this figure.
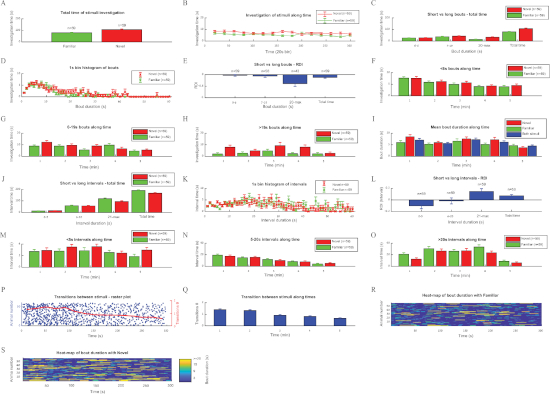
Figure 4: Analyzing the investigation behavior of SD rats during SNP test using the TrackRodent software. Plots for all optional analyses of the TrackRodentPopulationSummary software (Figure 2E) employed for a population of 59 SD adult male rats performing the SNP test. The various plots are displayed in a similar order and manner as they appeared on the computer screen (see the representative results section for a detailed explanation of each plot). (A-Q) Same as described in Figure 3. (R) Heat-map of bout duration with Familiar, (S) Heat-map of bout duration with Novel. Please click here to view a larger version of this figure.
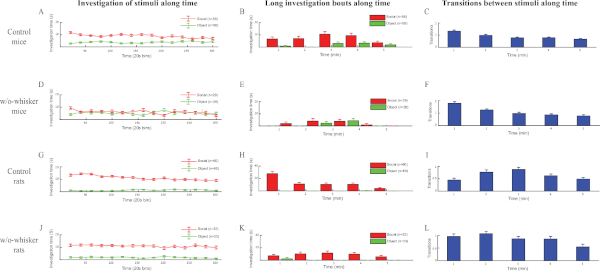
Figure 5: Whisker-trimming of mice and rats differentially impairs social behavior. (A) Plot of mean investigation time (measured in 20 s bins) during the SP test for male C57BL/6J mice (n = 58). (B) Distribution (1 min bins) of mean total time of long (>19 s) investigation bouts along the SP test shown in panel A for social and object stimuli. Note the much longer time of long investigation bouts towards the social stimulus compared to the object. (C) Distribution (1 min bins) of the transition rate along the time of the SP test shown in (A). (D-F) As shown in panels A-C, 29 mice's whiskers were trimmed about 1 week before the test. These animals lost their social preference, as reflected by the (D) general investigation time and (E) distribution of long bouts. They also displayed a higher general rate of transitions (F), suggesting less sustained interactions with the social stimulus. (G-I) As shown in panels A-C, 60 SD rats performed the SP test. Note the different dynamics of the rats' behavior compared to mice, with lower transition rates and longer periods of long investigation bouts in the early phase (2 min) of the test. (J-L) As shown in panels G-H, 33 rats were whisker-trimmed. Note that while the rats did not lose social preference following whisker-trimming as mice did, the dynamics of their social behavior in the first 2 min of the test was changed, showing more transitions and fewer long bouts. Please click here to view a larger version of this figure.
Discussion
The experimental system described here, which was designed as an alternative to the three-chamber apparatus2,5, allows performance of the same tests while solving some of its limitations. The use of triangular chambers, which are located in two opposite corners of the rectangular arena limits the subject-stimulus interaction area to a well-defined plane, thus enabling precise automated analysis of investigation behavior. One advantage is the use of the analysis software (TrackRodent) to calculate multiple behavioral parameters for each test. It also allows repositioning of the chambers in random opposite corners of the arena during the various stages of the paradigm, neutralizing any effects of spatial navigation skills.
Furthermore, the two corners of the arena, which are left empty during each stage, offer the subject a convenient place for resting and grooming and draw it away from the chambers, minimizing false-positive results. Thus, the system allows direct assessment of the subject's motivation to investigate each stimulus during the SP and SNP tests. Furthermore, as behavioral analysis of the videos is done in an automated manner by the TrackRodent software, an experimenter may load multiple movies for analysis, define the correct parameters for each, and let the software process overnight, which saves precious time. Notably, the system may be used for any type of social discrimination test, such as discrimination between male and female social stimuli or stimuli of distinct strains. Moreover, there are no limits (upper/lower) to the size of subjects that can be tracked using this system; thus, it may be used with juveniles or obese animals. This experimental system is inexpensive and simple to produce, and the computer codes used for analysis are publicly available as an open-source codes, allowing any laboratory to easily adopt the method.
The automated detection of investigation behavior in the system is based on detection of physical contact between the body contours of a subject and each of the areas defined by the experimenter as 'stimuli'. Thus, if the subject touches the 'stimuli' area with its body rather than head, this event is considered investigatory (i.e., a false positive). For this purpose, the head-directionality based algorithm was generated, which identifies the head of the subject and takes into account only contact between the head and 'stimuli'. However, this algorithm runs significantly slower than the body-based algorithms; hence, it is not recommended for analysis of a large number of movies, unless experimental conditions require it.
Similar results were observed (1.1% difference, n = 11 experiments, 5 min each, data not shown) between the two algorithms; therefore, the body-based algorithms were most often used. It should be noted that although analyses of large groups of animals are presented here, a much smaller group size is needed for observing a statistically significant difference between two stimuli, based on the total investigation time measured by the system. For example, power calculations revealed that sample sizes of only five and eight animals are required for the SP and SNP tests of mice, respectively (α = 0.05, power = 0.8). Yet, significantly larger groups of animals were used here to ensure observation of all the dynamics of social behavior.
The system requires that the experimenter manually defines the threshold for detection of the subject's body contours. Finding the right threshold to be use may require some experience; hence, most algorithms used in this system have regular, slower versions. These versions present the analyzed movie and detect investigation events online, while their fast versions do not enable online presentation. It is recommended that new users use the regular versions and monitor analyses online to ensure that it works properly, and that they use the "fast" algorithms only after gathering experience with the analysis procedure.
One advantage of this system over the three-chamber test is that it measures multiple parameters of investigation behavior, thus supporting more detailed analyses. This advantage is two-fold. First, the experimenter does not have to rely solely on the total investigation time to determine the preference. It was found that the long investigation bouts and long intervals are more sensitive than the total investigation time when detecting a preference of one stimulus over another. Regarding these parameters, it should be noted that their values during the last minute of the test are biased downwards, as the long investigation bouts and intervals are prematurely terminated at the end of the 5-min session.
Second, the multiple parameters allow detecting of subtle changes in the dynamics of social investigation following various manipulations. For example, it was found that C57BL/6J male mice lost their social preference following whisker-trimming. In contrast, SD male rats retained their social preference but changed social behavior dynamics as shown by shorter investigation bouts and higher level of transitions in the early phase of the test. Overall, these data suggest a reduction in the tendency of rats and mice for social interactions following whisker trimming. Thus, this system supports a rigorous analysis of the dynamics of social behavior, which may be highly sensitive to various manipulations.
In summary, we presented here a novel, simple, and affordable experimental system that supports automated analysis of social investigation behavior dynamics. This system will facilitate detailed analyses of social behavioral deficits in various strains and genetically modified lines of small rodents. Furthermore, the precise detection of investigation bouts demonstrated here, combined with the capability of this system to analyze behavior while subjects are connected to electrical cables or optical fibers, allows its use in experiments involving recording of brain activity associated with social behavior.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by The Human Frontier Science Program (HFSP grant RGP0019/2015), the Israel Science Foundation (ISF grants #1350/12, 1361/17), by the Milgrom Foundation and by the Ministry of Science, Technology and Space of Israel (Grant #3-12068).
Materials
| Flea3 1.3 MP Mono USB3 Vision | FLIR (formerly PointGrey) | FL3-U3-13Y3M-C | Monochromatic Camera |
| FlyCap 2.0 | FLIR (formerly PointGrey) | FlyCapture 2.13.3.61X64 | Video recording software |
| Home 5 minute Epoxy glue | Devocon | 20845 | For gluing the metal mesh to the Plexiglas stimuli chambers |
| Matlab 2014-2019 | MathWorks | R2014a – R2019a | Programming environment |
| Plexiglas boards (6 mm thickBlack or white) | Melina (1990) LTD, Israel | NaN | For arena and stimuli chambers construction |
| Red led strips (60 leds per meter) connected to a 12V power supply | 2012topdeal eBay supplier | NaN | For illumination of the acoustic chamber |
Referencias
- Insel, T. R. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 65 (6), 768-779 (2010).
- Moy, S. S., et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes, Brain and Behavior. 3 (5), 287-302 (2004).
- Carr, W. J., Yee, L., Gable, D., Marasco, E. Olfactory recognition of conspecifics by domestic Norway rats. Journal of Comparative and Physiological Psychoogyl. 90 (9), 821-828 (1976).
- Ferguson, J. N., Aldag, J. M., Insel, T. R., Young, L. J. Oxytocin in the medial amygdala is essential for social recognition in the mouse. Journal of Neuroscience. 21 (20), 8278-8285 (2001).
- Nadler, J. J., et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes, Brain and Behavior. 3 (5), 303-314 (2004).
- Page, D. T., Kuti, O. J., Sur, M. Computerized assessment of social approach behavior in mouse. Frontiers in Behavioral Neuroscience. 3, 48 (2009).
- Sankoorikal, G. M., Kaercher, K. A., Boon, C. J., Lee, J. K., Brodkin, E. S. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biological Psychiatry. 59 (5), 415-423 (2006).
- Martin, L., Sample, H., Gregg, M., Wood, C. Validation of operant social motivation paradigms using BTBR T+tf/J and C57BL/6J inbred mouse strains. Brain and Behavior. 4 (5), 754-764 (2014).
- Noldus, L. P. J. J., Spink, A. J., Tegelenbosch, R. A. J. EthoVision: A versatile video tracking system for automation of behavioral experiments. Behavior Research Methods Instruments & Computers. 33 (3), 398-414 (2001).
- Sams-Dodd, F. Automation of the social interaction test by a video-tracking system: behavioural effects of repeated phencyclidine treatment. Journal of Neuroscience Methods. 59 (2), 157-167 (1995).
- Netser, S., Haskal, S., Magalnik, H., Wagner, S. A novel system for tracking social preference dynamics in mice reveals sex- and strain-specific characteristics. Molecular Autism. 8, 53 (2017).

